Key Points
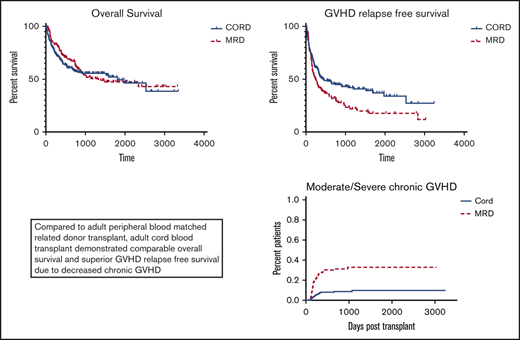
Adult CBT results in comparable overall survival and superior GRFS compared with matched sibling peripheral blood transplant.
In select situations, relapse rate may be lower with comparable transplant-related mortality comparing cord with peripheral blood transplant.
Abstract
We compared outcomes among adult matched related donor (MRD) patients undergoing peripheral blood stem cell transplantation and adult patients undergoing double unit cord blood transplantation (CBT) at our center between 2010 and 2017. A total of 190 CBT patients were compared with 123 MRD patients. Median follow-up was 896 days (range, 169-3350) among surviving CBT patients and 1262 days (range, 249-3327) among surviving MRD patients. Comparing all CBT with all MRD patients, overall survival (OS) was comparable (P = .61) and graft-versus-host disease (GVHD) relapse-free survival (GRFS) was significantly improved among CBT patients (P = .0056), primarily because of decreased moderate to severe chronic GVHD following CBT (P < .0001; hazard ratio [HR], 3.99; 95% confidence interval [CI], 2.26-7.04). Among patients undergoing our most commonly used MRD and umbilical cord blood (CB) myeloablative regimens, OS was comparable (P = .136) and GRFS was significantly improved among CBT patients (P = .006). Cumulative incidence of relapse trended toward decreased in the CBT group (P = .075; HR, 1.85; CI 0.94-3.67), whereas transplant-related mortality (TRM) was comparable (P = .55; HR, 0.75; CI, 0.29-1.95). Among patients undergoing our most commonly used nonmyeloablative regimens, OS and GRFS were comparable (P = .158 and P = .697). Cumulative incidence of both relapse and TRM were comparable (P = .32; HR, 1.35; CI, 0.75-2.5 for relapse and P = .14; HR, 0.482; CI, 0.18-1.23 for TRM). Our outcomes support the efficacy of CBT and suggest that among patients able to tolerate more intensive conditioning regimens at high risk for relapse, CB may be the preferred donor source.
Introduction
Umbilical cord blood (CB) is a well-established donor source for adult hematopoietic stem cell transplantation. Advantages of CB include rapid availability, less rigorous matching requirements than unrelated donors, decreased chronic graft-versus-host disease (cGVHD) compared with other donor sources, and, particularly for acute leukemias conditioned with at least moderately intensive conditioning regimens, decreased relapse rates.1-6 Challenges associated with cord blood transplantation (CBT) include delays in time to engraftment, delays in acquired immune reconstitution, increased risk of graft failure in high-risk circumstances, and increased acute costs of transplant resulting from CB acquisition costs and longer hospital stays early posttransplant.4,6-11
Numerous studies have demonstrated comparable overall survival (OS) following CB vs other donor source transplants.3-6 At our center, we have previously demonstrated comparable OS with significantly less cGVHD when comparing outcomes among CB and matched unrelated donors (MUDs).12 As a result of these findings, we have prioritized CB over MUD in most circumstances since 2014. Matched related donors (MRDs) have remained our first choice of donor source if available. In this report, we compare outcomes among MRD patients undergoing peripheral blood stem cell transplantation and CBT patients treated at our center between 2010 and 2017. To examine specific populations in whom CBT may be more or less beneficial, we also compare outcomes following our most commonly used MRD and CB myeloablative and nonmyeloablative conditioning regimens.
Methods
Patients
We compared outcomes in all consecutive adult patients undergoing first peripheral blood stem cell transplantation MRD transplant or first unmanipulated double unit CBT from January 2010 to December 2017. During this interval, no patients received single-unit CBT. All patients signed institutional review board-approved informed consent authorizing data collection for this analysis. GVHD-free, relapse-free survival (GRFS) was defined by events that included grade 3-4 acute GVHD (aGVHD), moderate to severe cGVHD, relapse, or death.13 cGVHD-free, relapse-free survival (CRFS) was defined by events that included moderate to severe cGVHD, relapse, or death.14 aGVHD was defined and graded according to Mount Sinai Acute GVHD International Consortium criteria.15 cGVHD and late aGVHD were defined and graded according National Institutes of Health consensus criteria for clinical trials in cGVHD.16 Graft failure was defined according to institutional guidelines and included: absence of 3 consecutive days with neutrophils ≥0.5 × 109/L at day +28 for MRD patients or day +42 for CB patients, less than 5% unsorted marrow or less than 5% sorted CD3 and CD33 donor-derived chimerism at day +28, or autologous count recovery (>95% recipient derived) with less than 5% donor CD3 peripheral blood chimerism without relapse.
Patient selection, GVHD prophylaxis, infectious disease prophylaxis, and donor selection were per institutional standards. Pretransplant performance status was evaluated using the Sorror hematopoietic stem cell transplant-specific (HSCT) comorbidity index.17 Conditioning regimen details are provided in Table 1. GVHD prophylaxis was cyclosporine and mycophenolate mofetil for CBT patients and tacrolimus and methotrexate for MRD patients undergoing reduced intensity or myeloablative conditioning and tacrolimus and mycophenolate mofetil for MRD patients undergoing nonmyeloablative conditioning with 90 mg/m2 fludarabine (FLU) and 2 to 3 Gy total body irradiation (TBI). For both cohorts, immunosuppression was scheduled to be tapered by approximately 6 months following transplant in the absence of GVHD. Standard infection prophylaxis for both CB and MRD patients included levofloxacin through neutrophil engraftment, fluconazole through 80 days (with mold active prophylaxis for patients with prolonged neutropenia or prior possible mold infection before transplant), sulfamethoxazole/trimethoprim through at least 6 months, acyclovir through at least 1 year for MRD patients and until varicella zoster vaccination for CB patients, and, for CB patients, high-dose acyclovir or letermovir through day 100 as previously published.18 Sibling donors were assessed for eligibility on the basis of physical examination, laboratory testing, and review of medical history. No upper or lower age limits were established a priori. Cord unit selection criteria evolved over time; initially, individual units were matched at a minimum of 4/6 loci (low-resolution HLA-A and HLA-B and high-resolution HLA-C) and, as possible, contained at least 2.0 total nucleated cells (TNC) × 107/kg. More recently, high-resolution typing and CD34 × 106/kg have also been considered. As possible, ≥8/10 and CD34 × 106/kg ≥0.1 were sought for each unit. Relapse was defined per standard morphologic criteria. Relapse risk was assigned according to the American Society for Blood and Marrow Transplantation Request for Information risk classification.19
Infections included all bacteremias, symptomatic culture positive urinary tract infections, symptomatic diarrhea with pathogens detected by polymerase chain reaction or culture, proven fungal infections, and symptomatic viral infections detected by polymerase chain reaction or culture.
Statistics
Patient characteristics and follow-up times were summarized using standard measures. Kaplan-Meier estimates were used to evaluate OS and GRFS. Kaplan-Meier curves were compared using log-rank test. Cumulative incidence estimates were used to compare relapse, transplant-related mortality (TRM), aGVHD, cGVHD, and neutrophil and platelet engraftment.20 For relapse, TRM was considered a competing risk, and for TRM, relapse was considered a competing risk. For aGVHD and cGVHD, relapse, TRM, and graft failure were considered competing risks. For neutrophil and platelet engraftment, relapse or TRM before day +28 for MRD and day +42 for CB were considered competing risks. Cumulative incidence curves were compared using the Gray test.
A multivariate analysis was used to assess OS. Variables in the model included donor source (MRD or cord), age, conditioning (high-definition TBI, myeloablative non-high-definition TBI, nonmyeloablative, and reduced intensity), HSCT-comorbidity index score (0-3, 4-5, >5), disease, and disease risk (low risk, intermediate risk, high risk, not applicable risk).
Infectious outcomes were examined among patients alive and not experiencing relapse at serial intervals (day 0-100, day 100-1 year, 1-2 years, and 2-3 years posttransplant). Patients were censored for infectious disease follow-up at the last date at which regular clinical documentation follow-up data were available. To ensure complete follow-up for the interval being analyzed, patients were only included if they had a censor date higher than the last day in the period. Patients experiencing relapse or TRM in a period were excluded from analysis in that period and subsequent periods (eg, patient experiencing relapse at 400 days posttransplant was included in day 0-100 and day 100-1 year analyses but excluded from 1-2 year and 2-3 year analyses). To compare whether an infection occurred per individual patient in a time period, analysis consisted of binomial logistic regression comparing CB and MRD patients for the odds of any infection occurring within the specified period. To compare the number of infections per patient per period, analysis consisted of t tests comparing the number of infections in a time period between CB and MRD.
For cumulative incidence curves, multivariate analysis, and infectious disease analysis, all data manipulation was carried out in R 3.6.0. All statistical analysis was carried out in SAS 9.4. For Kaplan-Meier curves, analysis was performed in GraphPad Prism 8. A significance threshold of 0.05 was used for all comparisons.
Results
Patient and graft characteristics
A total of 190 CBT patients were compared with 123 MRD patients. Details of patient characteristics are summarized in Table 1. Details of CB units are summarized in Tables 2 and 3. For MRD donors, median infused cells were 6.07 × 106 CD34/kg (range, 2.99-9.66). Age, diagnosis, conditioning regimen intensity, comorbidity index score, and relapse risk were all comparable between each group. Median follow-up was 896 days (range, 169-3350) among surviving CB patients and 1262 days (range, 249-3327) among surviving MRD patients. Shorter median follow-up among CB patients resulted because CB transplant numbers increased following 2014 when CB became our preferred donor over MUDs.
Engraftment
Cumulative incidence of neutrophil engraftment among CB patients was 92.6% at a median of 20 days and among MRD patients was 98.3% at a median of 17 days (time to engraftment favoring MRD P = .001; hazard ratio [HR], 1.974; 95% confidence interval [CI], 1.54-2.53). Cumulative incidence of platelet engraftment among CB patients was 84.1% at a median of 38 days and among MRD patients was 95.6% at a median of 17 days (time to engraftment favoring MRD P = .001; HR, 4.71; 95% CI, 3.03-7.30). Six CB patients experienced primary graft failure and no MRD patients experienced primary graft failure.
GVHD
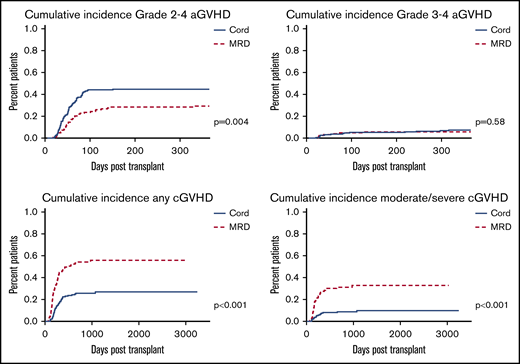
GVHD data are summarized in Figure 1. Cumulative incidence of grade II-IV aGVHD was higher among CBT then MRD patients (P = .004; HR, 0.571; 95% CI, 0.39-0.84), whereas cumulative incidence of grade III-IV aGVHD was comparable (P = .58; HR, 0.776; 95% CI, 0.31-1.9). Cumulative incidence of any as well as moderate to severe cGVHD was higher among MRD than CBT patients (P < .0001; HR, 2.77; 95% CI, 1.92-3.99 for any cGVHD and P < .0001; HR, 3.99; 95% CI, 2.26-7.04 for moderate to severe cGVHD).
Infections
Infection data are summarized in Table 4. Among patients surviving without relapse, the proportion of individual patients experiencing at least 1 infectious episode in intervals day 0-day 100, day 100-1 year, 1-2 years, and 2-3 years posttransplant was comparable between the CB and MRD groups. The number of infections per patient was increased among CB patients in the day 0-day 100 (P = .01) and day 100-1 year (P = .048) intervals but was comparable in the 1-2 years and 2-3 years intervals.
OS, GRFS, CRFS, relapse, and TRM
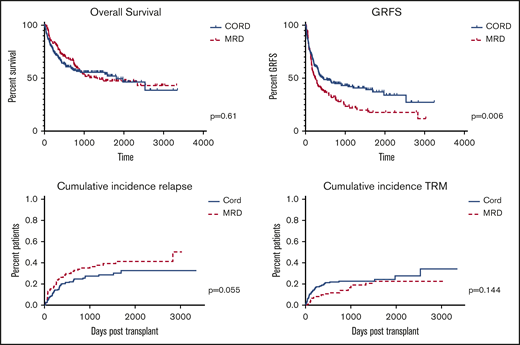
Outcomes are summarized in Figure 2. Comparing all CBT to all MRD patients, OS was comparable (P = .61) and GRFS and CRFS were significantly improved among CBT patients (P = .0056 and P = .0017, respectively). Cumulative incidence of relapse trended higher for MRD patients (P = .055; HR, 1.47; 95% CI, 0.99-2.19), whereas cumulative incidence of TRM was comparable (P = .144; HR, 0.69; 95% CI, 0.42-1.14). OS was comparable among CBT patients treated before 2014, when MUD donors were selected before CB donors, and after 2014, when CB was the first donor choice if no MRD was available (P = .78).
Multivariate analysis including donor source, age, conditioning intensity, comorbidity index, disease, and disease risk demonstrated that HSCT-comorbidity index scores 0-3 vs >5 and 4-5 vs >5 (HR, 0.20; P < .001; and HR, 0.22; P < .001) were associated with decreased survival and high-risk vs low-risk disease (HR, 1.56; P = .071) trended toward decreased survival.
Myeloablative outcomes
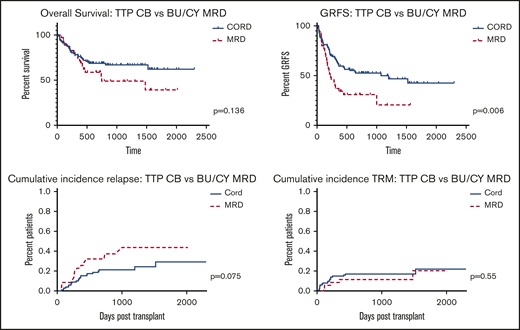
We compared outcomes between our most commonly used myeloablative CB and MRD regimens (for CB: FLU, 150 mg/m2; cyclophosphamide [CY], 50 mg/kg; thiotepa [TTP], 10 mg/kg; and 400 cGy TBI [TTP regimen] and for MRD: 12.8 mg/kg busulfan, 120 mg/kg CY [BU/CY regimen]). Outcomes are summarized in Figure 3. Among TTP regimen CB patients (n = 101) median age was 52 (range, 21-68) and median follow-up among survivors 818 days (range, 169-2300) vs median age 52 (range, 22-66) and median follow-up among survivors 715 days (range, 393-2018) among BU/CY (n = 35) MRD patients. Comparing TTP regimen CB to BU/CY MRD patients, OS was comparable (P = .136) and GRFS was significantly improved among CB patients (P = .006). Cumulative incidence of relapse trended toward significantly lower in the TTP regimen CB group (P = .075; HR, 1.85; 95% CI, 0.94-3.67), whereas cumulative incidence of TRM was comparable between each group (P = .55; HR, 0.75; 95% 95% CI, 0.29-1.95). Results were similar if FLU/melphalan (MEL) MRD patients (n = 30) were added to the BU/CY MRD group (OS P = .164).
Overall survival, GRFS, relapse, and TRM following myeloablative conditioning.
Nonmyeloablative outcomes
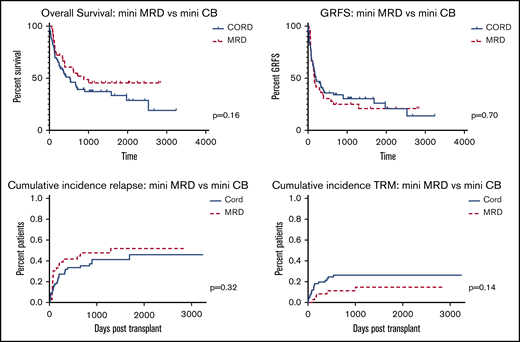
We compared outcomes between our most commonly used nonmyeloablative CB and MRD regimens (for CB: FLU 200 mg/m2, CY 50 mg/kg, and 2-3 Gy TBI [mini CB] and for MRD: 90 mg/m2 FLU and 2-3 Gy TB) [mini MRD]). Outcomes are summarized in Figure 4. Among mini CB patients (n = 66), median age was 68 (range, 23-73) and median follow-up among survivors 1063 days (range, 316-3244) vs median age 59 (range, 20-76) and median follow-up among survivors 1657 days (range, 262-2835) among mini MRD patients (n = 36). Comparing mini CB (n = 66) to mini MRD (n = 36) patients, OS and GFRS were comparable (P = .158 and P = .697). Cumulative incidence of relapse and TRM were comparable between each group (P = .32; HR, 1.35; 95% CI, 0.75-2.5 for relapse and P = .14; HR, 0.482; 95% CI, 0.18-1.23 for TRM).
Overall survival, GRFS, relapse, and TRM following nonmyeloablative conditioning.
Overall survival, GRFS, relapse, and TRM following nonmyeloablative conditioning.
Discussion
We compared outcomes of double unit CB and MRD patients undergoing transplant at our center between 2010 and 2017. Among all patients, we found comparable OS, but improved GRFS following CBT. Consistent with other reports, CBT was associated with significantly higher grade 2-4 aGVHD (but not grade 3-4 aGVHD), significantly less cGVHD (both any and moderate to severe cGVHD), decreased relapse, and a trend toward higher TRM compared with MRD.3-6,12
The significant split in GRFS favoring CBT developed at approximately 150 days posttransplant primarily because of the increased development of moderate to severe cGVHD among MRD patients. Although the use of peripheral blood (PB) among MRD donors may have resulted in higher cGVHD rates than would be seen with bone marrow, PB is a standard donor source in the setting of MRD transplantation for hematologic malignancies. The question of optimal donor source for MRD transplant is controversial, and some data suggest improved OS and decreased relapse using PB in this setting.21-24
The observation of reduced cGVHD in the CBT setting has important implications for several issues related to CBT. Double unit CBT is associated with higher acute transplant costs than other donor source transplants because of acquisition cost of CB units and longer initial inpatient stays.7,11 Over the long term, however, cGVHD is the primary driver of long-term posttransplant costs. Long-term costs of posttransplant care may therefore be higher for MRD transplants resulting in more comparable lifetime total costs. No robust data are available on this question, and it should be evaluated further. Additionally, improved GRFS is likely associated with improved long-term quality of life (QOL); studies are needed to assess long-term QOL following CBT vs other donors.
With respect to infections, CB is associated with slower neutrophil engraftment and delayed acquired immune reconstitution.4,10,25,26 Details of how CB transplants are conducted, combined with novel anti-infectious agents, may improve infectious outcomes. We use TBI for immunosuppression in all CBT conditioning regimens and avoid antithymocyte globulin (ATG) given the association of ATG with further delays in acquired immune reconstitution.27-29 Recent data suggest ATG-free adult CBT is associated with robust CD4+ T-cell recovery.30 The use of high-dose acyclovir to prevent CMV reactivation, and, more recently, the addition of letermovir has significantly reduced issues with CMV in the early posttransplant period.18 By prioritizing CB over MUD, we are typically able to identify optimal CB units with respect to size and matching, which may improve immune reconstitution. When comparing incidence of late infections between CB and MRDs, a higher incidence of cGVHD may result in higher incidence of late infection following MRD transplant. We found that the proportion of individual patients surviving without relapse who experienced infections posttransplant was comparable between the MRD and CB patients at intervals day 0-day 100, day 100-1 year, 1-2 years, and 2-3 years posttransplant, whereas the number of infections per patient was increased among CB patients between day 0-day 100 and day 100-1 year posttransplant and was comparable between 1-2 years and 2-3 years posttransplant. These data suggest that a subset of CB patients, rather than all patients, may experience poorer early immune reconstitution resulting in a higher incidence of infectious issues early posttransplant, but that over time the combination of immune recovery and reduced incidence of cGVHD result in comparable infections outcomes compared with MRD patients. Further studies to assess risk factors for poor early immune reconstitution following CBT and strategies to improve this issue are ongoing.
To further examine outcomes, we sought to explore specific patient populations that may particularly benefit from CBT compared with MRD transplant. Although each conditioning regimen has its own specific expected toxicities and anti-malignancy effects and direct comparisons between different regimens is imperfect, conditioning regimen intensity is particularly important when considering optimal patients for CBT. We and others have demonstrated more intensive conditioning regimens to be associated with markedly low relapse rates following CBT,1-4 whereas relapse rates have been high following the most commonly published nonmyeloablative CBT regimen, the mini cord regimen pioneered by the University of Minnesota.8 Concurrently, older or more comorbid patients in whom nonmyeloablative regimens are deemed more appropriate may be more susceptible to toxicity, especially delays in immune reconstitution, associated with CBT. To explore these issues in our experience, we compared outcomes with our most commonly used myeloablative CBT and MRD regimens and our most commonly used nonmyeloablative CBT and MRD regimens.
Among more intensively conditioned patients, there was a trend toward significant improvement in OS because of a trend toward significantly decreased relapse rates and significantly improved GRFS among CBT patients. These data suggest that CBT might be considered over MRD in selected patients, particularly those deemed at high risk for relapse.
In contrast, among patients undergoing nonmyeloablative conditioning, there was a trend toward improvement in OS in MRD patients and comparable GRFS between both groups. Both relapse rate and TRM were modestly increased in the CBT group, although neither reached statistical significance. Two-year survival among CBT patients receiving non-myeloablative conditioning was 34%, which is nearly identical to the 35% 2-year survival among CBT patients undergoing the same conditioning in the recently reported CTN 1101 trial comparing CBT with haploidentical transplant outcomes.19 Though OS was improved following haploidentical transplant in that study, our data affirm that the nonmyeloablative conditioning regimen used for CBT patients in that trial is associated with poor outcomes compared with other conditioning regimens and is not reflective of potential outcomes following CBT. Among these older and more comorbid patients, strategies to reduce both TRM and relapse are needed to improve outcomes. Given these patients’ vulnerability to TRM and relapse, strategies to reduce time to engraftment including haploidentical cord transplant31,32 or strategies to ex vivo expand CB units33,34 may be particularly valuable to enable intensification of conditioning in the CB setting to reduce relapse while hopefully simultaneously reducing early TRM.
Although our series is single center and retrospective, follow-up is durable. Given our institutional commitment to CB and our donor search algorithm that prioritizes CB over MUD in most circumstances, our CB population reflects a more representative group of transplant patients than centers who reserve CB for the highest risk patients with no alternative donors. Our algorithm results in rapid transplant turnaround for all patients, and because we are frequently transplanting patients with more common HLA typing than centers that reserve CB for patients lacking MUD options, we are able to identify optimal CB units for transplant in most circumstances. Median unit matching for our patients was 5/6 at low resolution and 8/10 at high resolution and median unit size was 2.7 TNC × 107/kg and 0.13 CD34 × 106/kg. Each of these parameters are well above recommended minimums for CB units selected for transplant.35 During the period of this study, we had no patients who lacked an MRD and did not have acceptable CB donors. We did not have different standards for comorbidities for consideration of MRD vs CB transplant. Our outcomes clearly support the efficacy of CBT, and suggest that among patients able to tolerate more intensive conditioning regimens, CB may be the preferred donor source.
We recognize the positive impact of posttransplant cyclophosphamide on cGVHD rates following haploidentical, and, increasingly, MRD and MUD transplant, and are currently exploring posttransplant cyclophosphamide at our center in the MRD setting in addition to exploring haploidentical transplantation when optimal CB units are not available. Though randomized trials of optimized transplant strategies are undoubtedly challenging, exploration of long-term QOL, long-term costs of transplants, and outcomes in the setting of measurable residual disease before transplant comparing CB to other donor sources are feasible and needed to better understand the relative risks and benefits of CB vs other donor sources.
For data sharing, e-mail the corresponding author, Jonathan A. Gutman (jonathan.gutman@ucdenver.edu).
Authorship
Contribution: P.S. and J.A.G. designed research, performed research, analyzed data, and wrote the paper; E.P., B.H., D.A.P., M.K., T.M., P.F., D.S., E.C., R.R., and C.A.S. performed research and wrote the paper; and A.H. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan A. Gutman, Division of Hematology, University of Colorado, 1655 Aurora Court, AOP 4327, Aurora, CO 80045; e-mail: jonathan.gutman@ucdenver.edu.