Key Points
CD47 is overexpressed on Sézary cells, which correlates with poor OS.
SIRPαFc depletes Sézary cells in patients with SS.
Abstract
Sézary syndrome (SS), the leukemic variant of cutaneous T-cell lymphoma, has limited treatment options and rare occurrences of long-term remission, thus warranting research into new treatment approaches. CD47 has emerged as a promising target for multiple tumor types, but its role in SS remains unknown. Here, we show that CD47 is highly expressed on Sézary cells in the peripheral blood and skin, and the high level of CD47 expression correlates with worse overall survival (OS) in patients with SS. We also demonstrate that CD47 expression on Sézary cells is under the influence of interleukin 4 (IL-4), IL-7, and IL-13. Signal regulatory protein αFc (SIRPαFc; TTI-621), a novel CD47 decoy receptor, triggers macrophage-mediated phagocytosis of Sézary cells and, when administered in clinical trial settings, results in significant tumor load reduction. We conclude that inhibition of the CD47-SIRPα signaling pathway has therapeutic benefit for patients with SS. This trial was registered at www.clinicaltrials.gov as #NCT02663518.
Introduction
Sézary syndrome (SS) is a rare and aggressive form of cutaneous T-cell lymphoma (CTCL), traditionally identified as a triad of erythroderma, generalized lymphadenopathy, and leukemic burden in the peripheral blood.1 The average survival of patients with SS is 2 to 4 years.2,3 Although durable remissions with conventional chemotherapy are rarely achieved,4-6 immunotherapies, including extracorporeal photopheresis and interferon α (IFN-α), are reported to lead to durable responses in select patients, suggesting that immune modulation is a useful strategy for the management of these patients.7 Recently, the innate checkpoint CD47 has been identified as a “do-not-eat” signal on tumor cells.8-10 CD47 is a ubiquitously expressed, heavily glycosylated member of the immunoglobulin superfamily.11 As a marker of self, CD47 contributes to the recognition of autologous cells through binding of signal regulatory protein α (SIRPα) on macrophages (Mϕs) and other myeloid cells thus inhibiting phagocytosis. Via the same mechanism, CD47 is involved in Mϕ-mediated clearance of senescent red blood cells due to loss of CD47.12 Notably, CD47 is overexpressed in hematologic and solid tumors, allowing evasion of immune surveillance through negative regulation of phagocytosis.10,13-23
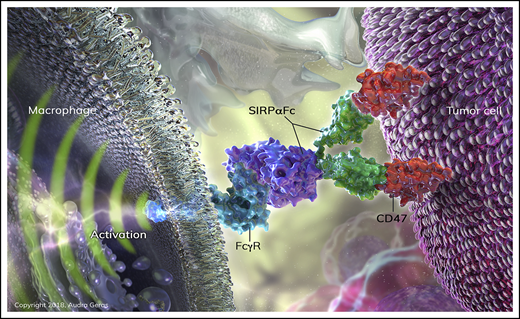
SIRPαFc (TTI-621) is a novel decoy receptor for CD47 that prevents the antiphagocytic signal derived from CD47-SIRPα interaction.24 The fusion protein is composed of the CD47-binding domain of human SIRPα linked to the Fc region of human immunoglobulin G1 (IgG1). It is designed as a dual-function molecule, neutralizing the suppressive CD47 signal and activating Mϕs through Fc receptors. TTI-621 is presently under investigation in relapsed and refractory hematologic malignancies using weekly IV infusion (NCT02663518), and in percutaneously accessible relapsed and refractory solid tumors and mycosis fungoides using intratumoral delivery (NCT02890368). TTI-621 has minimal binding to human erythrocytes in vitro, and early clinical results suggest no treatment-induced anemia in patients.25
In the present study, we offer new insight into the pathogenesis of SS by showing that overexpression of CD47 on Sézary cells is under the influence of T helper 2 (Th2) cytokines. In addition, we show that the expression level of CD47 is associated with overall survival (OS) in SS patients. Finally, targeting CD47 with TTI-621 promotes phagocytosis of patient Sézary cells in vitro, and a marked reduction of Sézary cells in SS patients receiving TTI-621. We conclude that CD47 is a novel therapeutic target in patients with SS.
Methods
SIRPαFc
TTI-621 consists of the N-terminal V domain of human SIRPα (GenBank AAH26692) fused to the human IgG1 Fc region (hinge-CH2-CH3, UniProtKB/Swiss-Prot, P01857). TTI-402, a human IgG1 Fc protein that lacks the SIRPα domain, was used as an isotype control.
Tissue bank
Sézary cells from 25 patients with an established diagnosis of SS were obtained from a biobank repository (University of Pittsburgh Institutional Review Board protocol PRO14030084). All patients provided written informed consent. Blood was collected only from treatment-naive patients or patients with progressive disease. Diagnosis of SS was established based on the constellation of clinical presentation, results of flow cytometry, and confirmed histologically by a dermatopathologist according to criteria proposed by the International Society of Cutaneous Lymphoma.26 Monoclonal T-cell receptor (TCR) gene rearrangement was detected in all patients by polymerase chain reaction (data not shown). Clinical characteristics are provided in Table 1.
Patients treated with TTI-621
Five patients with relapsed/refractory SS were treated with TTI-621 as a part of a phase 1a/1b dose escalation and expansion trial. Per the Declaration of Helsinki, all patients signed informed consent prior to being enrolled into the clinical trial. The median age of the patients was 67 years (range, 53-84 years). Screening modified Severity Weighted Assessment Tool (mSWAT) scores ranged from 3 to 200 (median, 95). All patients were dosed with 0.2 mg/kg TTI-621. The TCR Vβ of malignant clones was identified by flow cytometry from pretreatment peripheral blood on day 1 and subsequently assessed before the second infusion of TTI-621 on day 8.
Identification of the TCR Vβ family of the malignant clone
Staining of primary blood mononuclear cells was performed according to manufacturer instructions (IOTest β Mark TCRVβ repertoire kit; Beckman Coulter, Indianapolis, IN). The Vβ family of the malignant clone was identified as one that exceeded the average Vβ family size by 2 standard deviations (SDs) according to the data provided by Beckman Coulter.
Flow cytometry
Cell staining was performed with anti-CD4-V450 (RPA-T4), anti-CD47–phycoerythrin (2A3) from Caltag Laboratories (Burlingame, CA), and a corresponding anti-Vβ–fluorescein isothiocyanate from Beckman Coulter. The cells were evaluated using FACSAria and CellQuest software (Becton Dickinson, San Jose, CA). FlowJo software (TreeStar Inc, Ashland, OR) was used for analysis of the flow cytometric data.
Immunohistochemistry
Twenty formalin-fixed paraffin-embedded skin biopsies from patients with SS were collected from the tissue bank at the University of Pittsburgh. These 20 patients were part of 25 patients whose sera were obtained from the tissue bank. Commercial antibodies were used for CD47 staining (dilution 1/100; Prestige–Sigma-Aldrich, St. Louis, MO). Human bladder tissue was used as a positive control for CD47 staining. After deparaffinization, Borg epitope retrieval was conducted for 60 minutes at 95°C (Borg; Biocare Medical, Concord, CA). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 minutes. Endogenous avidin/biotin activity was quenched with a blocking kit (Background Sniper; Biocare Medical). Slides were incubated with primary antibodies for 60 minutes, 23 minutes with Mach 3 rabbit AP probe, 23 minutes with Mach 3 rabbit AP-polymer (Biocare Medical), and then 4 minutes with streptavidin-AP label (Biocare Medical). Warp red chromagen was applied for 15 minutes (Biocare Medical). Counterstaining was performed with Harris hematoxylin for 15 seconds.
CD47 expression after cytokine exposure
Primary blood mononuclear cells from 5 patients with SS were incubated with 1 of the following cytokines for 48 hours: interleukin 2 (IL-2; 200 IU/mL), IL-4 (20 ng/mL), IL-6 (20 ng/mL), IL-7 (20 ng/mL), IL-10 (20 ng/mL), IL-12 (50 ng/mL), IL-13 (20 ng/mL), IL-17 (20 ng/mL), IL-23 (20 ng/mL), IFN-γ (20 ng/mL), and tumor necrosis factor α (TNF-α; 20 ng/mL). CD47 expression was measured by flow cytometry on CD4+Vβ+ cells (malignant lymphocytes) and CD4+Vβ− cells (nonmalignant lymphocytes). The numbers of CD4+Vβ+ and CD4+Vβ− cells were measured to correlate with the level of CD47.
Transmission electron microscopy
At 0, 5, and 10 minutes after coexposure of Mϕs and Sézary cells to TTI-621 conjugated to 10-nm gold nanoparticles (Abcam, Cambridge MA), cells were fixed in cold 2.5% glutaraldehyde (25% glutaraldehyde EM grade; Taab Chemical) in 0.01 M phosphate-buffered saline, pH 7.3. The specimens were rinsed in phosphate-buffered saline, postfixed in 1% osmium tetroxide (osmium tetroxide crystals; Electron Microscopy Sciences) with 1% potassium ferricyanide (potassium ferricyanide; Fisher), dehydrated through a graded series of ethanol (30% to 90% reagent alcohol [Fisher] and 100% ethanol 200 proof [Pharmco]) and embedded in Epon (dodecenyl succinic anhydride, nadic methyl anhydride, Scipoxy 812 resin, and dimethylaminomethyl; Energy Beam Sciences). Semithin (300 nm) sections were cut on a Reichart Ultracut, stained with 0.5% toluidine blue (toluidine blue O and sodium borate; Fisher) and examined under the light microscope. Ultrathin sections (65 nm) were stained with 2% uranyl acetate (uranyl acetate dihydrate [Electron Microscopy Sciences] and methanol [Fisher]) and Reynold lead citrate (lead nitrate, sodium citrate, and sodium hydroxide [Fisher]). Observation was performed with a JEOL 1011 transmission electron microscope (Peabody, MA). Following collection, transmission electron microscopy (TEM) images were formatted using Adobe Photoshop for brightness and contrast.
Phagocytosis assay
Mϕ’s were derived from the buffy coat of healthy volunteers as previously described.24 Lymphocytes were obtained from the peripheral blood of SS patients. Malignant T cells were identified based on clonal expression of TCR Vβ using the IOTest β Mark TCRVβ repertoire kit (Beckman Coulter, Brea, CA). CD4+Vβ+ and CD4+Vβ− cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and added to IFN-γ primed Mϕs in 96-well plates at a 1:5 effector-to-target ratio. Mϕs and tumor cells were cocultured for 2 hours at 37°C in 5% CO2 in the presence of TTI-621, isotype control, or media alone. Cells were stained with HLA-DR to identify Mϕs, and the percentage of live, single, HLA-DR+, CFSE+ cells was determined by flow cytometry.
Luminex assay specifications and procedure
Sera from the peripheral blood of 11 patients with SS and 15 age- and sex-matched healthy volunteers were collected and stored at −80°C before the Luminex analysis. Written informed consent was obtained for all patients and healthy volunteers. Milliplex Map Human Th17 Magnetic Bead Panel and Milliplex Human Cytokine/Chemokine 19 Plex Magnetic Beal Panel (both from Millipore Sigma, Burlington, MA) were used to analyze supernatant by xMap technology27 according to the manufacturer’s instructions as previously described.28 Analysis of data was done using 4-parametric-curve fitting.29
Statistical analysis
The statistical analyses were based on the calculation of arithmetic mean and SD. The difference between 2 means was compared by a 2-tailed unpaired Student t test without the assumption of equal variances. The difference between >2 means was compared by 1-way analysis of variance with Tukey posttest. Pearson correlation (r) was measured to assess a linear dependence between 2 variables. P < .05 was considered statistically significant. OS was defined as the time from the first day of diagnosis to death from any cause. Patients without an event in OS were censored on the last day with valid information for the respective end point. OS was estimated according to Kaplan-Meier and compared by log-rank (Mantle-Cox) trend test. Prism software was used for all statistical analyses.
Results
CD47 expression on Sézary cells and its correlation with OS
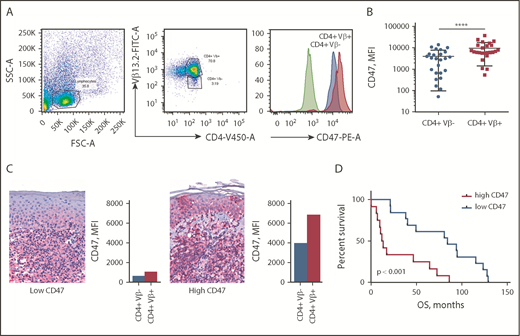
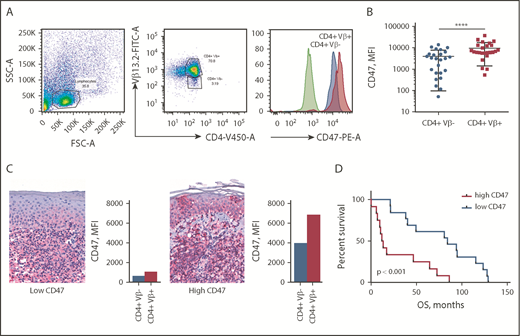
CD47 expression was determined by flow cytometry on peripheral blood of 25 patients with SS (Table 1). To establish whether CD47 expression was attributed to tumor cells or was associated with reactive T lymphocytes, we performed multicolor flow cytometry analysis. All patient samples were phenotyped to determine the dominant Vβ clone comprising the malignant cells (data not shown). The abnormal morphology of CD4+Vβ+ cells was confirmed by light microscopy. CD47 expression on Sézary cells (CD4+Vβ+) was compared with nonneoplastic CD4+ T cells (CD4+Vβ−) from the same individual (Figure 1A). Comparison of paired CD4+Vβ+ and CD4+Vβ− cells demonstrated that Sézary cells expressed a 2.5-fold higher level of CD47 than nonmalignant lymphocytes (9842 [95% CI, 6537-13 148] vs 3963 [95% CI, 2447-5479]; median fluorescent intensity [MFI]; P < .0001) (Figure 1B). Although the level of CD47 expression on Sézary cells was higher in comparison with nonmalignant lymphocytes, the CD47 expression on malignant lymphocytes varied among patients with SS, which was also observed in corresponding skin biopsies (Figure 1C). The mean OS of our patients was 54.7 months (95% CI, 33.8-71.6). When divided into CD47high (>6537 MFI) and CD47low groups (<6537 MFI; where 6537 MFI was the median of total expression among this population), Kaplan-Meier survival analysis indicated that SS patients with a high level of CD47 had 6.5 times (95% CI, 3.0-14.3) shorter OS than the CD47low SS patients (median OS, 84.0 vs 12.9 months; P < .001; Figure 1D). Together, these data indicate that overexpression of CD47 on Sézary cells correlates with worse OS in patients with SS.
CD47 is overexpressed on Sézary cells and correlates with worse OS. (A) Representative fluorescence-activated cell sorter (FACS) plots of gating strategy identifying CD47 expression on Sézary cells (with a dominant Vβ13.2+ T-cell population) in comparison with nonmalignant T cells (CD4+Vβ13.2−) in a patient with SS. CD47 expression was determined on Sézary cells (red histogram) compared with nonmalignant CD4+ cells (blue histogram) and isotype control (green histogram). (B) The MFI of CD47 on CD4+Vβ+ (Sézary cells, red) and matched CD4+Vβ− (nonmalignant lymphocytes, blue) cells in patients with SS (n = 25). Two-tailed unpaired Student t test (****P < .0001). (C) Representative immunohistochemistry (IHC) samples of the skin biopsy from a patient with SS with low CD47 expression (left panel) and 1 with high CD47 expression (right panel) (anti-CD47 with red chromagen stain; original magnification ×40). The level of expression of CD47 on Sézary cells in the peripheral blood determined by flow cytometry provided on the right of the corresponding skin biopsy. (D) Kaplan-Meier curves of OS (months) of patients with SS having a low CD47 expression on Sézary cells (<6537 MFI) vs high CD47 expression (>6537 MFI) on Sézary cells (n = 25). Log-rank (Mantel-Cox) test (P < .001). FITC-A, fluorescein isothiocyanate area; FSC-A, forward scatter area; PE-A, phycoerythrin area; SSC-A, side scatter area.
CD47 is overexpressed on Sézary cells and correlates with worse OS. (A) Representative fluorescence-activated cell sorter (FACS) plots of gating strategy identifying CD47 expression on Sézary cells (with a dominant Vβ13.2+ T-cell population) in comparison with nonmalignant T cells (CD4+Vβ13.2−) in a patient with SS. CD47 expression was determined on Sézary cells (red histogram) compared with nonmalignant CD4+ cells (blue histogram) and isotype control (green histogram). (B) The MFI of CD47 on CD4+Vβ+ (Sézary cells, red) and matched CD4+Vβ− (nonmalignant lymphocytes, blue) cells in patients with SS (n = 25). Two-tailed unpaired Student t test (****P < .0001). (C) Representative immunohistochemistry (IHC) samples of the skin biopsy from a patient with SS with low CD47 expression (left panel) and 1 with high CD47 expression (right panel) (anti-CD47 with red chromagen stain; original magnification ×40). The level of expression of CD47 on Sézary cells in the peripheral blood determined by flow cytometry provided on the right of the corresponding skin biopsy. (D) Kaplan-Meier curves of OS (months) of patients with SS having a low CD47 expression on Sézary cells (<6537 MFI) vs high CD47 expression (>6537 MFI) on Sézary cells (n = 25). Log-rank (Mantel-Cox) test (P < .001). FITC-A, fluorescein isothiocyanate area; FSC-A, forward scatter area; PE-A, phycoerythrin area; SSC-A, side scatter area.
Cytokine-mediated CD47 expression on Sézary cells
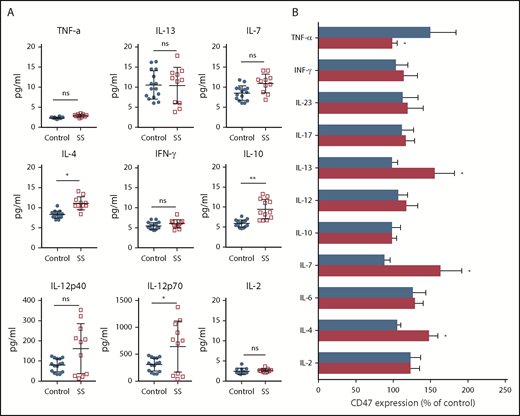
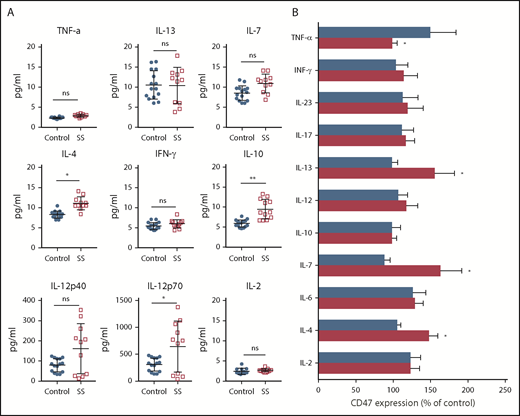
A variety of cytokines and mediators characterize the tumor microenvironment creating self-mediated inflammation that favors tumor development and progression.30 Considerable attention has been given to the Th1/Th2 axis because progression of CTCLs to the leukemic stage is accompanied by a switch from a predominantly Th1 cytokine profile to a Th2 cytokine profile.31 As expected, IL-4 was upregulated in patients with SS (Figure 2A). IL-10 production was also elevated (P < .01), whereas IFN-γ and TNF-α were comparable with age-matched healthy controls (P > .1). Although we did not observe a statistically significant difference between IL-12p40 in healthy controls and patients with SS (P > .01), we found that the level of IL-12p70 was significantly different. IL12-p70 also positively correlated with the number of Sézary cells in the peripheral blood (r = 0.71; P < .05; supplemental Figure 1). The influence of various cytokines on the CD47 expression on Sézary cells was evaluated ex vivo. Compared with nonmalignant lymphocytes, Sézary cells expressed more CD47 after coincubation with IL-4, IL-7, and IL-13, whereas only TNF-α decreased the level of CD47 expression on Sézary cells (Figure 2B). Although some cytokines are known for their proliferative (IL-2, IL-7, IL-13) and proapoptotic effects (TNF-α, IFN-γ), the number of Sézary cells did not increase after incubation with any cytokines in our study. In fact, the number of Sézary cells was slightly less than without stimulation and was on average 86.5% (95% CI, 80.0-93.4) of the original number after stimulation. These data suggest that CD47 expression on Sézary cells is under the influence of Th2 cytokines.
CD47 expression on Sézary cells is under influence of Th2 cytokines. (A) Cytokine concentration in peripheral blood of Sézary patients (n = 11) vs age-matched healthy volunteers (n = 15). Mean ± SD is depicted. Two-tailed unpaired Student t test (*P < .05; **P < .01). (B) Percentage of CD47 on the surface of Sézary cells (CD4+Vβ+, red) vs nonmalignant T cells from the same patients (CD4+Vβ−, blue) in relationship to nonstimulated cells following 48-hour incubation with IL-2 (200 IU/mL), IL-4 (20 ng/mL), IL-6 (20 ng/mL), IL-7 (20 ng/mL), IL-10 (20 ng/mL), IL-12 (50 ng/mL), IL-13 (20 ng/mL), IL-17 (20 ng/mL), IL-23 (20 ng/mL), IFN-γ (20 ng/mL), or TNF-α (20 ng/mL). Mean ± SD is depicted. Two-tailed unpaired Student t test (*P < .05). ns, not significant.
CD47 expression on Sézary cells is under influence of Th2 cytokines. (A) Cytokine concentration in peripheral blood of Sézary patients (n = 11) vs age-matched healthy volunteers (n = 15). Mean ± SD is depicted. Two-tailed unpaired Student t test (*P < .05; **P < .01). (B) Percentage of CD47 on the surface of Sézary cells (CD4+Vβ+, red) vs nonmalignant T cells from the same patients (CD4+Vβ−, blue) in relationship to nonstimulated cells following 48-hour incubation with IL-2 (200 IU/mL), IL-4 (20 ng/mL), IL-6 (20 ng/mL), IL-7 (20 ng/mL), IL-10 (20 ng/mL), IL-12 (50 ng/mL), IL-13 (20 ng/mL), IL-17 (20 ng/mL), IL-23 (20 ng/mL), IFN-γ (20 ng/mL), or TNF-α (20 ng/mL). Mean ± SD is depicted. Two-tailed unpaired Student t test (*P < .05). ns, not significant.
Phagocytosis of Sézary cells ex vivo after CD47 blockade with SIRPαFc
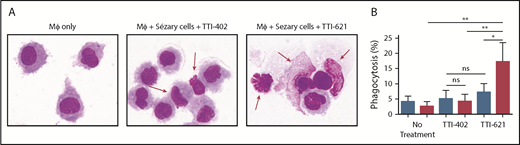
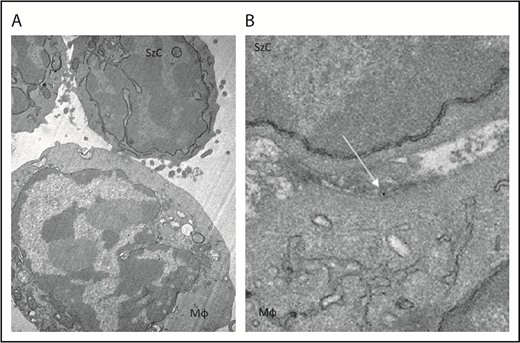
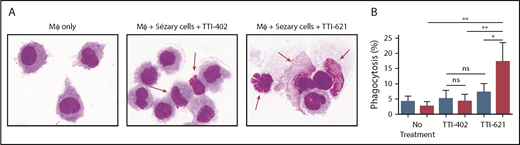
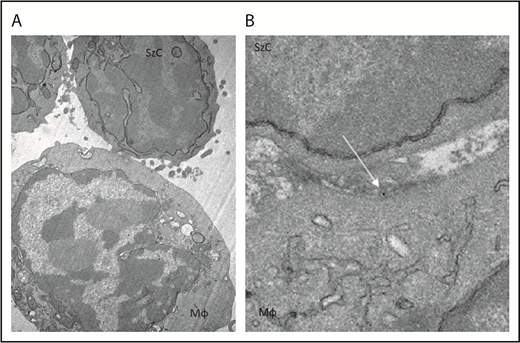
To determine whether CD47 blockade affects phagocytosis of Sézary cells ex vivo, Sézary cells were purified from peripheral blood of patients with SS based on dominant TCR Vβ expression. Because propagation of Mϕs from the peripheral blood of patients with SS was not possible due to the low absolute count of monocytes, Sézary cells were cocultured with Mϕs derived from the peripheral blood of healthy volunteers. Prior to Mϕ exposure, Sézary cells were incubated in the presence of SIRPαFc (TTI-621) or isotype control (TTI-402). TTI-621 has potent activity via both the CD47-binding domain and IgG1 Fc region.24 Although TTI-621 did not trigger phagocytosis of nonmalignant lymphocytes, the level of phagocytosis of Sézary cells was significantly higher in the presence of TTI-621 compared with isotype control or media alone (P < .01; Figure 3). Using TEM, gold-labeled TTI-621 was observed at the synaptic contact between Sézary cells and Mϕs (Figure 4). Thus, blockade of CD47 with SIRPαFc (TTI-621) selectively enhances phagocytosis of Sézary cells vs nonmalignant lymphocytes.
Blockade of CD47 with SIRPαFc (TTI-621) promotes phagocytosis of Sézary cells but not nonmalignant CD4+cells. (A) Cytospin preparation of representative samples 2 hours after phagocytosis in the presence of an IgG1 isotype control molecule (TTI-402) or TTI-621. Red arrows identify Sézary cells that are external to macrophages in the presence of isotype control but phagocytosed in the presence of TTI-621 (Romanowsky stain; original magnification ×100). (B) Macrophage-mediated phagocytosis of matched nonmalignant (blue) or Sézary cells (red) from 8 patients with SS in media (no treatment), 1 µM TTI-403 (isotype control), and 1 µM TTI-621. One-way analysis of variance with Tukey posttest (*P < .05; **P < .01).
Blockade of CD47 with SIRPαFc (TTI-621) promotes phagocytosis of Sézary cells but not nonmalignant CD4+cells. (A) Cytospin preparation of representative samples 2 hours after phagocytosis in the presence of an IgG1 isotype control molecule (TTI-402) or TTI-621. Red arrows identify Sézary cells that are external to macrophages in the presence of isotype control but phagocytosed in the presence of TTI-621 (Romanowsky stain; original magnification ×100). (B) Macrophage-mediated phagocytosis of matched nonmalignant (blue) or Sézary cells (red) from 8 patients with SS in media (no treatment), 1 µM TTI-403 (isotype control), and 1 µM TTI-621. One-way analysis of variance with Tukey posttest (*P < .05; **P < .01).
TTI-621 (arrow) can be observed at the interface between the Sézary cell and macrophage. Gold-labeled TTI-621 was incubated with Sézary cells and macrophages for 5 minutes before scanning electron microscopy. (A) Sézary cell (SzC) attached to Mϕ (original magnification ×15 000). (B) Gold-labeled TTI-621 at the interface between the Sézary cell and the Mϕ (original magnification ×50 000).
TTI-621 (arrow) can be observed at the interface between the Sézary cell and macrophage. Gold-labeled TTI-621 was incubated with Sézary cells and macrophages for 5 minutes before scanning electron microscopy. (A) Sézary cell (SzC) attached to Mϕ (original magnification ×15 000). (B) Gold-labeled TTI-621 at the interface between the Sézary cell and the Mϕ (original magnification ×50 000).
TTI-621 depletes Sézary cells in patients with SS
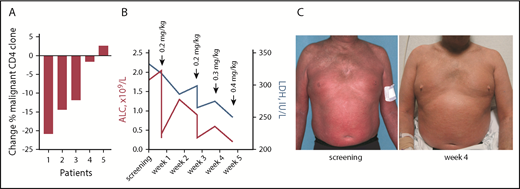
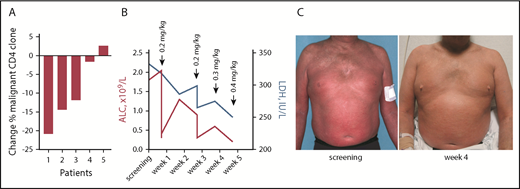
Five patients with SS were treated with TTI-621 as part of an open-label phase 1a clinical trial (NCT02663518). All subjects were heavily pretreated and exhibited erythroderma, a high leukemic count of Sézary cells, and lymphadenopathy. After a single 0.2 mg/kg infusion of TTI-621, 4 of 5 patients had a decrease in the dominant malignant clone 8 days after infusion as determined by flow cytometry (Figure 5A). Lactate dehydrogenase (LDH) has been proposed as an independent prognostic factor of disease severity32 and a marker of tumor burden.33,34 We observed a rapid decrease of LDH during treatment with TTI-621, which was accompanied by a decrease in the absolute lymphocyte count compared with baseline (Figure 5B) and correlated with skin improvement (Figure 5C). These data demonstrate the therapeutic benefit of TTI-621 in patients with SS.
Clinical benefit of TTI-621 in Sézary syndrome is accompanied by a decrease in circulating clone of Sézary cells. (A) Percentage change in clonal (Vβ+) CD4 cells in patients with Sézary syndrome (n = 5) who received a single dose of TTI-621. Comparison between day 1 pretreatment and 8 days after infusion. (B) Decrease in lactate dehydrogenase (LDH; right y-axis, blue) and absolute lymphocyte count (ALC; left y-axis, red) during treatment with TTI-621 (a representative patient). (C) A 52-year-old man with stage IVA1 (T4N1M0B2) SS enrolled in phase 1a of clinical trial NCT02663518. Images taken at screening and week 4 on study.
Clinical benefit of TTI-621 in Sézary syndrome is accompanied by a decrease in circulating clone of Sézary cells. (A) Percentage change in clonal (Vβ+) CD4 cells in patients with Sézary syndrome (n = 5) who received a single dose of TTI-621. Comparison between day 1 pretreatment and 8 days after infusion. (B) Decrease in lactate dehydrogenase (LDH; right y-axis, blue) and absolute lymphocyte count (ALC; left y-axis, red) during treatment with TTI-621 (a representative patient). (C) A 52-year-old man with stage IVA1 (T4N1M0B2) SS enrolled in phase 1a of clinical trial NCT02663518. Images taken at screening and week 4 on study.
Discussion
The identification of T-cell checkpoints, notably programmed cell death protein 1 (PD-1) and CTLA-4, has been key in our ability to control tumor growth via adaptive immunity. At the same time, there is emerging interest in engaging the innate immune system. In this context, the CD47-SIRPα axis has recently attracted considerable interest. We provide here the first evidence that CD47 is highly expressed on Sézary cells in the peripheral blood and skin, and that high levels of CD47 expression correlate with worse OS in patients with SS. We also show that CD47 expression on Sézary cells is under the influence of IL-4, IL-7, and IL-13. SIRPαFc (TTI-621), a novel CD47 decoy receptor, triggers Mϕ-mediated phagocytosis of Sézary cells. When administered in a clinical trial setting, TTI-621 results in a significant reduction in tumor load, indicating that inhibition of the CD47-SIRPα signaling pathway may have therapeutic benefit for patients with SS.
Previous work has implicated CD47 as a crucial immune escape mechanism for several tumors. Abnormally high expression of CD47 has been found in hematopoietic stem cells and leukemia cells, human acute myeloid leukemia stem cells, hepatocellular carcinoma, and ovarian clear cell carcinoma.8,35,36 Additionally, several studies have shown that high CD47 expression in tumors correlates with poor prognosis.37 However, little is known about the role of CD47 in SS. Using TCR Vβ staining to identify the dominant clone, we demonstrate that malignant lymphocytes (clonal Vβ+CD4+) express high levels of CD47 compared with nonmalignant lymphocytes in the same patient with SS. Furthermore, we show that the high expression of CD47 on malignant T cells correlates with worse OS in patients with SS. Survival analysis of groups stratified according to the level of expression of CD47 on Sézary cells showed that patients with low expression of CD47 have a better OS than patients with high CD47 expression. Thus, evaluating the expression of CD47 on Sézary cells could be used as a noninvasive method to identify patients with a worse prognosis.
Our studies, as well as previous data, failed to demonstrate the difference in serum levels of IL-12p40 between patients with SS and controls.38,39 We observed a subgroup of patients with very high leukemic count whose IL-12p70 level was significantly elevated and correlated with a number of Sézary cells (r = 0.71; P < .05). Unusually high levels of IL-12p70 were observed in patients with the number of Sézary cells >5000/mm3. Possibly, earlier studies in SS were done in patients with lower disease burden. A recent study suggested that T cells may develop immunosuppressive functions when exposed to IL-4 and IL-12.40 Correspondingly, we observed significantly higher levels of both IL-10 and IL-4 in the serum of SS patients and it is conceivable that the combination of IL-12 and IL-4 plays a role in SS.
We found that CD47 expression on Sézary cells is under the influence of Th2 cytokines IL-4, IL-7, and IL-13. Although the serum levels of IL-7 and IL-13 in patients with SS were not different from age-matched healthy volunteers, IL-4 levels were significantly elevated in the peripheral blood of SS patients, consistent with a previous publication,41 suggesting the potential to influence CD47 expression on Sézary cells. This result is consistent with another recent study showing a correlation between serum IL-4 levels and CD47 expression in other types of cancers.37 IL-13 was recently demonstrated to be an autocrine factor for Sézary cells that potentiates the phosphorylation of STAT6 along with IL-4.42 IL-13 is homologous to IL-4, and may act similarly with respect to CD47 expression. Although IL-7 is known for its proliferative effects on Sézary cells, we used a concentration of IL-7 that is 3 times lower than reported for stimulation of Sézary cells.43 After coincubation with low-dose IL-7, we found a significant increase in surface CD47 expression on Sézary cells, in the absence of proliferation. Interestingly, the expression of CD47 may also affect cytokine production by T cells. Studies with CD47 KO mice demonstrated that CD47+ T cells produce significantly less IFN-γ than CD47− T cells thereby skewing cells toward a Th2 phenotype.44 Thus, CD47 may serve as a negative regulator of Th1 cytokines and sensitize cells to IL-4.
We have previously shown that Sézary cells are resistant to TNF-α–induced apoptosis due to the loss of TNFR1.45 However, it was remarkable to find that TNF-α decreases the level of CD47 expression, which may facilitate clearance of Sézary cells via phagocytosis. Phagocytosis of Sézary cells after exposure to SIRPαFc was not related to opsonization because the isotype control IgG did not affect the extent of phagocytosis. Previous work demonstrated that inhibition of phagocytosis protects cancer cells that were coincubated with anti-CD47 antibodies from death, indicating that phagocytosis was required for anti-CD47–mediated apoptosis.46
In this study, we used normal donor monocytes as we were not able to isolate a sufficient number of autologous monocytes for phagocytosis assays, contrary to reported findings of a comparable number of monocytes47 or even an enlarged population of monocytes in the blood of patients with SS.48 However, these monocytic fractions in automated blood counts are, in fact, Sézary cells that have similar forward- and side-scatter properties leading to incorrect automatic estimation.48 Further negative selection of monocyte from the peripheral blood with CD14 microbeads confirmed those findings in our patients’ samples (data not shown). However, the majority of macrophage-mediated phagocytosis likely occurs in the spleen and liver, where macrophages are abundant.49
As CD47 is ubiquitously expressed on normal cells throughout the body,50 high expression of CD47 in tumor tissue presents an opportunity for therapeutic selectivity. A number of clinical trials are evaluating anti-CD47 therapies in hematologic malignancies as well as CTCL.51 Our results demonstrate that Sézary cells are a target for CD47 blockade, and that TTI-621 has clinical utility in SS patients by reducing the number of Sézary cells in peripheral blood. Further clinical testing is ongoing to determine whether this approach is applicable to other types of CTCL.
Presented in oral form at the 2017 European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Task Force (EORTC-CLTF) Meeting on Cutaneous Lymphoma, London, United Kingdom, 14 October 2017.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Marie Acquafondata for excellent assistance with the CD47 immunohistochemistry analysis, Ming Sun and Donna Stolz for assistance with TEM, and Denise Posser and Anna Lokshin for assistance with the Luminex assay.
This work was supported by research funding from Trillium Therapeutics Inc (O.E.A.).
Authorship
Contribution: L.D.S.J., O.E.A., R.A.U., and M.W. participated in research design and execution of experiments, analyzed and interpreted data, and wrote the manuscript; S.B. and O.K. performed flow cytometry, cell culture experiments, and experiments with human samples; O.E.A., S.M.H., A.L., J.Z., C.Q., R.C., C.O., A.S., and O.A.O. enrolled the patients with SS and treated with TTI-621; N.N.V. participated in conducting experiments and revising the manuscript; E.L.S. initiated this research project; Y.S. continued to execute the clinical part of this project; and E.L.S. and Y.S. revised the manuscript.
Conflict-of-interest disclosure: L.D.S.J., N.N.V., E.L.S., Y.S., R.A.U., and M.W. are employees of Trillium Therapeutics Inc. O.E.A. has received research funding from Trillium Therapeutics Inc. S.M.H., A.L., J.Z., C.Q., R.C., C.O., A.S., O.A.O., and O.E.A. are clinical investigators on a trial sponsored by Trillium Therapeutics Inc. S.M.H. has received both research funding/grant support and honoraria/consulting fees from ADCT Therapeutics, Aileron, Forty-Seven Infinity/Verastem Kyowa-Hakka-Kirin, Millennium/Takeda, and Seattle Genetics; research/grant support from Celgene and Trillium Therapeutics Inc; and honoraria consulting fees from Affimed, Angimmune, Beigene, Corvus, Innate Pharma, Kura, Merck, Miragen, Mundipharma, Portola, and Syros Pharmaceutical. The remaining authors declare no competing financial interests.
Correspondence: Oleg E. Akilov, Department of Dermatology, University of Pittsburgh, 200 Lothrop St, Biomedical Science Tower, Room E1157, Pittsburgh, PA 15261-2109; e-mail: akilovoe@upmc.edu.