Key Points
Flu/Cy/ATG and Cy/ATG regimens offer the best survival for matched-sibling BMT.
Transplantation in patients aged ≥30 years is associated with higher mortality after matched-sibling and unrelated donor BMT.
Abstract
Allogeneic bone marrow transplantation (BMT) is curative therapy for the treatment of patients with severe aplastic anemia (SAA). However, several conditioning regimens can be used for BMT. We evaluated transplant conditioning regimens for BMT in SAA after HLA-matched sibling and unrelated donor BMT. For recipients of HLA-matched sibling donor transplantation (n = 955), fludarabine (Flu)/cyclophosphamide (Cy)/antithymocyte globulin (ATG) or Cy/ATG led to the best survival. The 5-year probabilities of survival with Flu/Cy/ATG, Cy/ATG, Cy ± Flu, and busulfan/Cy were 91%, 91%, 80%, and 84%, respectively (P = .001). For recipients of 8/8 and 7/8 HLA allele-matched unrelated donor transplantation (n = 409), there were no differences in survival between regimens. The 5-year probabilities of survival with Cy/ATG/total body irradiation 200 cGy, Flu/Cy/ATG/total body irradiation 200 cGy, Flu/Cy/ATG, and Cy/ATG were 77%, 80%, 75%, and 72%, respectively (P = .61). Rabbit-derived ATG compared with equine-derived ATG was associated with a lower risk of grade II to IV acute graft-versus-host disease (GVHD) (hazard ratio [HR], 0.39; P < .001) but not chronic GVHD. Independent of conditioning regimen, survival was lower in patients aged >30 years after HLA-matched sibling (HR, 2.74; P < .001) or unrelated donor (HR, 1.98; P = .001) transplantation. These data support Flu/Cy/ATG and Cy/ATG as optimal regimens for HLA-matched sibling BMT. Although survival after an unrelated donor BMT did not differ between regimens, use of rabbit-derived ATG may be preferred because of lower risks of acute GVHD.
Introduction
Allogeneic transplantation with an HLA-matched sibling donor is widely regarded as first-line treatment for children and young adults with severe aplastic anemia (SAA).1-3 When an HLA-matched sibling is not available, treatment with immunosuppressive agents is the first-line therapy, and allogeneic transplantation from an unrelated donor (URD) is generally offered only after failure of immunosuppressive treatment.1-3 For adults aged >40 years, immunosuppression is typically first-line treatment, and allogeneic transplantation is reserved for those who do not respond to immunosuppression.1-4 Although survival after allogeneic transplantation in children is excellent, in adults, organ toxicity and graft failure are higher and add to the burden of morbidity and mortality.2,5,6 Consequently, there have been several phase 2 clinical trials of transplantation aimed at lowering toxicity and improving survival.7-12 Given the rarity of SAA, there are no randomized trials that have compared transplant-conditioning regimens. Cyclophosphamide (Cy) at 200 mg/kg or a lower dose and antithymocyte globulin (ATG) with or without fludarabine (Flu) are most often used for HLA-matched sibling transplantation.2 Low-dose total body irradiation is often added to Cy and ATG for URD transplantation.7-10 Reports from the European Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research support bone marrow as the preferred graft choice for HLA-matched sibling and URD transplantation.13-15 In those reports, transplantation of peripheral blood was associated with more frequent chronic graft-versus-host disease (GVHD) and lower survival. In the current analyses, we evaluated the effect of conditioning regimens on transplant outcomes after HLA-matched sibling and 8/8 or 7/8 HLA-matched URD bone marrow transplantation for SAA.
Methods
Patients
Data on consecutive allogeneic bone marrow transplantations for SAA were reported to the Center for International Blood and Marrow Transplant Research. For this study, transplant data (2000-2014) were reported by 142 transplant centers worldwide. Donor-recipient pairs of URD transplants were matched at the allele-level at HLA-A, HLA-B, HLA-C, and HLA-DRB1 or mismatched at a single HLA-locus. Patients were followed up longitudinally until death or lost to follow-up. Recipients of unrelated umbilical cord blood (n = 9) and peripheral blood (n = 216) were excluded because earlier studies have shown an association with greater GVHD and mortality.13-15 Other exclusions included regimens used during the early study period and not thereafter (n = 58 total body irradiation [TBI] or total lymphoid irradiation regimens for HLA-matched sibling; n = 110 high-dose TBI regimens for unrelated donor transplant), Flu, and Cy + alemtuzumab (n = 16 HLA-matched sibling; n = 19 unrelated donor transplant; and n = 90 ≥2 loci HLA-mismatched transplants).
Patients or their legal guardians provided written informed consent for data collection and analysis. The Institutional Review Board of the National Marrow Donor Program approved this study.
End points
The primary end point was overall survival. Death from any cause was considered an event, and surviving patients were censored at last follow-up. Secondary end points included neutrophil recovery, platelet recovery, graft failure, and grade II to IV acute and chronic GVHD. Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) ≥0.5 × 109/L for 3 consecutive days. Platelet recovery was defined as achieving platelet counts ≥20 × 109/L unsupported for a minimum of 7 days. Graft failure was defined as failure to achieve ANC ≥0.5 × 109/L for 3 consecutive days or ANC declines to <0.5 × 109/L without recovery after having achieved ANC ≥0.5 × 109/L or myeloid donor chimerism (<5%) or a second transplant.16 For acute and chronic GVHD assignment, standard criteria were used based on reports from transplant centers.17,18
Statistical methods
Separate analyses were performed for HLA-matched sibling and URD transplantation. The incidence of neutrophil recovery, platelet recovery, and acute and chronic GVHD were calculated by using the cumulative incidence estimator to accommodate competing risks.19 A Cox regression model was built to identify factors associated with overall survival and the probability of overall survival calculated from the final Cox model.20,21 Fine and Gray models were built to identify factors associated with acute and chronic GVHD.22 Variables tested in the multivariate models included conditioning regimen, age, sex, performance status, cytomegalovirus (CMV) serostatus, time from diagnosis to transplant, donor–recipient sex match, donor–recipient HLA match (URD only), GVHD prophylaxis, ATG source (horse vs rabbit), and transplant period. Age was treated as a binary variable (≤30 years vs >30 years). The age cut-point was determined statistically by using the minimum P value approach. Variables that attained P ≤ .01 were considered significant and held in the final model, with the exception of conditioning regimen, which was retained in the final model regardless of the level of significance. P ≤ .01 was chosen to accommodate the comparison of 4 conditioning regimen groups for each donor type (0.05/4 = .01). There were no first-order interactions between conditioning regimen and other variables in the final model. An effect of transplant center on survival was examined by using the frailty approach.23 All P values were 2-sided, and analyses were performed by using SAS version 9.3 (SAS Institute, Inc.).
Results
HLA-matched sibling transplant
Patient and transplant characteristics
A total of 955 patients received bone marrow grafts from HLA-matched siblings (Table 1). Cy with ATG (Cy/ATG, n = 593) was the predominant regimen, accounting for 62% of all transplants. Flu/Cy/ATG (n = 135) was the second most commonly used regimen. ATG was not used with the 2 other regimens, Cy ± Flu and busulfan (Bu)/Cy, accounting for one-quarter of the HLA-matched sibling transplants. Conditioning regimens also varied by transplant period, with Cy/ATG and Flu/Cy/ATG more likely to be used during the 2009 to 2014 period. The use of rabbit-derived ATG (r-ATG) and equine-derived ATG (h-ATG) was confounded according to regimen. h-ATG was predominantly used with Cy/ATG (68%), and r-ATG was predominantly used with Flu/Cy/ATG (66%). Forty-nine percent of patients were aged <18 years, and 51% were aged ≥18 years. Most patients were CMV seropositive and reported a performance score of 90 to 100. In a subset of patients (591 of 955, transplantations after 2007), comorbidity scores were ≤2 for 503 (85%) and ≥3 for 88 (15%). The distribution of comorbidity scores did not differ according to conditioning regimen (P = .14). All patients received a calcineurin inhibitor (CNI) containing GVHD prophylaxis. Cyclosporine was used most often (748 of 955 [78%]), usually with methotrexate (670 of 955 [70%]).
Outcomes
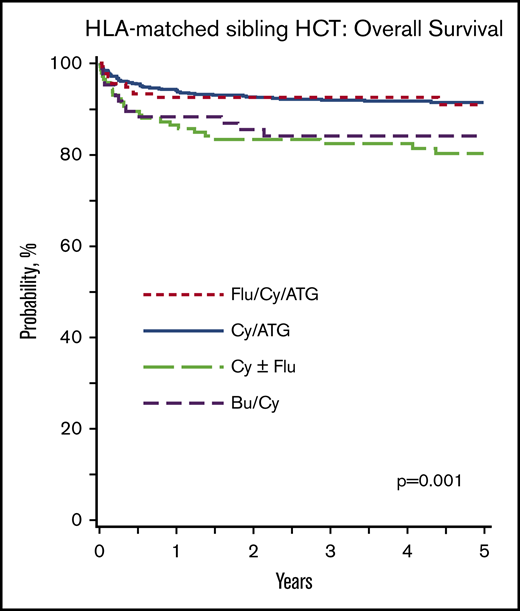
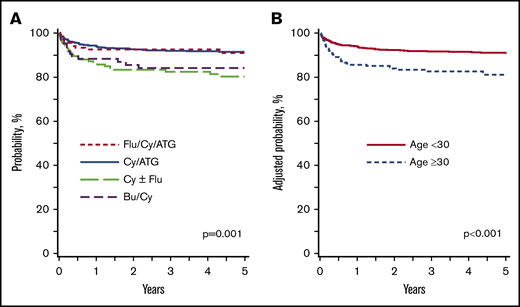
The median time to neutrophil recovery was 17 days (interquartile range, 12-22 days); for platelet recovery, it was 24 days (interquartile range, 19-30 days). The day 28 incidence of neutrophil recovery with Cy/Flu/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy was 96%, 87%, 88%, and 87%, respectively (P = .12). The day 100 incidence of platelet recovery with Cy/Flu/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy was 97%, 94%, 91%, and 93% (P = .40). Graft failure at 1 year was higher after Cy ± Flu (16%; 95% confidence interval [CI], 10-22) compared with Cy/Flu/ATG (8%; 95% CI, 4-13) and Cy/ATG (7%; 95% CI, 5-9; P = .02). Graft failure rates did not differ between Cy ± Flu and Bu/Cy (9%; 95% CI, 4-17; P = .26). The 5-year probabilities of survival after Flu/Cy/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy were 91% (95% CI, 85-96), 91% (95% CI, 89-94), 80% (95% CI, 73-87), and 84% (95% CI, 75-91) (P = .001) (Figure 1A).
Overall survival: HLA-matched sibling transplant. (A) HLA-matched sibling transplant: survival according to conditioning regimen adjusted for age and recipient CMV serostatus. The 5-year probabilities of survival after Flu/Cy/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy were 91% (95% CI, 85-96), 91% (95% CI, 89-94), 80% (95% CI, 73-87), and 84% (95% CI, 75-91), respectively (P = .001). (B) HLA-matched sibling transplant: survival according to age adjusted for conditioning regimen and recipient CMV serostatus. The 5-year probabilities of survival in patients aged ≤30 years and >30 years were 91% (95% CI, 89-93) and 81% (95% CI, 76-87; P < .001).
Overall survival: HLA-matched sibling transplant. (A) HLA-matched sibling transplant: survival according to conditioning regimen adjusted for age and recipient CMV serostatus. The 5-year probabilities of survival after Flu/Cy/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy were 91% (95% CI, 85-96), 91% (95% CI, 89-94), 80% (95% CI, 73-87), and 84% (95% CI, 75-91), respectively (P = .001). (B) HLA-matched sibling transplant: survival according to age adjusted for conditioning regimen and recipient CMV serostatus. The 5-year probabilities of survival in patients aged ≤30 years and >30 years were 91% (95% CI, 89-93) and 81% (95% CI, 76-87; P < .001).
Results of multivariate analyses for overall survival and acute and chronic GVHD are shown in Table 2. Survival was lower with the Cy ± Flu regimen compared with Cy/Flu/ATG and Cy/ATG (hazard ratio [HR], 2.08; 95% CI, 1.30-3.33; P = .002). Survival was also lower with the Bu/Cy regimen compared with the Cy/Flu/ATG and Cy/ATG regimens (HR, 1.76; 95% CI, 0.97-3.33; P = .06). There were no differences in survival between Cy/ATG and Cy/Flu/ATG. Other factors associated with lower survival independent of conditioning regimen were older age (≥30 years) and recipient CMV seropositivity. In patients aged ≥30 years, the 5-year survival after Cy/ATG was 81% (95% CI, 72-88) and after Flu/Cy/ATG, it was 86% (95% CI, 74-94; P = .10). There were very few patients who received Cy ± Flu (n = 23, 15 alive, 65%) and Bu/Cy (n = 11, 9 alive, 82%). The 5-year probabilities of survival according to patient age, adjusted for conditioning regimen and CMV serostatus, is shown in Figure 1B. There were no differences in survival between transplant centers.
Conditioning regimen was not associated with the incidence of grade II to IV acute GVHD. However, the incidence of acute GVHD was higher in the 2009 to 2014 period. The 6-month incidence of grade II to IV acute GVHD with Cy/Flu/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy regimens was 11% (95% CI, 6-17), 13% (95% CI, 10-16), 11% (95% CI, 6-16), and 11% (95% CI, 5-18), respectively. The corresponding incidence of grade III to IV acute GVHD was 7% (95% CI, 3-12), 4% (95% CI, 3-6), 5% (95% CI, 2-9), and 6% (95% CI, 2-12). Chronic GVHD risks were higher with the Cy/ATG and Bu/Cy regimens compared with the Cy/Flu/ATG regimens. Although risks were higher with Cy ± Flu, this finding did not meet the level of significance set for the study. Chronic GVHD risks did not differ between the Cy/ATG, Cy ± Flu, and Bu/Cy regimens (data not shown). The 5-year incidence of chronic GVHD with the Cy/Flu/ATG, Cy/ATG, Cy ± Flu, and Bu/Cy regimens were 9% (95% CI, 4-14), 18% (95% CI, 15-21), 16% (95% CI, 10-23), and 21% (95% CI, 13-30). Among patients who developed chronic GVHD, there was no difference in severity of chronic GVHD according to regimen (data not shown). Chronic GVHD was more frequent in patients aged ≥30 years.
URD transplant
Patient and transplant characteristics
A total of 409 patients received bone marrow grafts from HLA-matched or mismatched URDs (Table 3). Most transplantations (78%) used donors who were HLA-matched at A, B, C, and DRB1 at the allele-level, and the remaining 22% were 7/8 matched. Flu/Cy/ATG/TBI 200 cGy (n = 172; 42%) and Cy/ATG/TBI 200 cGy (n = 120; 29%) were the predominant regimens used. These regimens were used more often during the 2009 to 2014 period. ATG was included with all regimens; overall, 62% used r-ATG and 38% used h-ATG. Thus, ATG type was confounded with regimen as h-ATG was mostly used with Cy/ATG/TBI 200 cGy (69%), and r-ATG was mostly used with Flu/Cy/ATG/TBI 200 cGy (73%), Flu/Cy/ATG (71%), and Cy/ATG (56%). Most patients were CMV seropositive and reported a performance score of 90 or 100. In a subset of patients (293 of 409, transplantations after 2007), comorbidity scores were ≤2 for 225 (75%) and ≥3 for 68 (23%). The distribution of comorbidity scores did not differ according to conditioning regimen (P = .09). All patients received a CNI-containing GVHD prophylaxis, usually with methotrexate.
Outcomes
The median time to neutrophil recovery was 19 days (interquartile range, 15-22 days); for platelet recovery, it was 27 days (range, 20-35 days). The day 28 incidence of neutrophil recovery with Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG were 89%, 90%, 92%, and 90%, respectively (P = .11). The day 100 incidence of platelet recovery with Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG was 86%, 88%, 82%, and 80% (P = .11). Graft failure at 1 year did not differ according to conditioning regimen (P = .18). Graft failure at 1 year was 9% (95% CI, 5-15), 8% (95% CI, 4-12), 15% (95% CI, 8-25), and 12% (95% CI, 4-21) after Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG (P = .18).
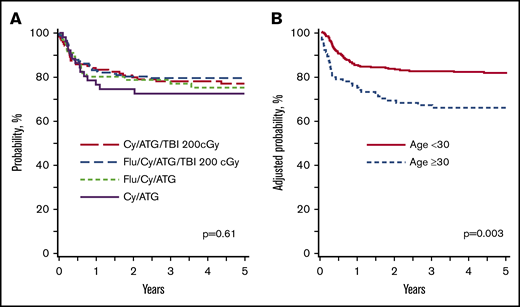
The results of multivariate analysis for survival and acute and chronic GVHD are shown in Table 4. The 5-year probabilities of survival with Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG were 77% (95% CI, 69-84), 80% (95% CI, 73-85), 75% (95% CI, 64-85), and 72% (95% CI, 59-84), respectively (P = .61) (Figure 2A). Transplant conditioning regimens and ATG type were not associated with survival. Survival was lower for patients aged ≥30 years regardless of conditioning regimen. In patients aged ≥30 years, the 5-year survival after the Cy/ATG/TBI regimen was 68% (95% CI, 52-82) and after the Flu/Cy/ATG/TBI regimen, it was 63% (95% CI, 47-78; P = .91). There were very few patients who received Flu/Cy/ATG (n = 14, 9 alive, 64%) and Cy/ATG (n = 18, 13 alive, 72%). The 5-year probabilities of survival according to patient age, adjusted for conditioning regimen and ATG type, are shown in Figure 2B.
Overall survival: URD transplant. (A) URD transplant: survival according to conditioning regimen adjusted for age and ATG source. The 5-year probabilities of survival with Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG were 77% (95% CI, 69-84), 80% (95% CI, 73-85), 75% (95% CI, 64-85), and 72% (95% CI, 59-84), respectively (P = .61). (B) URD transplant: survival according to age adjusted for conditioning regimen and ATG source. The 5-year probabilities of survival in patients aged ≤30 years and >30 years were 81% (95% CI, 76-85) and 66% (95% CI, 57-75; P = .003).
Overall survival: URD transplant. (A) URD transplant: survival according to conditioning regimen adjusted for age and ATG source. The 5-year probabilities of survival with Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG were 77% (95% CI, 69-84), 80% (95% CI, 73-85), 75% (95% CI, 64-85), and 72% (95% CI, 59-84), respectively (P = .61). (B) URD transplant: survival according to age adjusted for conditioning regimen and ATG source. The 5-year probabilities of survival in patients aged ≤30 years and >30 years were 81% (95% CI, 76-85) and 66% (95% CI, 57-75; P = .003).
Neither grade II to IV acute GVHD nor chronic GVHD was associated with conditioning regimen. Although the risk of grade II to IV GVHD was lower with the Flu/Cy/ATG regimen compared with Cy/ATG/TBI 200 cGy, this finding did not meet the level of significance set for the study. However, grade II to IV acute GVHD was lower with r-ATG independent of regimen. ATG type was not associated with chronic GVHD (data not shown). The 6-month incidence of grade II to IV acute GVHD with Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG regimens was 48% (95% CI, 39-57), 30% (95% CI, 24-37), 26% (95% CI, 16-37), and 37% (95% CI, 25-51), respectively. The corresponding incidence of grade III to IV acute GVHD was 15% (95% CI, 9-22), 10% (95% CI, 6-14), 8% (95% CI, 3-15), and 20% (95% CI, 10-32). Older patients were at higher risk for chronic GVHD; they were also at higher risk of use of CNI alone or CNI with mycophenolate for GVHD prophylaxis (Table 4). The 5-year incidence of chronic GVHD with the Cy/ATG/TBI 200 cGy, Flu/Cy/ATG/TBI 200 cGy, Flu/Cy/ATG, and Cy/ATG regimens was 40% (95% CI, 31-49), 39% (95% CI, 32-47), 33% (95% CI, 21-45), and 28% (95% CI, 16-42). Because HLA 7/8-matched transplants were uncommon (n = 66; 16%), we studied transplant outcomes according to conditioning regimen for 8/8 HLA-matched transplants, and the findings were consistent with the main analysis (supplemental Table 1).
Discussion
We analyzed transplant conditioning for HLA-matched sibling and URD bone marrow transplantation for SAA. In recipients of HLA-matched sibling bone marrow transplantation, the Flu/Cy/ATG and Cy/ATG regimens were associated with excellent survival independent of age at transplantation and recipient CMV serostatus: 93% and 91%, respectively. Graft failure was also infrequent with the Flu/Cy/ATG and Cy/ATG regimens that support in vivo T-cell depletion for HLA-matched sibling transplants for SAA. Our findings are in contrast to the European Society for Blood and Marrow Transplantation guidelines favoring Flu/Cy/ATG in patients aged ≥30 years and the British Society for Haematology guidelines favoring Flu/Cy + alemtuzumab.24,25 However, 42% of patients who received Flu/Cy/ATG in the current analysis were aged ≥30 years compared with only 18% of patients who received Cy/ATG. The Cy ± Flu and Bu/Cy regimens led to poorer outcomes, and our data do not support the use of these regimens for HLA-matched sibling bone marrow transplantation for SAA. The majority of HLA-matched sibling transplantations used cyclosporine and methotrexate, and we were therefore unable to provide recommendations on an optimal GVHD prophylaxis regimen. In recipients of URD bone marrow transplantation, we observed no differences in survival or graft failure according to conditioning regimen. However, others have recorded survival differences.7,11,12 A North American Cy dose-finding trial recorded higher mortality with Cy 150 mg/kg compared with Cy 50 mg/kg or 100 mg/kg in combination with Flu/ATG/TBI 200 cGy.11,12 Similarly, a European trial with Flu/Cy/ATG was also modified for graft failure to add TBI 200 cGy for older patients.7 In the current analysis, for ∼70% of URD transplants, ATG/Cy/TBI 200 cGy or Flu/ATG/Cy/TBI 200 cGy were the regimens of choice. The Cy dose for ATG/Cy/TBI 200 cGy was predominantly 200 mg/kg, and the Cy dose for Flu/ATG/Cy/TBI 200 cGy was 50, 100, or 150 mg/kg; only 37 (22%) of 172 patients received 150 mg/kg. However, 14 (38%) of 37 patients are dead compared with 28 (21%) of 133 for Cy doses 50 mg/kg and 100 mg/kg. Although it could not be proven in multivariate modeling, use of Cy 150 mg/kg along with TBI 200 cGy/Flu/ATG warrant caution because the excess mortality with this regimen is in keeping with the phase 2 Cy dose de-escalation trial.11 All regimens used for URD transplants included ATG. Consistent with a previous Center for International Blood and Marrow Transplant Research study, survival was slightly but not significantly higher for r-ATG compared with h-ATG for URD transplantations.26 However, acute grade II to IV GVHD was substantially lower with r-ATG and thus beneficial for a disease that does not benefit from a graft vs tumor effect. The data do not support use of calcineurin alone or with mycophenolate for unrelated donor transplantation as this use increases the risk of chronic GVHD.
Consistent with other reports, age at transplantation is an important predictor for survival, with worse survival in those aged ≥30 years.2 In our analysis, the risk of mortality was twice as high for patients aged ≥30 years compared with younger patients and was independent of conditioning regimen. Age is a biologic variable, and epidemiologic studies confirm a biphasic distribution for aplastic anemia with 2 peaks: 15 to 25 years and >60 years. The most recent natural history study of aplastic anemia from Sweden recorded higher mortality in patients aged >40 years regardless of treatment.27 The young median age of our study population for matched sibling and URD transplants suggest transplantation is mainly offered to children and young adults. In a phase 2 trial of 50 patients with SAA that studied a Flu/Cy/alemtuzumab conditioning regimen in an older population (median age, 35 years), survival was 95% after HLA-matched sibling transplant and 83% after URD transplant. Similarly, the North American phase 2 Cy de-escalation study for URD transplantation that treated 96 patients at 50 mg/kg Cy or 100 mg/kg also recorded survival of 92% and 86%, respectively. This outcome compares favorably to the Flu/Cy/alemtuzumab regimen that included both HLA-matched sibling and URD transplants. Apart from age, donor-recipient HLA-matching is relevant for URD transplantation, especially in terms of graft failure, which is higher with HLA mismatching.28 Because few URD transplantations were mismatched, we were unable to study the effect of HLA disparity on graft failure in this analysis. We examined for an effect of poor performance score on survival and found none. Because our study included transplantations conducted before 2008, we could not study for an effect of comorbidity on survival.
Our study has limitations, beginning with the retrospective nature of the study population and our inability to comment on why a regimen was chosen for any given patient. The choice of regimen was dependent on physician or institutional preference and may reflect unknown or unmeasured factors that may have influenced the outcomes recorded. However, there are patterns regarding use of conditioning regimens. In the setting of HLA-matched sibling transplants, Flu/Cy/ATG and Cy/ATG are the predominant regimens. Although Flu/Cy/ATG was more commonly used for older patients and Cy/ATG for younger patients, both regimens are associated with excellent survival independent of age. In the setting of unrelated donor transplants, ATG/Cy/TBI 200 cGy and Flu/ATG/Cy/TBI 200 cGy are the predominant regimens with comparable survival, the exception being the use of Cy 150 mg/kg with the Flu/ATG/Cy/TBI 200 cGy regimen.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported by U24-CA76518 from the National Institutes of Health, National Cancer Institute, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases, and HHSH 250201200016C from Health Services Research Administration, Department of Health and Human Services.
The content is solely the responsibility of the authors and does not represent the official policy of the National Institutes of Health or the Health Resources and Services Administration or any other agency of the US Government.
Authorship
Contribution: N.B. had primary responsibility for drafting the manuscript; and all authors interpreted the results and reveiwed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Eapen, Center for International Blood and Marrow Transplant Research, Department of Medicine, Medical College of Wisconsin, 9200 W Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: meapen@mcw.edu.
References
Author notes
The full-text version of this article contains a data supplement