Key Points
Use of ATG was associated with reduced incidence of graft-versus host disease in the patients undergoing allo-HCT with TBI.
Use of ATG for GVHD prophylaxis did not impact disease relapse or NRM.
Abstract
The impact of the use of antithymocyte globulin (ATG) following a total body irradiation (TBI)–based myeloablative conditioning regimen has been poorly explored. We retrospectively analyzed 724 patients who underwent a first allogeneic hematopoietic cell transplantation (allo-HCT) following a TBI-based conditioning regimen for acute myeloid leukemia (AML) and compared the outcomes of 251 (35%) patients who received ATG (ATG group) with 473 (65%) patients who did not (non-ATG group). Median follow-up of surviving patients was 59 months (interquartile range, 28-83). The cumulative incidence of grade II-IV acute graft-versus-host disease (aGVHD) for non-ATG and ATG groups in the first 100 days was 33% vs 24%, respectively (P = .0098). The 2-year cumulative incidence of chronic graft-versus-host disease (cGVHD) was reduced significantly in the ATG group in comparison with the non-ATG group (46% vs 34%, P = .003). Using multivariate analysis, in vivo T-cell depletion (ATG group) was independently associated with a decreased incidence of grade II-IV aGVHD (hazard ratio [HR], 0.28; P < .001), grade III-IV aGVHD (HR, 0.21; P < .001), cGVHD (HR, 0.63; P = .02), and nonrelapse mortality (NRM) (HR, 0.54; P = .02). Relapse risk, overall survival, and leukemia-free survival were similar between the 2 groups. Our results suggest that the addition of ATG to TBI-based myeloablative conditioning for allo-HCT in AML patients results in a significant reduction in aGVHD and cGVHD, translating into a significant reduction in NRM without increasing the relapse rate.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) cures a wide range of hematological disorders. Allo-HCT with a myeloablative conditioning (MAC) regimen remains the treatment of choice for high-risk acute myeloid leukemia (AML).1-3 However, the use of peripheral blood stem cell (PBSC) grafts from HLA-matched related donors (MRDs) or HLA-matched unrelated donors (MUDs) with MAC increases the risk of chronic graft-versus-host disease (cGVHD).4-6 cGVHD is the leading cause of morbidity and mortality in long-term survivors after allo-HCT6-9 (40%-60%) and leads to decreased quality of life.9,10 The incidence of cGVHD showed a gradual increase from 1995 to 2007.11
The use of in vivo T-cell depletion (TCD) with anti–T-cell or antithymocyte globulin (ATG) products has been reported to be associated with decreased graft-versus-host disease (GVHD).12,13 A number of phase 2 studies have evaluated the effect of ATG in patients receiving unmanipulated bone marrow (BM) grafts after MAC.13 These studies showed that ATG decreased the risk of acute graft-versus-host disease (aGVHD) and cGVHD, without increasing nonrelapse mortality (NRM), and improved quality of life. ATG has also been shown, in 2 randomized trials, to decrease the incidence of cGVHD in long-term survivors without adversely affecting the incidence of disease relapse, serious infection, or overall survival (OS)14-18 ; however, ATG has not been adopted routinely.
The above-mentioned prospective randomized studies have also shown that in vivo TCD with ATG reduces the incidence of cGVHD without increasing the risk of relapse in allo-HCT with PBSC grafts from MRDs or MUDs after conventional cyclophosphamide-based MAC regimens for AML.14,15,19 Heterogeneity between groups in terms of conditioning or types of disease limits the interpretation of these data. Also, the number of patients receiving total body irradiation (TBI)–based ablative regimens in these studies was small, regardless of graft source or donor type. This raises questions about the impact of the use of ATG in TBI-based MAC regimens, in which the balance between GVHD and graft-versus-leukemia (GVL) effects of allo-HCT might be more sensitive to in vivo TCD. Very limited data exist on the potential effect of ATG in this context.
With the objective to explore the impact of the use of ATG on the TBI-based MAC regimen, we chose to retrospectively analyze a cohort of 724 adult patients receiving allo-HCT for AML following a TBI-based MAC regimen. In this homogeneous cohort of patients, we compared transplant outcomes of 251 patients who had received ATG for GVHD prophylaxis with the 473 patients who had not. The analysis tests the hypothesis that ATG before allo-HCT decreases the risk of GVHD without compromising GVL effects.
Methods
Study design and data collection
This is a retrospective multicenter analysis using the data set of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) group registry. The EBMT is a voluntary working group of >600 transplant centers that are required to report all consecutive stem cell transplantations and follow-ups once a year. Audits are routinely performed to determine the accuracy of the data. The study was planned and approved by the Acute Leukemia Working Party of the EBMT. Informed consent was obtained from all patients to use their personal information for research purposes. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Eligibility criteria for this analysis included patients older than 18 years of age with AML who underwent a first allo-HCT from a matched sibling (n = 412) or 9/10 or 10/10 MUD (n = 312), following a TBI (≥8-Gy)-based MAC regimen, between 2008 and 2016. Exclusion criteria were previous allogeneic or cord blood transplantation, ex vivo T-cell–depleted hematopoietic cell graft, and lack of information on cytogenetics. Data collected included recipient and donor characteristics (age, sex, cytomegalovirus [CMV] serostatus), disease characteristics and status at transplant; year of transplant and interval from diagnosis to transplant, and transplant-related factors, including conditioning regimen, use and dose of ATG for pretransplant in vivo TCD, stem cell source (BM or peripheral blood [PB]), and posttransplant GVHD prophylaxis. GVHD prophylaxis regimens were dependent on the centers’ protocols. We did not have information about the brand of ATG formulation (thymoglobin versus Grafalon). Grading of aGVHD was performed using established criteria.20 cGVHD was classified as limited or extensive, according to published criteria.21 For the purpose of this study, all necessary data were collected according to the EBMT guidelines, using the EBMT minimum essential data forms. The list of institutions reporting data included in this study is provided in the supplemental data.
Statistical analysis
Study end points were engraftment, incidence, and severity of aGVHD and cGVHD, primary disease relapse incidence (RI), NRM, leukemia-free survival (LFS), OS, and GVHD and relapse-free survival (GRFS). Start time was the date of transplant for all end points. LFS was defined as survival without relapse or progression, NRM was defined as death without relapse/progression, and GRFS was defined as survival with no evidence of relapse/progression, grade III-IV aGVHD, or severe cGVHD, as defined by Ruggeri et al for registry-based studies.22 Cumulative incidence functions were used to estimate RI and NRM in a competing risk setting, because death and relapse compete with each other. For estimating the cumulative incidence of aGVHD and cGVHD, we considered relapse and death to be competing events. Groups were compared using the χ2 test for qualitative variables, and the Mann-Whitney U test was applied for continuous parameters. Univariate comparisons were performed using the log-rank test for OS, LFS, and GRFS and using Gray’s test for RI, NRM, and GVHD cumulative incidences. Multivariate analyses were performed using the Cox proportional hazards model for all end points. Factors differing in terms of distribution between the groups, factors associated with 1 of the outcomes in univariate analysis, and all factors known to be potential risk factors were included in the final model. To test for a center effect, we introduced a random effect or frailty for each center into the model, as described previously.23 All tests were 2-sided. The type I error rate was fixed at 0.05 for the determination of factors associated with time-to-event outcomes. Statistical analyses were performed with SPSS 24.0 (IBM Corp., Armonk, NY) and R 3.4.0 software (R Development Core Team, Vienna, Austria) packages.
Results
Patient, transplant, and disease characteristics
Patient, disease, and transplant characteristics are summarized in Table 1.
A total of 724 patients with AML who had received an allo-HCT from a matched sibling (n = 412) or a 9/10 or 10/10 MUD (n = 312), following a TBI (≥8-Gy)-based MAC regimen, between 2008 and 2016 was included in the study. Among the total population of patients, 473 (65%) did not receive ATG within the conditioning regimen (non-ATG group), whereas 251 (35%) received ATG (ATG group). Other than ATG, GVHD prophylaxis was mainly based on the association of cyclosporine and methotrexate in both groups (74% and 72% in the non-ATG and ATG groups, respectively). The choice of GVHD prophylaxis was dependent on transplant center protocols and strategies for transplantation.
There were no significant differences in patient and disease characteristics between the ATG and non-ATG groups (Table 1). There was no difference in terms of the cytogenetic risk category based on European Leukemia Net stratification24 between the 2 groups. Cyclophosphamide and TBI (Cy-TBI) was used more frequently in the non-ATG group (93% vs 84% in the ATG group, P < .0001). Unrelated donor was used for 89% of the patients in the ATG group vs 19% in the non-ATG group (P < .001). There were more patients in the ATG group with 9/10 MRDs compared with the non-ATG group (24% vs 3%). The majority of the patients received PB as the stem cell source (78% in the non-ATG group vs 83% in the ATG group, P = not statistically significant). The median follow-up was 59 months (interquartile range [IQR], 28-83) and was similar in the non-ATG group (59 months; IQR, 32-84) and the ATG group (58 months; IQR, 24-83).
Impact of ATG on engraftment and GVHD
Engraftment and incidences of acute and chronic GVHD are shown in Tables 1 and 2. There was no difference with regard to engraftment rate between the non-ATG and ATG groups (99% in both groups). The median time to neutrophil engraftment (first day of 3 consecutive days with absolute neutrophil count >0.5 × 109/L) was longer in the ATG group (19 days vs 17 days in non-ATG group, P < .001). The day-30 cumulative incidence of neutrophil engraftment was 98% in the non-ATG group and 96% in the ATG group. Two ATG recipients and 3 patients in the non-ATG group died before engraftment before day 28.
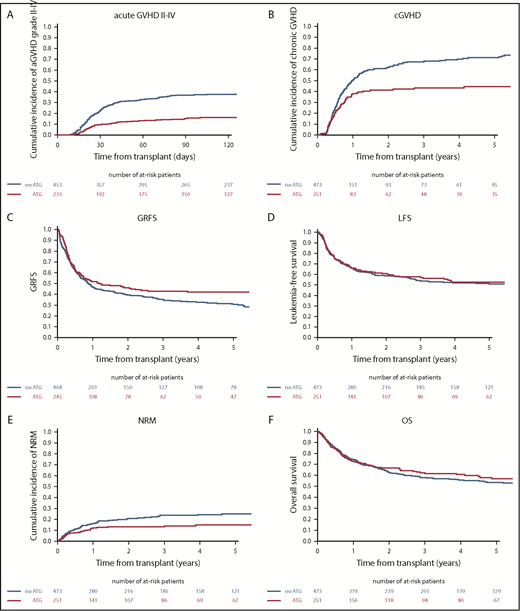
Using univariate analysis, in vivo TCD (ATG group) was significantly associated with a reduced risk for grade II-IV and grade III-IV aGVHD (Table 2). Day-100 cumulative incidences of grade II-IV and grade III-IV GVHD were 33% vs 24% (P = .01) and 13% vs 7% (P = .0092), respectively, in the non-ATG and ATG groups. Using multivariate analysis, in vivo TCD (ATG group) was independently associated with a decreased risk for grade II-IV aGVHD (hazard ratio [HR], 0.28; 95% confidence interval [CI], 0.18-0.44; P < .0001) and grade III-IV aGVHD (HR, 0.21; 95% CI, 0.10-0.43; P < .0001). The other factors significantly associated with aGVHD are summarized in Tables 2 and 3.
Two-year cumulative incidences of overall and extensive cGVHD were significantly reduced in the ATG group in comparison with the non-ATG group (46% vs 34% for overall cGVHD, P = .003; 22% vs 16% for extensive cGVHD, P = .01) (Table 2; Figure 1). As shown in Table 4, GVHD-related deaths accounted for 23% (n = 42) and 20% (n = 20) of all causes of death in the non-ATG and ATG groups, respectively (P = .66). On multivariate analysis, the use of ATG was associated with a reduced risk for cGVHD development (overall, HR, 0.63; 95% CI, 0.42-0.94; P = .02; extensive, HR, 0.69; 95% CI, 0.38-1.23; P = .21) (Table 3; Figure 1). The other factors associated with cGVHD are summarized in Tables 2 and 3.
Impact of ATG on transplant outcome after TBI-based myeloablative regimens. (A) Adjusted grade II-IV aGVHD. (B) cGVHD. (C) GVHD and GRFS. (D) LFS. (E) NRM. (F) OS.
Impact of ATG on transplant outcome after TBI-based myeloablative regimens. (A) Adjusted grade II-IV aGVHD. (B) cGVHD. (C) GVHD and GRFS. (D) LFS. (E) NRM. (F) OS.
Toxicity and NRM
Two-year NRM for the entire cohort was 16% (95% CI, 13.6-19.1). Using univariate analysis, the cumulative incidence of 2-year NRM was similar between the groups (16%; 95% CI, 12.8-19.6 for non-ATG group and 17%; 95% CI, 12.2-21.7 for ATG group) (Table 2). Other than GVHD, the main causes of death from NRM in the non-ATG and ATG groups were infections, in 21 (11%) and 14 (14%) patients, respectively, and veno-occlusive disease, in 4 (2%) and 1 (1%) patient, respectively (Table 4). One patient in each group died from an unspecified lymphoproliferative disorder, and 1 patient in the ATG group died from posttransplant lymphoproliferative disease. We have included a detailed description of other causes of death in supplemental Table 2. Using multivariate analysis, in vivo TCD (ATG group) was independently associated with a decreased risk for NRM (HR, 0.54; 95% CI, 0.32-0.91; P = .02). The other factors associated with NRM are summarized in Tables 2 and 3.
Use of ATG had no impact on relapse incidence
Two-year cumulative incidence of relapse in the entire cohort of patients was 24.5% (95% CI, 22.2-28.8) and represented the main cause of death in both groups: 53% of all causes of death in the non-ATG group and 47% of all causes of death in the ATG group (Table 4). Using univariate analysis, the use of ATG had no impact on the 2-year cumulative incidence of relapse, which was 25% (95% CI, 21.1-29.3) of the patients in the non-ATG group and 26% (95% CI, 20.6-31.9) of those in the ATG group (P = .78) (Table 2; Figure 1). The absence of the impact of ATG on relapse risk was confirmed using multivariate analysis (HR = 1.45, 95% CI, 0.90-2.34; P = .13). The other factors associated with RI are summarized in Table 2 and Table 3.
Survival: OS, LFS, and GRFS
Two-year OS rate for the entire cohort was 63.2% (95% CI, 59.5-66.9). On univariate analysis, OS was not different between the groups (2-year: non-ATG 64%, 95% CI, 59.1-68.2; ATG: 62%, 95% CI, 56.1-68.6). (Table 2; Figure 1). Two-year LFS for the entire cohort was 58% (95% CI, 54.5-62.1). Using univariate analysis, 2-year LFS was not different between the groups (non-ATG: 59%, 95% CI, 54.2-63.5; ATG: 57%, 95% CI, 50.9-63.6) (Table 2; Figure 1). Two-year GRFS for the entire cohort was 42% (95% CI, 37.9-45.5). Using univariate analysis, 2-year GRFS was not different between the groups (non-ATG: 41%, 95% CI, 36.2-45.6; ATG: 43%, 95% CI, 36.8-49.8) (Table 2; Figure 1).
Multivariate analysis adjusted for age, recipient and donor sex, disease, disease risk, Karnofsky performance score, conditioning regimen, cytogenetic risk, CMV serostatus, and graft source confirmed that there was no impact of ATG on OS, LFS, or GRFS. The other factors associated with OS, LFS, and GRFS are summarized in Tables 2 and 3.
Discussion
The impact of the use of ATG following a TBI-based MAC regimen has been poorly explored. A recent prospective double-blind phase 3 study of ATG vs placebo in patients with myelodysplastic syndrome or acute leukemia who underwent allo-HCT from a MUD following MAC regimens reported inferior PFS and OS in the ATG arm, especially in the TBI cohort. However, the study was limited by the small number of patients receiving a TBI-based conditioning regimen (placebo, n = 37 vs ATG group, n = 31).25 In the absence of large prospective studies and to better understand the impact of ATG on a TBI-based MAC regimen in AML, we analyzed the effect of ATG in a large homogenous cohort of AML patients undergoing allo-HCT with TBI, with and without ATG. Contrary to previous reports on small series, our data showed that addition of ATG to a TBI-based conditioning regimen, followed by allo-HCT for AML, resulted in a significant reduction in aGVHD and cGVHD, translating into a significant reduction in NRM without increasing the relapse rate. These findings become more important when one considers that most patients in the ATG group were transplanted from an unrelated donor compared with the non-ATG group. This finding is of great importance, because cGVHD and the associated immunosuppression are the primary causes of morbidity and mortality in long-term survivors after allo-HCT and contribute directly or indirectly to most late complications.
The use of TBI-based MAC regimens has been decreasing as a result of equally effective chemotherapy-based regimens and possibly because of concerns about an increased risk for late effects with TBI-based regimens.26 However, TBI remains an integral part of the conditioning in hematopoietic cell transplantation for high-risk malignancies, especially advanced leukemias. It is possible that integration of ATG into a TBI-based regimen might be beneficial to long-term follow-up by preventing late relapse, as well as decreasing late complications after hematopoietic cell transplantation, but this needs further study.
The traditional preparative myeloablative regimens for patients with AML include Cy-TBI and the combination of busulfan + cyclophosphamide or busulfan + fludarabine.27-29 Several retrospective registry-based and randomized studies compared Cy-TBI with busulfan + cyclophosphamide or busulfan + fludarabine in patients with AML and reported conflicting results regarding outcome and toxic effects.2,27,30 However, no large study has directly compared the long-term outcome between chemotherapy-based conditioning regimens and TBI-based MAC regimens including ATG.
Our data indicate that TBI + ATG is an effective conditioning regimen for allo-HCT in patients with AML, with a lower short-term toxicity profile compared with a regimen without ATG. This makes TBI + ATG potentially applicable to high-risk patients with advanced disease. Further studies are needed to confirm the superiority of TBI + ATG over TBI-based regimens without ATG or chemotherapy-based myeloablative regimens.
The increased use of PBSC grafts (nearly 80% of stem cell grafts now used in Europe)4,5,31 is also associated with a higher incidence of aGVHD and cGVHD, even with HLA-identical sibling donors.4-6,11 It may be appropriate to consider the use of ATG, especially in patients receiving TBI-based PBSC grafts, and it might improve overall outcome by decreasing GVHD and related late complications. As reported in other settings of allo-HCT,12-15,17,32-36 this study confirms that the addition of ATG significantly reduces, after adjustment for other factors, the risk of developing aGVHD (HR, 0.28; P < .001) and cGVHD (HR, 0.627; P = .02). Such a reduction in GVHD incidence was not associated with a reduced effect of GVL, because the use of ATG did not impact RI.
A recently published25 prospective randomized double-blind trial showed decreased OS and PFS when ATG was added to tacrolimus and methotrexate as an immunosuppressive regimen, in recipients of MAC MUD allo-HCT. However, several other open-label randomized trials14,18,19 have reported a benefit of ATG with a lower incidence of cGVHD without negatively impacting PFS or OS. It is unclear why the study by Soiffer et al25 did not recapitulate outcomes from prior studies. An important explanation for the difference in outcomes may be related to the impact of the conditioning regimen on absolute lymphocyte count (ALC) and the interaction between ALC and ATG. Multiple studies, including the 1 by Soiffer et al,25 found a striking relationship between low ALC (more commonly seen with TBI-based regimens) at the time of ATG administration and inferior PFS and OS. The low ALC before allo-HCT likely resulted in less binding of ATG to lymphocytes, with subsequently delayed clearance and higher concentrations of ATG after allo-HCT.37-39
Routine use of lower-dosing (30 mg/kg total) schedules of ATG, as in the study by Kröger et al,19 could potentially maintain protective effects against cGVHD but not increase mortality.12,13 In our study, most patients had received an ATG dose < 6 mg/kg (median dose in our study was 5 mg/kg), so we could not analyze the impact of the ATG dose on outcomes in our series. The question remains whether we can decrease the ATG dose with TBI-based MAC regimens. Kennedy et al37 have shown that the PB ALC on the day of ATG administration and the total dose of ATG (range, 428.8-833.6 mg) interacted to predict transplant outcomes. For low-recipient ALC (10th percentile, or 0.56 × 102/μL), a higher total ATG dose was associated with a greater risk for death, whereas for high-recipient ALC (90th percentile, or 24.96 × 102/μL), a higher total ATG dose was associated with a lower risk for death. Patients receiving TBI-based ablation are likely to develop early lymphopenia due to the strong myeloablative and lymphoablative effects of TBI compared with chemotherapy-based MAC regimens, and transplant outcome can be improved further by lowering the ATG dose in this group of patients. The interaction between ATG and its target, the recipient lymphocyte, could represent a new paradigm for ATG dosing, and it may now be timely to explore the individualized ATG dosing in patients receiving TBI-based MAC regimens based on real-time ALC measurements to further improve transplant outcomes.37 Another potential variable is the difference in ATG formulations (thymoglobin versus Grafalon) used across the studies.40,41 It is unknown whether in vivo TCD is comparable between these 2 formulations because of the absence of dose equivalence and head-to-head comparisons between thymoglobin and Grafalon. In a meta-analysis of prospective studies, Gagelmann et al showed that Grafalon was associated with improved GVHD (acute and chronic) rates compared with thymoglobin.40 We did not have information about the brand of ATG used in the current analysis, which is a limitation of this study. A prospective study will be required to define the optimal dose and formulation of ATG.
Why does the significant reduction in aGVHD and cGVHD not translate into better OS in our series? The most likely explanations could be the improved supportive treatment and the handling of immunosuppressive measures over time that resulted in better survival, despite an increased risk for aGVHD and cGVHD, in the non-ATG group.4,6,7,32,42,43 Although cGVHD has been associated with GVL effects,4,5,44 it is also the leading cause of mortality/morbidity in long-term survivors and impairs quality of life.7-9,45 Given the long-term of effects of cGVHD, extended follow-up might yield different results; specifically, quality-of-life measures, together with other patient-reported outcomes, will be important end points to analyze at a future date. As other strategies for TCD (posttransplant cyclophosphamide) are being developed, the role of ATG in GVHD prevention will need to be validated in a prospective fashion.
In summary, incorporation of ATG-based in vivo TCD in TBI-based MAC for allo-HCT in AML resulted in lower GVHD rates and improved NRM, without increasing the disease relapse rate. Although this is a registry-based observational study, it is the largest analysis of its kind and it demands a prospective validation of the role of ATG in TBI-based MAC. Future studies exploring ALC-based dynamic dosing of ATG may establish in vivo TCD as an important GVHD-prevention strategy.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the centers of the EBMT and national registries for contributing patient information and collecting data. Supporting information is available on the EBMT Web site. Reporting institutions included in this study are available as supplemental data.
Authorship
Contribution: A.N., M.M., and B.N.S. conceived and designed the study; M.L. analyzed and interpreted data; B.D., A.N., B.N.S., M.L., and M.M. wrote the manuscript; R.N., J.M., X.P., J.C., P.R., J.H.B., Y.B., R.M., T.K., and W.S. critically reviewed the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: M.M. has received research support and honoraria from Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Bhagirathbhai Dholaria, Vanderbilt University Medical Center, 2220 Pierce Ave, PRB 686, Nashville, TN 37232; e-mail: bhagirathbhai.r.dholaria@vumc.org.