Key Points
Survival outcomes were significantly worse in patients with PBSCs compared with those with BM in FtoM HCT.
In FtoM HCT, BM would result in better outcomes than PBSCs.
Abstract
The use of granulocyte colony-stimulating factor–mobilized peripheral blood stem cells (PBSCs) and sex-mismatched hematopoietic cell transplantation (HCT), especially with female donors and male recipients (FtoM), is known to be associated with an increased risk of chronic graft-versus-host disease (GVHD) compared with transplantation with bone marrow (BM). This raises the question of whether the use of PBSCs in FtoM HCT might affect allogeneic responses, resulting in fatal complications. Using a Japanese transplantation registry database, we analyzed 1132 patients (FtoM, n = 315; MtoF, n = 260; sex-matched, n = 557) with standard-risk diseases who underwent HCT with an HLA-matched related donor without in vivo T-cell depletion between 2013 and 2016. The impact of PBSC vs BM on transplantation outcomes was separately assessed in FtoM, MtoF, and sex-matched HCT. Overall survival (OS) and nonrelapse mortality (NRM) at 2 years post-HCT were significantly worse in patients with PBSCs vs those with BM in FtoM HCT (2-year OS, 76% vs 62%; P = .0084; 2-year NRM, 10% vs 21%; P = .0078); no differences were observed for MtoF or sex-matched HCT. Multivariate analyses confirmed the adverse impact of PBSCs in FtoM HCT (hazard ratio [HR] for OS, 1.91; P = .025; HR for NRM, 3.70; P = .0065). In FtoM HCT, patients with PBSCs frequently experienced fatal GVHD and organ failure. In conclusion, the use of PBSCs in FtoM HCT was associated with an increased risk of NRM in the early phase, resulting in inferior survival. This suggests that, when we use female-related donors for male patients in HCT, BM may result in better outcomes than PBSCs.
Introduction
Hematopoietic cell transplantation (HCT) is a widely accepted curative procedure for hematological malignant diseases. However, patients frequently experience various adverse complications after HCT, including infection, graft-versus-host disease (GVHD), and disease relapse. Several clinical variables have been established as risk factors for GVHD or inferior survival: ages of the patient and donor, disease, disease status, HLA mismatch, and unrelated donor.1-6 Among these risk factors, sex-mismatched HCT, especially HCT with female donors and male recipients (FtoM), is well known to be associated with a higher incidence of GVHD or inferior survival.3-10 This adverse effect of FtoM HCT is thought to result from an allogeneic immune response against minor histocompatibility antigens encoded on the Y chromosome of the male recipient (HY antigens).7,11-16 In fact, the cumulative number of anti–HY antigen antibodies (HY Abs) was shown to be significantly associated with increased risks of chronic GVHD and nonrelapse mortality (NRM).14
In contrast, advances regarding cell sources such as peripheral blood stem cells (PBSCs) mobilized by granulocyte-colony stimulating factor have contributed to the worldwide adoption of HCT. Over the past decade, PBSCs have been widely used as a cell source.17 However, a PBSC graft contains many more T cells than bone marrow (BM) or cord blood,17 which has led to concerns about GVHD. In fact, PBSCs are well known to increase the risk of chronic GVHD compared with other sources.4,5,18-20 The impact of PBSCs compared with BM on survival is still controversial.19,21-24 This background raises the question of whether the use of PBSCs in FtoM HCT might affect allogeneic responses, resulting in fatal complications. Therefore, we hypothesized that BM could contribute to better survival than PBSCs in FtoM HCT by reducing NRM.
Patients and methods
Patient selection
Clinical data of recipients who underwent HCT were collected by the Japan Society for Hematopoietic Cell Transplantation and the Japanese Data Center for Hematopoietic Cell Transplantation with the Transplant Registry Unified Management Program.25-27 Using the Japan Society for Hematopoietic Cell Transplantation registry database, we retrospectively analyzed adult and adolescent recipients (age >15 years) with standard-risk diseases who underwent their first HCT with an HLA-matched related donor between 2013 and 2016. For recipients to be considered eligible, data on age, sex, HLA, donor source, disease status at HCT, and survival status at the end of follow-up were required. Patients who underwent in vivo T-cell depletion were excluded. This retrospective analysis was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board at Jichi Medical University Saitama Medical Center.
Definitions of categories
Conditioning regimens were classified as myeloablative or reduced-intensity conditioning based on the report by Giralt et al.28 Briefly, conditioning regimens that included >8 Gy of total body irradiation, ≥140 mg/m2 of melphalan, or ≥9 mg/kg of oral busulfan (≥7.2 mg/kg of IV busulfan) were classified as myeloablative conditioning, and other regimens were classified as reduced-intensity conditioning. Standard-risk diseases were defined as follows: acute leukemia in first or second complete remission, chronic myeloid leukemia (CML) in first or second chronic phase, myelodysplastic syndrome other than refractory anemia with excess blasts, and lymphoma in complete or partial remission. Other disease status was classified as high risk. HLA match was defined as a 6/6 serological match at HLA-A, -B, and -DR loci. Refined disease risk index (rDRI) was estimated according to the previous report.29 HCT-specific comorbidity index (HCT-CI) was calculated as described previously.30 The diagnosis and severity of acute GVHD (aGVHD) were reported based on traditional grading scores,31 and those of chronic GVHD (cGVHD) were reported based on the classical Seattle criteria.32 Causes of death were determined based on the primary cause of death reported by the attending physicians. When the secondary cause of death was GVHD, relapse, or graft failure, the cause of death was reclassified respectively.
Statistical analysis
Overall survival (OS) from HCT was calculated by the Kaplan-Meier method with a 95% confidence interval (CI) and compared by the log-rank test. The cumulative incidences of aGVHD and cGVHD were estimated and compared by Gray’s method, where death or relapse without these events was considered as a competing risk. Relapse and NRM were also estimated by Gray’s method, considering the other as a competing risk. Multivariate analyses were performed by a Cox proportional hazard model, and the hazard ratio (HR) of PBSCs was adjusted for the ages of the patient and donor, serostatus of cytomegalovirus, conditioning type, disease, rDRI, GVHD prophylaxis, HCT-CI, performance status, and use of total body irradiation. The impact of PBSC vs BM on HCT outcomes was separately assessed in the individual cohorts of FtoM, MtoF, and sex-matched HCT. P < .05 was considered statistically significant. All data management and statistical calculations were performed using Stata (version 12.0; Stata Corp., College Station, TX) and EZR, which is a graphical user interface for R (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria).33
Results
Patient characteristics
The overall HCT cohort was classified into FtoM (n = 315; BM, n = 95 and PBSCs, n = 220), MtoF (n = 260; BM, n = 90 and PBSCs, n = 170), and sex-matched (n = 557; BM, n = 176 and PBSCs, n = 381) HCT cohorts. The median age of the patients was 44, 45, and 44 years, respectively. There were no significant differences in age, disease, rDRI, HCT-CI, or conditioning intensity between the BM and PBSC groups in each cohort, but more recipients tested positive for cytomegalovirus in the PBSC group of the FtoM cohort. In addition, the BM group tended to receive more cyclosporine-based GVHD prophylaxis in the 3 cohorts (Table 1). In total, each cohort classified according to sex-mismatch type was considered to have a similar background in the BM and PBSC groups, although cyclosporine was more frequently used as GVHD prophylaxis in the BM group than in the PBSC group in all 3 cohorts (Table 1). The median duration of follow-up for survivors was 755 days.
OS in HCT with BM vs PBSCs in the cohorts classified according to sex-mismatch type
In the FtoM HCT cohort, 2-year OS in the BM group was superior to that in the PBSC group (76%; 95% CI, 65%-84% vs 62%; 95% CI, 54%-68%; P = .0084; Figure 1A). In contrast, no significant differences were observed between the BM and PBSC groups in the MtoF HCT cohort (81%; 95% CI, 70%-89% vs 73%; 95% CI, 65%-80%; P = .12; Figure 1B) or the sex-matched HCT cohort (73%; 95% CI, 65%-80% vs 73%; 95% CI, 67%-77%; P = .98; Figure 1C).
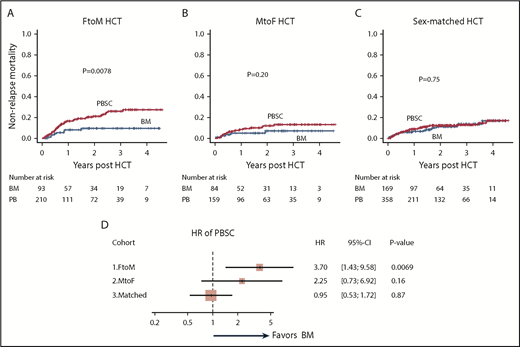
OS in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. FtoM HCT (A), MtoF HCT (B), and sex-matched HCT (C). (D) Forest plots of the impact of PBSCs on OS in FtoM, MtoF, and sex-matched HCT.
OS in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. FtoM HCT (A), MtoF HCT (B), and sex-matched HCT (C). (D) Forest plots of the impact of PBSCs on OS in FtoM, MtoF, and sex-matched HCT.
Multivariate analyses confirmed that the use of PBSCs was significantly associated with inferior survival in the FtoM HCT cohort (HR, 1.91; 95% CI, 1.09-3.36; P = .025), whereas no significant associations between cell source and OS were observed in the other HCT cohorts (Figure 1D).
NRM and relapse incidence in HCT with BM vs PBSCs classified according to sex-mismatch type
In the FtoM HCT cohort, 2-year NRM in the BM group was lower than that in the PBSC group (10%; 95% CI, 4%-17% vs 21%; 95% CI, 16%-28%; P = .0078; Figure 2A). In contrast, the 2-year NRM values in the BM and PBSC groups were similar in both the MtoF HCT cohort (7%; 95% CI, 3%-15% vs 12%; 95% CI, 7%-18%; P = .20; Figure 2B) and sex-matched HCT cohort (11%; 95% CI, 7%-17% vs 12%; 95% CI, 9%-16%; P = .75; Figure 2C).
NRM in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. FtoM HCT (A), MtoF HCT (B), and sex-matched HCT (C). (D) Forest plots of the impact of PBSC on OS in FtoM, MtoF, and sex-matched HCT.
NRM in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. FtoM HCT (A), MtoF HCT (B), and sex-matched HCT (C). (D) Forest plots of the impact of PBSC on OS in FtoM, MtoF, and sex-matched HCT.
Multivariate analyses also demonstrated that the use of PBSCs was significantly associated with an increased risk of NRM in the FtoM HCT cohort (HR, 3.70; 95% CI, 1.43-9.58; P = .0069), whereas cell source was not associated with any risk of NRM in either the MtoF or sex-matched HCT cohort (Figure 2D).
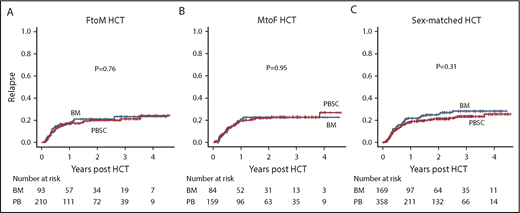
Regarding the incidence of relapse, no difference was observed between the BM and PBSC groups in any cohort stratified according to sex-mismatch type (Figure 3A-C).
Cumulative incidences of relapse in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. FtoM HCT (A), MtoF HCT (B), and sex-matched HCT (C).
Cumulative incidences of relapse in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. FtoM HCT (A), MtoF HCT (B), and sex-matched HCT (C).
In summary, the use of PBSCs in the FtoM HCT cohort was associated with an increased risk of NRM, which led to inferior OS, whereas cell source had no effect in the other cohorts.
Neutrophil engraftment and aGVHD and cGVHD in HCT with BM vs PBSC classified according to sex-mismatch type
Next, we explored what kinds of adverse events caused the increased NRM in the PBSC group in the FtoM HCT cohort and also checked the prevalence of these events in the MtoF and sex-matched HCT cohorts as reference. Expectedly, the PBSC group significantly more rapidly achieved neutrophil engraftment in the FtoM HCT cohort as well as the other HCT cohorts, but >95% of patients succeeded in engraftment 30 days after HCT in all groups.
In the FtoM HCT cohort, the cumulative incidence of grade 2 to 4 aGVHD was 28% (95% CI, 19%-37%) in the BM group vs 34% (95% CI, 28%-41%) in the PBSC group 100 days after HCT (P = .16; Figure 4A). Multivariate analysis revealed that the use of PBSCs was not associated with an increased risk of grade 2 to 4 aGVHD in the FtoM HCT cohort (HR, 1.34; 95% CI, 0.83-2.16; P = .23). In contrast, the PBSC groups in both the MtoF HCT cohort (29%; 95% CI, 20%-38% vs 37%; 95% CI, 30%-45%; P = .048; Figure 4B) and sex-matched HCT cohort (21%; 95% CI, 16%-28% vs 31%; 95% CI, 26%-35%; P = .032; Figure 4C) seemed to frequently experience grade 2 to 4 aGVHD.
Cumulative incidences of aGVHD in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. Grade 2 to 4 (A-C) and grade 3 to 4 (D-F) aGVHD in FtoM HCT (A,D), MtoF HCT (B,E), and sex-matched HCT (C,F).
Cumulative incidences of aGVHD in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. Grade 2 to 4 (A-C) and grade 3 to 4 (D-F) aGVHD in FtoM HCT (A,D), MtoF HCT (B,E), and sex-matched HCT (C,F).
The cumulative incidence of grade 3 to 4 aGVHD seemed similar between the BM and PBSC groups in the FtoM HCT cohort (10%; 95% CI, 5%-17% vs 34%; 95% CI, 9%-18%; P = .39; Figure 4D) as well as the MtoF HCT cohort (Figure 4E). Multivariate analyses also failed to show an association between cell source and any risk of grade 3 to 4 aGVHD in either the FtoM or MtoF HCT cohort. However, in the sex-matched HCT cohort, the PBSC group experienced significantly more grade 3 to 4 aGVHD, although the incidence was low (Figure 4F).
As expected, among 3-month survivors without relapse (n = 269, 217, and 463 in FtoM, MtoF, and sex-matched HCT, respectively), the PBSC group experienced significantly more cGVHD than the BM group in the FtoM HCT cohort (60%; 95% CI, 52%-67%; P = .0014; Figure 5A) as well as the MtoF and sex-matched HCT cohorts (Figure 5B-C). In fact, the use of PBSCs was significantly associated with an increased risk of cGVHD in the FtoM HCT cohort (HR, 2.57; 95% CI, 1.63-4.06; P < .001) as well as the MtoF (HR, 2.28; 95% CI, 1.30-3.99; P = .0042) and sex-matched (HR, 1.41; 95% CI, 1.00-1.99; P = .049) HCT cohorts. The HR for the use of PBSCs in the FtoM HCT cohort seemed to be greater than those in the other cohorts.
Cumulative incidences of cGVHD in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. Any-grade (A-C) and extensive (D-F) cGVHD in FtoM HCT (A,D), MtoF HCT (B,E), and sex-matched HCT (C,F).
Cumulative incidences of cGVHD in the BM and PBSC groups in HCT cohorts classified according to sex-mismatch type. Any-grade (A-C) and extensive (D-F) cGVHD in FtoM HCT (A,D), MtoF HCT (B,E), and sex-matched HCT (C,F).
The PBSC group also tended to show a higher incidence of extensive cGVHD (Figure 5D-F). However, multivariate analyses suggested that the use of PBSCs was significantly associated with an increased risk of extensive cGVHD only in the FtoM HCT cohort (HR, 1.89; 95% CI, 1.11-3.22; P = .019), but not in the other cohorts. Figure 6 summarizes the impact of the use of PBSCs on clinical outcomes in individual cohorts according to sex-mismatch type. Conversely, supplemental Table 1 shows clinical outcomes of sex-mismatched HCT in the BM or PBSC cohort as reference.
Summary of the impact of PBSCs on clinical outcomes in the HCT cohorts classified according to sex-mismatch type. aGVHD24, grade 2 to 4 aGVHD; aGVHD34, grade 3 to 4 aGVHD; ex.cGVHD, extensive cGVHD.
Summary of the impact of PBSCs on clinical outcomes in the HCT cohorts classified according to sex-mismatch type. aGVHD24, grade 2 to 4 aGVHD; aGVHD34, grade 3 to 4 aGVHD; ex.cGVHD, extensive cGVHD.
Causes of death
In the FtoM HCT cohort, the cause of nonrelapse death was GVHD related in 19, organ failure in 23, infection in 12, and other in 4 patients, whereas death resulting from relapse was observed in 45 patients. The PBSC group experienced fatal GVHD and organ failure more often than the BM group in the FtoM HCT cohort (17% vs 5%; P = .006; Figure 7). In contrast, in the other cohorts, there was no difference in adverse events, including fatal GVHD and organ failure, between the BM and PBSC groups (MtoF HCT cohort: 6% vs 9%; P = .47; sex-matched HCT cohort: 10% vs 9%; P = .88; Figure 7).
Incidences of fatal events in the groups with BM and PBSCs in HCT cohorts classified according to sex-mismatch type. IPS, idiopathic pneumonia syndrome; SOS, sinusoidal obstruction syndrome; TMA, thrombotic microangiopathy.
Incidences of fatal events in the groups with BM and PBSCs in HCT cohorts classified according to sex-mismatch type. IPS, idiopathic pneumonia syndrome; SOS, sinusoidal obstruction syndrome; TMA, thrombotic microangiopathy.
Discussion
In the FtoM HCT cohort only, the PBSC group exhibited inferior survival and higher NRM, whereas no differences in survival were observed between the BM and PBSC groups in either MtoF or sex-matched HCT. The PBSC group in the FtoM HCT cohort frequently experienced fatal GVHD or organ failure compared with the BM group. These findings support our hypothesis that PBSC use in FtoM HCT might deteriorate allogeneic responses in the early phase, resulting in fatal complications.
To our knowledge, this is the first report to separately assess the impact of BM vs PBSCs in cohorts classified according to sex-mismatch type. PBSCs have been established as a risk factor for the development of cGVHD.4,5,18-20,23,24 However, it remains a matter of debate whether the increased incidence of GVHD results in inferior survival, because GVHD may also be associated with graft-versus-leukemia effects. In fact, PBSCs were previously reported to have a lower incidence of relapse than BM.34-36 Several meta-analyses using prospective studies have addressed the question of whether BM or PBSCs are a better cell source.19,23,24 The Stem Cell Trialists’ Collaborative Group reported no significant difference in OS between the BM and PBSC groups when 9 trials were combined.19 Similarly, there was still no difference in OS when 17 trials were reanalyzed.24 However, subgroup analyses demonstrated that PBSCs were associated with superior OS in patients with advanced-stage disease or CML.19 However, no difference was observed in patients with early-stage disease.19 In the 2000s or earlier, a major indication for HCT was CML. However, the progress made with tyrosine kinase inhibitors has reduced the indication of HCT for CML patients. Therefore, the impact of PBMCs vs BM might differ according to the disease, disease risk, and year the study was performed. In the current study, by focusing on patients who recently underwent HCT and those with standard-risk diseases, we could more accurately assess the impact of PBSCs on clinical outcomes.
FtoM HCT is also recognized as a significant risk factor for the development of cGVHD.3-10 Biologically, naïve female-donor T or B cells are considered to recognize HY proteins as alloantigens and attack tissues of male recipients, eventually leading to cGVHD development or deterioration.7,11-16 In fact, HY Abs were detected in half of male recipients with female donors 3 months after HCT, and the cumulative number of HY Abs 3 months after HCT predicts subsequent cGVHD development. In addition, an increased cumulative number of HY Abs 3 months after HCT was associated with the severity of cGVHD and NRM.14 Furthermore, B cells specific to DBY-2, an immune-dominant peptide of HY antigens, are also detected 6 to 12 months after FtoM HCT and are associated with cGVHD development.12 Taken together, these results strongly support the notion that there are allogeneic responses specific to FtoM HCT, but it is still unclear whether PBSCs react more aggressively against these HY antigens because of more lymphocytes or whether BM could induce tolerance because of more regulatory cells. Therefore, the current study might shed light on the difference in HY immunity according to cell source, and additional basic research is warranted.
This study has several limitations resulting from its retrospective nature. The selection of donor sources may have been decided partially based on several donor factors, including age, comorbidity, and donor preference. The registry database does not include information on the reason why BM or PBSCs were selected. Therefore, there may be some selection preference by the participating institutions or bias resulting from the various patient and donor backgrounds. Furthermore, the current analysis is limited to patients with standard-risk diseases. Practically, HCT for patients with high-risk diseases might attract more attention from clinicians. Therefore, the difference in the impact of cell source according to sex-mismatch type should be addressed in patients with high-risk diseases, unrelated donors, or minimal conditioning intensity in future studies, because the magnitude of graft-versus-leukemia or GVHD according to sex mismatch or cell source might differ according to these factors and affect survival outcomes.37 However, the current analyses using a large population made it possible to identify which patients with standard-risk diseases may benefit from the selection of BM as a cell source. In Japan, in vivo T-cell depletion is rarely used for HCT with an HLA-matched related donor. Therefore, these findings may inspire a prospective observational study or clinical trial for an interventional approach to reduce the adverse events in FtoM HCT with PBSCs by using antithymocyte globulin or rituximab.
In conclusion, the use of PBSCs in FtoM HCT was associated with an increased risk of NRM even in the early phase, resulting in inferior survival. The PBSC group in FtoM HCT frequently experienced fatal GVHD or adverse complications. However, the use of PBSCs did not seem to affect survival outcomes in MtoF or sex-matched HCT. Therefore, when we have to select female-related donors for male patients, BM may result in better outcomes. A prospective trial is necessary before we can draw definitive conclusions.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the physicians and data managers at the centers that contributed valuable data on transplantation to the Japan Society for Hematopoietic Cell Transplantation (JSHCT) and also thank all of the members of the Transplant Registry Unified Management committees at JSHCT for their dedicated data management.
H. Nakasone was a recipient of a grant from Takeda Science Foundation.
Authorship
Contribution: H. Nakasone designed the study, analyzed data, and wrote the manuscript; K.K., K.Y., A.S., Y.I., and S.S. advised on methods and wrote the manuscript; M.T., K.O, S.O., N.U., T.F., H. Nakamae, and K.M. collected data and revised the manuscript; J.K. and T.I. collected data, revised the manuscript, and were responsible for data management at JSHCT; Y.A. managed the unified registry database and revised the manuscript; and F.K. and M.O. designed the study, advised on the methods, revised the manuscript, and were responsible for the project.
Conflict-of-interest disclosure: T.I. received research funding from Astellas Pharma, Chugai Pharmaceutical Co., CSL Behring, Eisai Co., Kyowa Hakko Kirin Co., Ono Pharmaceutical Co., Pfizer, Nippon Shinyaku Co., MSD, Otsuka Pharmaceutical Co., Repertoire Genesis Inc., Sumitomo Dainippon Pharma Co., Taiho Pharmaceutical Co., Takeda Pharmaceutical Co., and Zenyaku Kogyo Co., and honoraria from Alexion Pharmaceuticals, Bristol-Myers Squibb, Celgene, JCR Pharmaceuticals, Janssen Pharmaceutical K.K., Mundipharma, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Hideki Nakasone, Division of Hematology, Jichi Medical University Saitama Medical Center, 1-847 Amanuma-cho Omiya-ku, Saitama 330-8503, Japan; e-mail: nakasone-tky@umin.ac.jp.