Abstract
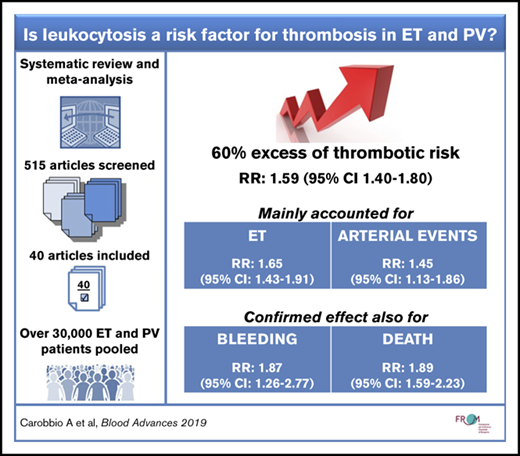
In the last years, a growing amount of evidence has been produced regarding the role of leukocytosis as a risk factor for thrombosis in patients with myeloproliferative neoplasms, predominantly in polycythemia vera (PV) and essential thrombocythemia (ET). Results from epidemiologic studies on this issue, however, are inconclusive. We conducted a systematic review and meta-analysis of articles published in the last 12 years addressing the issue, according to a predefined protocol. Forty-one articles analyzing >30 000 patients met our inclusion criteria and were deemed of acceptable methodologic quality. In addition to data on thrombosis, data were collected on bleeding, hematologic evolution, secondary cancer, and death. The relative risk (RR) of thrombosis in the presence of leukocytosis was 1.59 (95% CI, 1.40-1.80), mainly accounted for by ET (RR, 1.65; 95% CI, 1.43-1.91) and arterial thrombosis (RR, 1.45; 95% CI, 1.13-1.86) subgroups; the effect was not significant in venous thrombosis alone. Sensitivity analyses considering recurrent events as well as white blood cell estimates adjusted or unadjusted for confounding factors confirmed the primary results. In addition, the pooled RR of studies that tested white blood cell counts in time-dependent models suggested a causative effect of leukocytes in the mechanism that triggers thrombosis. The effect of leukocytosis on bleeding (RR, 1.87; 95% CI, 1.26-2.77) and death (RR, 1.89; 95% CI, 1.59-2.23) was confirmed, whereas conclusions on hematologic evolutions and solid tumors were uncertain. To confirm the accuracy of these results, an investigation on individual patient data in a large collective archive of homogeneous patients is warranted.
Introduction
Essential thrombocythemia (ET) and polycythemia vera (PV) are chronic myeloproliferative neoplasms (MPNs) characterized by clonal proliferation of the erythroid, myeloid, and megakaryocyte lineages. Their natural history is marked by cardiovascular events, bleeding, transformation to myelofibrosis, and acute myeloid leukemia. Advanced age and/or previous cardiovascular events identify patients at high risk of thrombosis for whom cytoreductive therapy is indicated.1,2
In ET and PV, new factors have been proposed as candidate biomarkers for predicting postdiagnosis vascular events. In the International Prognostic Score for Essential Thrombocythemia (IPSET)–thrombosis, the presence of cardiovascular risk factors and JAK2V617F mutation resulted in thrombosis-independent risk factors, in addition to age and previous events.3,4 In PV, an additional “intermediate” risk category can be identified by the presence of cardiovascular risk factors such as hypertension in otherwise low-risk patients.5 To refine these risk classes, the role of leukocytosis as a prognostic/causative factor has been the object of numerous investigations, but to date no definitive conclusion has been drawn on the matter.6-9 Although a number of studies suggested a role for leukocytosis in the pathogenesis of thrombosis, both on the basis of statistical association10-12 and biological plausibility,13-15 leukocytosis has never been formally included in risk models and prognostic scores.16 This uncertainty is due to inconsistencies in the definition of leukocytosis, lack of a clear cutoff value for white blood cell (WBC) counts, and heterogeneity in methods for its assessment. Furthermore, in all clinical studies available to date, designs were never specifically aimed at assessing the role of leukocytosis; as a consequence, available studies are highly heterogeneous in terms of size, statistical power, and duration of follow-up and exposure, ultimately leading to inconclusive evidence.
A systematic review and meta-analysis of available evidence can provide an answer to this unmet clinical need, whereas new studies will be needed to accurately quantify the role of leukocytosis and grant its inclusion in prognostic scores. The goals of the current study were: (1) to assess whether leukocytosis is associated with a higher risk of arterial as well as venous major thrombotic events in adult patients with PV or ET; and (2) to investigate the effect of leukocytosis on secondary outcomes (major bleeding, hematologic evolutions, solid tumors, and mortality).
Methods
Protocol registration
The protocol for this review was registered in advance in the International Prospective Register of Systematic Reviews (PROSPERO registration number, CRD42019122292).19
Type of studies and participants
This study included only controlled and noncontrolled trials and prospective and retrospective cohort studies with at least 20 participants reporting data on incident (after diagnosis) outcomes. The following studies were excluded: case reports, expert opinion papers, editorials, and narrative reviews.
Studies on adult (age ≥18 years), nonpregnant participants diagnosed with both ET and PV based on World Health Organization (WHO) criteria were included.
Types of exposures
WBC counts measured both at diagnosis and at enrollment into a clinical trial and/or an observational study were considered.
Outcomes
The primary outcome of the current review was major thrombotic event associated with leukocyte count. Major thromboses were defined as fatal or nonfatal arterial (eg, myocardial infarction, stroke, transient ischemic attack, peripheral and visceral thromboembolism) or venous (eg, deep vein thrombosis, pulmonary embolism, cerebral venous sinus thrombosis, splanchnic circulation thrombosis) thrombotic events occurring after the diagnosis of ET or PV.
Secondary outcomes were defined as: (1) major bleeding events; (2) hematologic transformations and/or solid neoplasms; and (3) overall mortality.
Identification and selection of studies
Using MEDLINE electronic databases (from 2005-October 2018), all studies that reported the relative risk of thrombosis estimates for leukocytosis were identified. We developed 2 search strategies with the aim of identifying direct and indirect reporting of WBC risk estimates. For the first strategy, the following Medical Subject Headings and text words were used: “leukocytes,” “leukocytes count,” and “leukocytosis” together with “Essential Thrombocythemia” or “Polycythemia Vera.” For the second strategy, we used “causative factor” and “risk factor” together with “Essential Thrombocythemia” or “Polycythemia Vera,” and we manually screened the presence of WBC risk estimates.
Working independently and in duplicate, 2 reviewers (A.C. and A.F.) examined all titles and abstracts, obtained full texts, and determined their eligibility. In case of disagreement, a third author (T.B.) was involved.
Data extraction
Two reviewers (A.C. and A.F.), working independently and in duplicate, extracted data using a data collection sheet reporting study details (year of publication and design), population characteristics (patient numbers, diagnosis phenotypes, mean/median age, mean/median leukocyte count at diagnosis/enrollment, and low-/high-risk status), how WBC effect was assessed (using a prespecified cutoff, as a continuous variable, or other), type of statistical model used to obtain the risk estimate (univariate/multivariable), and site of thrombosis (arterial/venous).
When outcome data were not reported, the authors were contacted and asked to supply the missing information.
Risk of bias assessment
The quality of the studies (good, fair, and poor) was rated by awarding stars in each domain following the guidelines of the Newcastle-Ottawa Scale (NOS).20 NOS comprises 8 items with 3 subscales (selection, comparability, and outcomes), and the total maximum score of these 3 subsets is 9. A “good” quality score required 3 or 4 stars in “selection,” 1 or 2 stars in “comparability,” and 2 or 3 stars in “outcomes.” A “fair” quality score required 2 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcomes. A “poor” quality score reflected 0 or 1 star in selection, or 0 stars in comparability, or 0 or 1 star in outcomes.
We interpreted the results of the meta-analysis taking into due account the risk of bias of the studies included.
Statistical analysis
Differences in the outcomes among groups were expressed as pooled risk ratios (RRs) with corresponding 95% confidence intervals (CIs), which were calculated by using a random effects model.21 This approach takes into account any difference between studies even if there is no statistically significant heterogeneity.
Statistical heterogeneity was evaluated by using the I2 statistic, which assesses the appropriateness of pooling the individual study results. The I2 value provides an estimate of the amount of variance across the studies as a result of heterogeneity rather than chance alone: I2 <30% indicates mild heterogeneity, 30% to 50% moderate heterogeneity, and >50% severe heterogeneity. A random effects model was used in cases of moderate or severe heterogeneity. A meta-regression model was fitted to examine the impact of moderator variables on effect size using regression-based techniques.
Publication bias was assessed by visual inspection of the funnel plots of the effect size versus its standard error, coupled with the Egger test.22 The Duval and Tweedie nonparametric “trim and fill” method23 to adjust for publication bias in meta-analysis was used in case of evidence of publication bias. The method, a rank-based data augmentation technique, formalizes the use of funnel plots, estimates the number and outcomes of missing studies, and adjusts the meta-analysis to incorporate the theoretical missing studies.
All data were analyzed by using STATA software release 13.
Subgroup and sensitivity analyses
The following preplanned analyses were performed on subgroups of interest: diagnosis (ET or PV), type of thrombosis (arterial or venous), time of thrombosis (first event or recurrent event), time of WBC measurement (at diagnosis or time-dependent), and type of estimates (adjusted or unadjusted). We interpreted subgroup analyses with caution because performing multiple analyses can lead to inflation of type I error.24
Results
Study selection
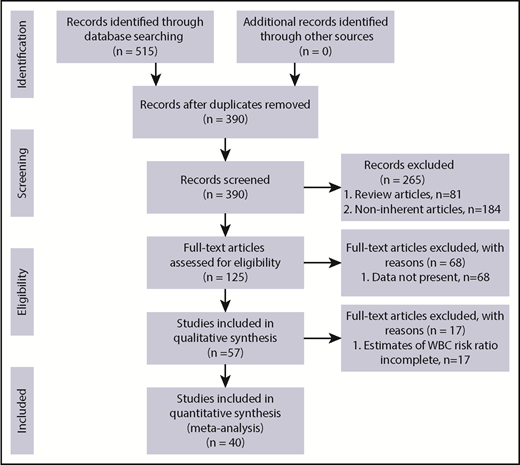
The process of study selection is outlined according to the PRISMA flowchart (Figure 1). We identified 262 potentially relevant articles with the first electronic search strategy and 263 ones with the second search, for a total of 515 articles after removal of duplicates. A total of 265 articles were excluded, based on title and abstract, as they were review or noninherent articles (n = 81 and n = 184, respectively), leaving 125 full-text articles for more detailed assessment.
Eighty articles were further excluded for the following reasons: WBC estimates were not reported (n = 68) or WBC estimates were not complete (n = 17). Forty articles were finally included in this systematic review, 32 of which were selected for the primary outcome (major thrombotic event).
Characteristics of studies included
Characteristics of the studies included are reported in supplemental Table 1. The publications refer mainly to the last 10 years. All the studies included were observational, except 1 randomized clinical trial,25 and were conducted in ET (n = 20),10,26-44 PV (n = 11),8,12,25,45-52 or ET + PV (n = 9).31,53-60 Of note, in 1 case, a small cohort of patients with myelofibrosis was also included.58
The association of leukocyte count with risk of thrombosis was tested by using a cutoff value (expressed as the increase of risk above the cutoff) in the majority of cases. In a few cases, it was tested as a continuous variable (expressed as the increase of risk in the increase of 1 unit of value) or by using a delta value (expressed as the difference in thrombosis risk following an increase or a decrease in WBC count).
Risk of bias assessment
Quality of the studies included was rated applying the NOS for case-control and cohort studies (supplemental Table 2). Thirty-seven of 41 studies obtained a total NOS score between 6 and 9, scoring as good; the remaining 4 studies were ranked with fair quality, with a total score of 4.
Primary analysis
Fifty WBC RR estimates for thrombosis were extracted from 32 studies. In the majority of cases, WBC count was measured at diagnosis or enrollment (n = 44 [88%]), the first event was considered as opposed to recurrences (n = 42 [84%]), and overall major thrombosis was evaluated without differentiating between arterial and venous events (n = 38 [76%]). Considering this set of conditions, the forest plot (Figure 2) of the random effects model included 25 studies in which leukocytosis was defined as WBC count above a cutoff. The estimated pooled RR of overall thrombosis by leukocytosis was 1.59 (95% CI, 1.40-1.80). The I2 statistic was 11.5% (P = .300). Cutoffs used for the definition of leukocytosis ranged from 8.4 × 109/L to 15.0 × 109/L in ET, from 9.5 × 109/L to 25.0 × 109/L in PV, and from 11.0 × 109/L to 25.0 × 109/L when the 2 diagnoses were considered together.
Forest plot of the random effects meta-analysis assessing the pooled RR of leukocytosis on the primary outcome (thrombosis). Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamond represents the pooled RR, with the center representing the point estimate and the width representing the associated 95% CIs.
Forest plot of the random effects meta-analysis assessing the pooled RR of leukocytosis on the primary outcome (thrombosis). Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamond represents the pooled RR, with the center representing the point estimate and the width representing the associated 95% CIs.
Sensitivity and subgroup analyses
MPN diagnosis.
The significant association of leukocyte count with thrombosis shown in the primary analysis was confirmed stratifying according to disease, even though a moderate (I2 = 14.4% in ET and 51.1% in PV) heterogeneity was highlighted: the pooled RRs were 1.65 (95% CI, 1.43-1.91) and 1.34 (95% CI, 1.08-1.66) in ET and PV patients, respectively (Figure 3).
Forest plot of the subgroup analysis on the primary outcome according to MPN diagnosis. Results are presented in the subgroups of patients with ET or PV. Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamonds represent the pooled RRs, with the center representing the point estimate and the width representing the associated 95% CIs.
Forest plot of the subgroup analysis on the primary outcome according to MPN diagnosis. Results are presented in the subgroups of patients with ET or PV. Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamonds represent the pooled RRs, with the center representing the point estimate and the width representing the associated 95% CIs.
Type of thrombosis.
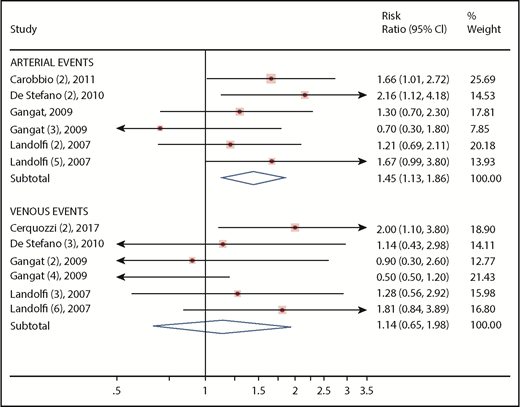
By limiting the model to the occurrence of arterial or venous thrombosis, different results were obtained. Venous events were not predicted according to leukocytosis (RR, 1.14; 95% CI, 0.65-1.98), whereas it was confirmed as a risk factor for arterial events (RR, 1.45; 95% CI, 1.13-1.86) (Figure 4).
Forest plot of the subgroup analysis on primary outcome according to type of thrombosis. Results are presented in the subgroups of patients with arterial or venous events. Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamonds represent the pooled RRs, with the center representing the point estimate and the width representing the associated 95% CIs.
Forest plot of the subgroup analysis on primary outcome according to type of thrombosis. Results are presented in the subgroups of patients with arterial or venous events. Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamonds represent the pooled RRs, with the center representing the point estimate and the width representing the associated 95% CIs.
Other sensitivity analyses.
The association of leukocyte count with thrombosis was confirmed in the following subgroups: recurrent events (RR, 1.92; 95% CI, 1.42-2.59), studies using time-dependent WBC measurements (RR, 1.81; 95% CI, 1.42-2.31), and estimates adjusted or unadjusted for confounding factors (RR, 1.51 [95% CI, 1.34-1.69]; RR, 1.59 [95% CI, 1.40-1.80], respectively).
Publication bias
The funnel plot suggests the occurrence of publication bias (Egger test: slope = .98; P = .041) (supplemental Figure 1). After applying the Duval and Tweedie trim and fill approach, we found that the adjusted analysis differed slightly from the original one toward a slightly lower effect size (25 observed and 4 trimmed studies; RR, 1.51; 95% CI, 1.31-1.75). Supplemental Figures 2 and 3 show the point estimates and CIs of observed and trimmed studies and the related funnel plot.
Meta-regression analysis
A meta-regression model was fitted to examine the impact of moderator variables on effect size.
Confounding effects that were controlled for in meta-regression were MPN phenotype (ET or PV) and type of event (arterial or venous) because they turned out to be the major source of heterogeneity in subgroup analyses. The results are detailed in supplemental Table 3. The model confirmed that the leukocytosis-associated thrombosis risk differed between ET and PV patients (RR, 0.71; 95% CI, 0.50-0.99; P = .043) and between arterial and venous events (RR, 0.68; 95% CI, 0.47-0.99; P = .046).
Secondary analyses
Publication details included for the analysis of secondary outcomes are listed in supplemental Table 1.
Bleeding.
By performing a random effects model with 4 studies in which leukocytosis was tested as a risk factor for major bleeding, a pooled estimate of 1.87 (95% CI, 1.26-2.77) was obtained.
Hematologic evolutions and solid tumors.
We were unable to retrieve sufficient data for an analysis of these outcomes.
Overall mortality.
A statistically significant increase in risk of death associated with leukocytosis was reported in a pooled analysis of 11 estimates (RR, 1.89; 95% CI, 1.59-2.23) (Figure 5).
Forest plot of the random effects meta-analysis assessing the pooled RRs of leukocytosis on a secondary outcome (death). Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamond represents the pooled RR, with the center representing the point estimate and the width representing the associated 95% CIs.
Forest plot of the random effects meta-analysis assessing the pooled RRs of leukocytosis on a secondary outcome (death). Squares and horizontal lines represent the point estimates and associated 95% CIs. The diamond represents the pooled RR, with the center representing the point estimate and the width representing the associated 95% CIs.
Discussion
In the last decade, several studies have investigated the association between leukocytosis and risk of thrombosis in patients with MPN, but the conclusions were not univocal.6-9 Furthermore, even in studies concluding that leukocytosis was associated with thrombosis, no consensus was found on the numerical cutoff that should be used to define leukocytosis.
Twelve years after the first studies reporting on the role of leukocytosis in risk of thrombosis in MPNs were published,12,29 we offer the first complete assessment of evidence about this association by means of a systematic literature review and meta-analysis of >30 000 patients with ET or PV. The overall quality of the studies was good as assessed according to the NOS, although it must be considered that very few studies included evaluation of leukocytosis as a risk factor for thrombosis or other outcomes as their primary objective.
A remarkable effort was made in extracting data about study-specific characteristics to address the issue of study heterogeneity. Indeed, the final selection of studies included in the primary analysis regarding thrombosis outcome was homogeneous, with no evidence of significant heterogeneity (I2 = 11.5%; P = .300). This finding strengthens the conclusions from the pooled analysis by showing the significant association between leukocytosis and thrombosis with an RR of 1.60, concurrently providing a reasonably precise relative risk estimate with narrow CIs (95% CI, 1.40-1.80).
We were unable, however, to identify the best WBC cutoff useful to predict vascular complications with a formal prognostic accuracy meta-analysis because no data about true- and false-positive outcomes and negative rates were retrievable. This was because the design was never specifically aimed at assessing the role of leukocytosis, and the WBC cutoffs used had hardly ever been selected by using appropriate techniques for evaluation of diagnostic/prognostic accuracy (receiver-operating characteristic curve or other).
Subgroup analyses revealed that the effect of leukocytosis was stronger in ET (RR, 1.65; 95% CI, 1.43-1.91) compared with PV (RR, 1.34; 95% CI, 1.08-1.66), and it seems exclusively related to arterial events (RR, 1.45; 95% CI, 1.13-1.86) because no effect was found in venous events (RR, 1.14; 95% CI, 0.65-1.98). This outcome offers a plausible explanation for the exclusion of leukocytosis from the IPSET–thrombosis score,3 which considered overall thrombosis without differentiating between arterial and venous events.
Our sensitivity analyses, considering recurrent events as well as WBC estimates adjusted or unadjusted for confounding factors, confirmed the role of leukocytosis as an established, and probably independent, risk factor for thrombosis. Only 5 studies12,25,28,29 tested WBC in time-dependent models, with a pooled RR of 1.81 (95% CI, 1.42-2.31), suggesting also a causative effect of these cells in the mechanism that triggers thrombosis.
The main limitation of the current study, as highlighted by the funnel plot and by the Egger test, is the presence of remarkable publication bias. Many of the studies we excluded did not report any or incomplete estimates of RR, and this issue was more frequent in studies in which leukocytosis was not found to be a risk factor for thrombosis. Conversely, we applied a statistical method to adjust for publication bias that ultimately confirmed the results from the original analysis.
An analysis on secondary outcomes was also performed. We confirmed an association between leukocytosis and bleeding both in PV and ET, but the low number of studies reporting on this factor did not allow for an estimate (RR, 1.87; 95% CI, 1.26-2.77) as precise as the one for thrombosis. We stress that the most frequent type of bleeding was gastrointestinal, followed by skin or muscle hematoma and hemarthrosis.
Concerning survival, this meta-analysis validated a survival disadvantage of patients with leukocytosis. Of note, leukocytosis, in addition to advanced age and history of thrombosis, has already been incorporated into an international prognostic score for shortened survival in ET (IPSET-survival).61
In conclusion, a 60% expected increase of thrombosis risk in the presence of leukocytosis is a consistent estimate with non-negligible clinical relevance that should be taken into account in classifying the thrombotic risk of these patients for arterial events and in ET cases. However, a consensus definition of leukocytosis for the best prediction of vascular events requires further research. To determine the WBC cutoff that provides the best prognostic accuracy, analysis on individual patient data merged in a large collective archive of patients with predefined inclusion criteria is warranted.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Veronika Buxhofer-Ausch (Ordensklinikum Linz, Linz, Austria), and Steffen Koschmieder and Judith Gecht (RWTH Aachen University, Aachen, Germany) for providing details on the estimates required by the meta-analysis and not present in their original articles.
Authorship
Contribution: A.C. examined all titles and abstracts, obtained full texts and determined their eligibility, extracted data and performed the analysis, and wrote the paper; A.F. examined titles and abstracts and wrote the paper; A.M., A.G., and G.B. reviewed and approved the paper; and T.B. conceived the research, examined titles and abstracts, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tiziano Barbui, FROM Research Foundation, Papa Giovanni XXIII Hospital, Piazza O.M.S., 1, 24127 Bergamo (BG), Italy; e-mail: tbarbui@fondazionefrom.it.