Key Points
ATLL presents more aggressively in US Afro-Caribbean than Japanese patients and continues to have a poor outcome despite modern therapies.
AZT-IFN is a reasonable up-front option for aggressive, nonbulky, leukemic ATLL subtypes, resulting in long PFS after a complete response.
Abstract
Adult T-cell leukemia/lymphoma (ATLL) is a fatal disease caused by human T-cell leukemia virus type 1 (HTLV-1). We retrospectively analyzed 195 patients with ATLL (lymphomatous n = 96, acute n = 80, unfavorable chronic n = 7, chronic n = 5, smoldering n = 3, and unclassified n = 4) diagnosed between 1987 and 2016 (median age 52 years, 77% Afro-Caribbean). Hypercalcemia was associated with acute ATLL (65%, vs 23% lymphomatous) (P = .012). The median survival for patients treated with modern therapies between 2000 and 2016 was 4.1 months for acute, 10.2 months for lymphomatous, 72 months for chronic/smoldering, and not reached for unfavorable chronic type, with 4-year survival rates of 10%, 4%, 60%, and 83%, respectively. The overall response rate (ORR) after first-line multiagent chemotherapy was 78% (complete response [CR] 39%) for acute vs 67% (CR 33%) for lymphomatous ATLL. First-line zidovudine interferon-α (AZT-IFN) resulted in ORR of 56% (CR 23%) for acute (n = 43), 33% (CR 16.5%) for lymphomatous (n = 6), and 86% (CR 29%) for unfavorable chronic ATLL. The median progression-free survival (PFS) in patients with aggressive ATLL who achieved CR after AZT-IFN was 48 months vs 11 months after chemotherapy (P = .003). Allogeneic hematopoietic stem cell transplant (allo-HSCT) resulted in a PFS of 24 and 28 months in 2 patients with lymphomatous ATLL. Our results suggest high-dose AZT-IFN is a reasonable up-front option for patients with aggressive leukemic ATLL followed by chemotherapy switch in nonresponders, whereas chemotherapy should be used in lymphomatous type followed by allo-HSCT when feasible.
Introduction
Adult T-cell leukemia/lymphoma (ATLL) is a mature, peripheral T-cell neoplasm caused by human T-cell leukemia virus type 1 (HTLV-1).1,2 The virus is primarily transmitted via breastfeeding, blood transfusion, sharing of needles, and sexual intercourse. HTLV-1 infects up to 10 million people worldwide and is most endemic in southwestern Japan, the Caribbean, South America, and west Africa.3 In America, the highest prevalence of HTLV-1 is found in Haiti, Jamaica, Dominican Republic, northeastern Brazil, and Peru.3 South Florida is the continental US region most proximal to the Caribbean; therefore, HTLV-1–associated diseases, including ATLL and HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP), are commonly encountered in Miami, FL.4,5 HTLV-1–related diseases may also affect US-born African Americans.5
HTLV-1 establishes lifelong latency in human T cells. Malignant transformation leading to ATLL occurs in HTLV-1–infected individuals with a cumulative lifetime risk of 4% to 7%.6 ATLL occurs predominantly in adults between the sixth and seventh decades.6,7 ATLL is classified into 4 clinical subtypes, namely acute, lymphomatous, chronic, and smoldering, as defined by Shimoyama criteria.8 The most aggressive acute and lymphomatous forms are by far the most common, and patients frequently present with lymphadenopathy, hepatomegaly, splenomegaly, hypercalcemia, and involvement of the skin, lung, bones, and other organs. The lymphomatous type often presents with extensive lymphadenopathy and a relative absence of ATLL cells in the peripheral blood (<1%). The acute type usually presents with leukemia and high levels of serum lactose dehydrogenase (LDH). The smoldering and chronic forms present with <4 × 109 or ≥ 4 × 109 lymphocytes/L in the peripheral blood, respectively; normal or elevated LDH (<1.5 or 1.5-2 times the upper normal value, respectively); involvement of lung, skin, or liver (in chronic only), but no other extranodal sites; and no hypercalcemia. Comorbid opportunistic infections are often seen in ATLL patients as a result of immunosuppression caused by dysfunctional HTLV-1–infected T cells. Parasitic infections, especially strongyloidiasis, and fungal infections are frequently associated with all forms of ATLL.9-11
ATLL carries a dismal prognosis and is incurable by conventional drugs. Patients with acute and lymphomatous types had median survival (MS) times of just 6.2 and 10.2 months, respectively, in Japan between 1984 and 1987.8 The largest updated retrospective Japanese study that included 1594 patients treated with modern aggressive therapies between 2000 and 2009 reported MS times of 8.3 for acute, 10.6 months for lymphomatous, 31.5 months for chronic, and 55 months for smoldering ATLL, with 4-year overall survival (OS) rates of 11%, 16%, 36%, and 52%, respectively.12 Only allogeneic hematopoietic stem cell transplantation (allo-HSCT) appeared to be curative, with a 4-year OS of 26% in 227 patients and an MS of 5.9 months, in part due to disease relapse or transplant-related mortality.12
Phase 2 studies performed outside of Japan, including at our center, have demonstrated the efficacy of zidovudine and interferon-α (AZT-IFN) in patients with chronic and aggressive leukemic ATLL types.13-15 A recent retrospective multicenter analysis suggested that AZT-IFN may be more effective in leukemic ATLL forms in terms of long-term outcome, as several patients with acute type remained progression-free under maintenance therapy for several years.16 AZT-IFN is now considered a first-line treatment option for nonlymphomatous ATLL17 and is recommended under National Comprehensive Cancer Network treatment guidelines. Despite this, the long-term prognosis of ATLL remains poor.
This study describes the epidemiology, clinical features, treatment, and outcome of patients with ATLL encountered over the past 3 decades at the University of Miami and affiliated hospitals in a region that has a relatively high prevalence of HTLV-1–related diseases.5 The clinical information gathered from this retrospective study should provide invaluable insight and guidance into the diagnosis and treatment of this relatively rare and challenging disease in the United States.
Methods
Patients
We conducted a retrospective analysis of patients diagnosed with ATLL at the University of Miami–affiliated hospitals and clinics in Miami, FL, between January 1987 and December 2016. Patient demographics and clinical data were obtained from available medical records. This study was approved by the joint University of Miami and Jackson Memorial Hospital Institutional Review Board.
ATLL diagnosis and classification
The diagnosis of ATLL was based on serologic evidence of HTLV-1 by enzyme-linked immunosorbent assay, confirmed by reflex western blot, and identification of clonal CD4+CD7−CD25+/− T cells in peripheral blood or tissues as determined by histology, immunophenotyping, and gene rearrangement studies; 1 case with bone lymphoma was CD4−CD8+. Patients with aggressive ATLL had at least 1 of the typical disease features, including blood-circulating pathognomonic “flower-like” cells with convoluted nuclei, elevated LDH, generalized lymphadenopathy, hepatosplenomegaly, multivisceral involvement, cutaneous lesions, and hypercalcemia. Patients were classified according to the Shimoyama criteria into acute, lymphomatous, chronic, and smoldering ATLL.8 Chronic ATLL with LDH elevation < 2 times (2N) the upper normal limit value was classified as unfavorable chronic. Patients with lymphoma features and absolute lymphocyte count <4 × 109/L were classified as lymphomatous type.
Treatment regimens
The following treatment approaches were used as first-line therapy: (1) chemotherapy alone, (2) combined chemotherapy with AZT-IFN (concurrently or sequentially), and (3) AZT-IFN alone. As a local institutional practice, high-dose AZT (1.5 g or, more recently, 750 mg/m2, twice daily) and interferon-alfa-2b (IFN-α; 5-10 million units twice daily) were initiated and administered IV on an inpatient basis as previously reported.18 In general, patients who responded continued to receive oral AZT 600 mg twice daily and subcutaneous IFN-α 5 million units once or twice daily as outpatients. For patients with prolonged clinical responses, these drugs were eventually tapered down to as low as AZT 300 mg twice daily and subcutaneous IFN-α 3 million units three times weekly as maintenance therapy. The chemotherapy regimens used at any time included cyclophosphamide, vincristine, doxorubicin and prednisone (CHOP)–like regimens, etoposide, cyclophosphamide, vincristine, doxorubicin and prednisone (EPOCH) and other etoposide-based regimens, platinum-based regimens, including vincristine, cyclophosphamide, doxorubicin and prednisone (VCAP-AP-VECP; modified version of LSG-15 Japanese regimen using vincristine in place of vindesine and no ranimustine, which is not available in the United States), other non–platinum-based regimens, single-agent chemotherapy, single investigational or biological agents, and allo-HSCT. Regimens were selected by the treating physician based on ATLL subtype and according to institutional practices (Table 1).
Response criteria
Treatment responses were assessed according to the 2009 consensus report for ATLL by Tsukasaki et al.17 For computed tomography imaging, Cheson criteria19 was used to assess response. In patients with leukemic phase, complete response (CR) required a decrease in the abnormal peripheral blood absolute lymphocyte count to <4 × 109/L; partial response (PR) was defined as a ≥50% reduction and progressive disease (PD) as a ≥50% increase in abnormal absolute lymphocyte count at any time. Stable disease (SD) was defined when PR was not attained in the absence of PD. To be classified as such, CR, PR, and SD had to persist for a period of at least 4 weeks.
Statistical analysis
Demographics, clinical features, and treatment received were summarized using descriptive statistics. The main study patient outcomes were treatment response, progression-free survival (PFS) and OS. PFS was defined as the time from treatment initiation until PD, relapse, or death from any cause. Event-free patients were censored at the date of last clinical follow-up. OS was defined as the elapsed time from diagnosis until death from any cause. Alive patients were censored at last follow-up in clinic or by telephone. Survival estimates were calculated by Kaplan-Meier method and compared using the log-rank test. Statistical analysis was performed using IBM SPSS Statistics version 23.
Results
Demographics and clinical features
A total of 195 patients identified between 1987 and December 2016 had sufficient data for partial analysis from existing medical records. There were sizeable time gaps in cases due to missing records between 1987 and 1998, while most patients included (n = 147) were diagnosed between 1999 and 2016. Demographic characteristics of ATLL patients are shown in Figure 1 and Table 2.
Geographical distribution and originating countries of ATLL patients encountered in Miami, FL (N = 195).
Geographical distribution and originating countries of ATLL patients encountered in Miami, FL (N = 195).
Of 195 patients, the vast majority (n = 176, 90.3%) had aggressive ATLL (lymphomatous n = 96, 49%; acute n = 80, 41%). Four cases (2%) were unclassifiable due to lack of sufficient laboratory or imaging information. There were 7 (4%) unfavorable chronic, 3 (1.7%) smoldering, and 5 (2%) chronic cases. Six previously treated patients (3 lymphomatous, 1 unfavorable chronic, 1 smoldering, and 1 chronic) transformed and relapsed as acute, while 4 cases (3 acute and 1 unfavorable chronic) relapsed as lymphomatous. The median age at diagnosis for all patients was 52 years, and a female predominance was observed in aggressive ATLL cases (61% in acute vs 55% in lymphomatous, P = .38).
The great majority of patients were Afro-Caribbean (77%) originating from Haiti (39%) and Jamaica (24%), and the rest from the smaller Caribbean islands (Figure 1). Other patients were US-born African American (12%) or Latino (Hispanic, Amerindian, or African mixed [11%]) originating from the Dominican Republic and South America. The country of origin was unknown for 1 patient. Six patients (3.1%) had first-degree relatives (offspring and/or siblings) with hematologic malignancy diagnosed or suspected to be ATLL.
The clinical characteristics of ATLL patients are summarized in Table 2. Ann Arbor staging was only applicable to lymphomatous cases, with 74% stage III to IV and 14% stage I to II; sufficient staging information was not available in 13 unclassified patients. Acute, chronic, and smoldering cases were stage IV by definition due to peripheral blood involvement. Hypercalcemia was highly associated with acute ATLL as compared with lymphomatous ATLL (65% vs 23%, respectively) (P = .022). Within the acute group, 73% had serum LDH ≥2N and 10% had normal LDH vs 35% and 17%, respectively, in lymphomatous ATLL.
Among active infectious comorbidities, HIV was observed in 9% (n = 17), parasitic infections (strongyloidiasis, neurocysticercosis, scabies, toxoplasmosis) in 4.6% (n = 9), viral hepatitis (hepatitis B and C) in 2.6% (n = 5), and opportunistic fungal infections (Pneumocystis or Cryptococcus) in 2.1% (n = 4). HAM/TSP was observed in 3% (n = 6). The most commonly affected extranodal sites across all subtypes, excluding peripheral blood and bone marrow, were skin (28%), bone with lytic lesions (14%), lung (13%), and liver (12%). Central nervous system (CNS) involvement was present at diagnosis in 7% (n = 12). Patients who presented or relapsed with CNS disease are summarized in supplemental Table 1.
Disease outcome
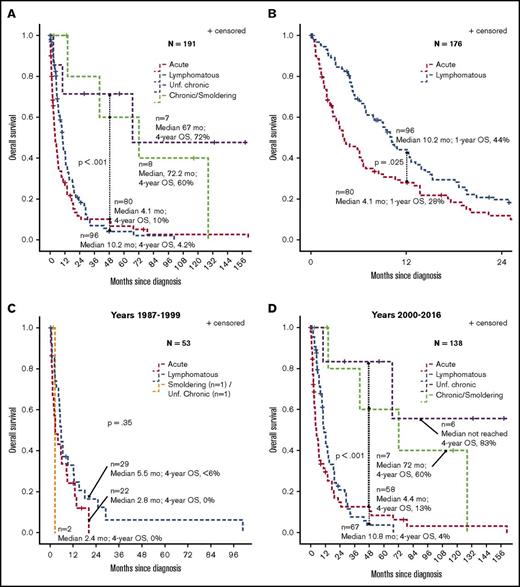
The median and 4-year OS rates from time of diagnosis in 191 assessable patients were 4.1 months and 10% for acute (n = 80), 10.2 months and 4.2% for lymphomatous (n = 96), 67 months and 72% for unfavorable chronic (n = 7), and 72 months and 60% for chronic/smoldering combined ATLL (n = 8) (log-rank P < .001 for acute and lymphomatous vs chronic and smoldering forms) (Figure 2A). The 1-year OS rates in acute vs lymphomatous were 28% vs 44%, respectively (P = .025) (Figure 2B). Separate survival analyses were performed for 1987-1999 (n = 53) vs 2000-2016 (n = 138) time periods and showed median OS rates of 5.5 months vs 10.8 months for lymphomatous, 2.8 months vs 4.4 months for acute, 2.4 months for smoldering/unfavorable chronic (n = 2) vs not reached for unfavorable chronic (n = 6), and 72 months (6 years) for chronic/smoldering ATLL (n = 7), respectively (Figure 2C-D). The median OS was <6 months in all subtypes between 1987 and 1999 (P = .35; Figure 2C). Among patients diagnosed between 2000 and 2016, the 4-year OS rates were 13% for acute, 4% for lymphomatous, 83% for unfavorable chronic, and 75% for chronic/smoldering (log-rank P < .001 for aggressive types vs chronic and smoldering forms; Figure 2D).
Survival of ATLL patients according to clinical subtype. (A) OS by ATLL subtype in all patients. (B) OS in acute vs lymphomatous subtypes. (C) OS for patients diagnosed between 1987 and 1999. (D) OS for patients diagnosed between 2000 and 2016. Survival estimates were calculated by Kaplan-Meier method and compared using the log-rank test.
Survival of ATLL patients according to clinical subtype. (A) OS by ATLL subtype in all patients. (B) OS in acute vs lymphomatous subtypes. (C) OS for patients diagnosed between 1987 and 1999. (D) OS for patients diagnosed between 2000 and 2016. Survival estimates were calculated by Kaplan-Meier method and compared using the log-rank test.
Treatment administered
The median number of treatment regimens administered in all patients was 3. The regimens used at any point during the first 3 lines of therapy for all patients are summarized in Table 1. AZT-IFN alone was given in 96 patients, concurrent with chemotherapy (n = 9), or sequentially after (n = 8). A total of 175 patients received multiagent chemotherapy at any point, 23 single-drug chemotherapy, and 24 biological agents. Five patients underwent allo-HSCT.
AZT-IFN
Sixty-three evaluable patients received high-dose AZT-IFN alone as first-line treatment (Table 3). The ORR was 55% (CR 24%) for acute (n = 42), 30% (CR 10%) for lymphomatous (n = 10), and 86% (CR 29%) for unfavorable chronic ATLL (n = 7). SD was observed in 100% chronic and smoldering types (n = 4 total). The ORR in patients with aggressive ATLL receiving AZT-IFN after chemotherapy failure (n = 18) was 27% for acute (2 CRs) and 0% in lymphomatous (n = 7) (Table 3). The 2 patients who achieved CR were treated with AZT-IFN within 3 to 6 weeks after initiation of chemotherapy that resulted in kinetic failure, and they achieved prolonged PFS of 2.2 years and 5.9 years.
Chemotherapy-based regimens
A total of 111 evaluable patients received multiagent chemotherapy as first line (n = 69; 50 lymphomatous, 18 acute, and 1 chronic) or after failing AZT-IFN (n = 35; 23 acute, 7 lymphomatous, 3 unfavorable chronic, 1 chronic, and 1 smoldering), and 7 patients received first-line chemotherapy concurrent with AZT-IFN (n = 2) or followed by maintenance AZT-IFN (n = 5) (sequential treatment) (Table 3). The OR rates for lymphomatous (n = 50) and acute (n = 18) after first-line chemotherapy alone was 72% (CR 36%) vs 77% (CR 33%), respectively. The ORR after the first chemotherapy attempt in patients who had received AZT-IFN as the first line of treatment was 73% (CR 35%) for acute (n = 23) vs 43% (all PR) for lymphomatous (n = 7) vs 0% in chronic/smoldering types (n = 5). The ORR resulting from sequential chemotherapy followed by AZT-IFN was 100% (CR 40%) in patients with lymphomatous ATLL (n = 5) and 100% (all PR) in patients with aggressive ATLL treated with chemotherapy concurrent with AZT-IFN (n = 2). The OR rates after the first chemotherapy line using CHOP-like regimens (n = 32), EPOCH-based regimens (n = 29), and modified Japanese LSG-15 (VCAP-AP-VECP) (n = 17) were 75% (CR 34%) vs 79% (CR 24%) vs 65% (CR 41%), respectively. The median PFS rates for these 3 different regimens were 6.4 months vs 4.2 months vs 8.2 months, respectively (log-rank P = .623; supplemental Figure 1). The CR rates for CHOEP (n = 4) and oral etoposide (n = 5) were 50% and 60%, respectively, compared with 0% for those treated with hyper-CVAD (n = 5).
AZT-IFN vs chemotherapy
AZT-IFN and chemotherapy exhibited no statistically significant difference in PFS in patients with acute ATLL, but there was a trend toward a better PFS with the use of chemotherapy (median PFS 6.2 months [1 year 34%] vs 1.8 months [19%]; P = .115; Figure 3A); however, this analysis included early crossovers from AZT-IFN to chemotherapy (physician decision, and nonresponders with clinically stable disease) usually within a few days (<1 week) of treatment, while patients treated with chemotherapy were generally assessed for response several weeks after starting treatment (ie, after ≥2 cycles of treatment). In the longer term, the PFS was significantly longer in complete responders with aggressive ATLL types (acute, lymphomatous, and unfavorable chronic) treated with AZT-IFN than in those treated with chemotherapy alone, with median PFS of 48 months vs 11 months, respectively, and 1- and 3-year PFS of 69% vs 48% and 61% vs 0%, respectively (P = .003) (Figure 3C). Similarly, acute ATLL cases who achieved CR after AZT-IFN had a superior median PFS of 40 months (vs 8.2 months after chemotherapy alone) and 1-year and 3-year PFS of 69% and 59% (vs 31% and 0% after chemotherapy alone, respectively) (P = .035) (Figure 3D). By contrast, patients with lymphomatous ATLL who received chemotherapy as first-line treatment had better 1-year PFS (24%) than those treated with AZT-IFN (19%; P = .045), with a median PFS of 6.3 months vs 0.7 months, respectively (Figure 3B). The clinical characteristics of all patients who achieved a first CR are summarized in Table 4.
PFS in ATLL. (A-B) PFS in acute (A) and lymphomatous ATLL (B) cases according to first-line treatment (AI [AZT-IFN] vs chemotherapy alone). (C) PFS in 51 aggressive ATLL cases who achieved CR altogether (28 acute, 2 UC, and 21 lymphomatous). (D) PFS in 27 acute ATLL cases who achieved CR. Survival estimates were calculated by Kaplan-Meier method and compared using the log-rank test.
PFS in ATLL. (A-B) PFS in acute (A) and lymphomatous ATLL (B) cases according to first-line treatment (AI [AZT-IFN] vs chemotherapy alone). (C) PFS in 51 aggressive ATLL cases who achieved CR altogether (28 acute, 2 UC, and 21 lymphomatous). (D) PFS in 27 acute ATLL cases who achieved CR. Survival estimates were calculated by Kaplan-Meier method and compared using the log-rank test.
CNS involvement
CNS involvement was present at diagnosis in 7% (n = 12), while 6 additional patients relapsed with CNS disease after treatment (supplemental Table 1). In general, CNS imaging and/or cerebrospinal fluid analysis was performed only in patients who had symptoms suggestive of neurologic disease. Lumbar puncture was commonly avoided in asymptomatic patients with leukemia to prevent CNS seeding. CNS disease generally responded to intrathecal (IT) and/or high-dose MTX-based chemotherapy (supplemental Table 1).
HSCT
Five patients with aggressive ATLL (2 acute and 3 lymphomatous) underwent allo-HSCT at our institution. One 47-year-old Jamaican woman with acute-type ATLL who had progressed after AZT-IFN achieved a CR after VCAP-AP-VECP and received a conditioning regimen with fludarabine (FLU) and melphalan (MEL) followed by allo-HSCT from a matched sibling; PFS was 7 months before isolated CNS/leptomeningeal relapse, and she eventually succumbed to her disease. Two lymphomatous cases (36-year-old and 47-year-old Jamaican women) were transplanted in first remission after receiving multiagent chemotherapy (EPOCH and VCAP-AP-VECP) and conditioning regimen consisting of FLU/MEL/anti-thymocyte globulin (ATG); 1 patient received double umbilical cord blood allo-HSCT and died due to infection 2 months post-HSCT; the other patient had a PFS of 28 months prior to systemic relapse before succumbing to her disease. A 62-year-old Jamaican man with lymphomatous ATLL previously treated with EPOCH, followed by ICE as salvage with remission, underwent conditioning with FLU/MEL/ATG followed by matched unrelated donor HSCT with a PFS of 24 months post-HSCT at the time of this report. Finally, a 43-year-old African American woman with acute ATLL who failed AZT-IFN achieved remission after CHOEP and underwent conditioning with busulfan/FLU/ATG followed by matched unrelated donor HSCT resulting in graft failure with subsequent relapse and death.
Discussion
This study provides a comprehensive assessment of the diagnosis, treatment, and outcome of ATLL encountered at our center over the past 3 decades. The median age of 52 at diagnosis was similar to previous US reports describing Afro-Caribbean patients and approximately a decade younger than Japanese patients.6,7,12,20 However, in our patient population, lymphomatous ATLL was more common (49.2%) than acute ATLL (41%), which differed from patients presenting in New York City, where 68% of those encountered had acute ATLL, and Japan, where acute ATLL was also more common (56%, vs 22% lymphomatous).7,12,20 Despite of variable ethnic populations encountered in New York, NY (a larger Caribbean/Hispanic group), Miami, FL (a large number of Haitians), and an unrelated ethnic group in Japan, a plausible explanation for such discrepancy could be differences in ATLL subclassification methods across centers. Shimoyama criteria excludes patients with ≥1% circulating ATLL cells as lymphomatous, so in the absence of a carefully designed prospective immunophenotypic and clonal analysis of peripheral blood, the distinction between lymphomatous ATLL with disseminated cells and other subtypes is often difficult to make. HTLV-1 carriers without ATLL can have up to 5% of blood-circulating atypical cells, which exposes one of the limitations of Shimoyama criteria, prompting clinicians to classify lymphomatous ATLL (often with bulky disease) with circulating atypical cells as acute. Our study is limited due to its retrospective nature. Patients were classified using archived records and laboratory and pathology reports. Ultimately, there was a clear distinction between acute and lymphomatous groups, including significantly higher rates of hypercalcemia, elevated LDH levels, and poorer outcome in acute ATLL. The 1-year OS rates of 28% for acute ATLL vs 44% in lymphomatous ATLL (P = .025) are consistent with the natural history of ATLL.
In our study, female predominance and hypercalcemia rates were similar to other US reports.7,20 Hypercalcemia and elevated LDH (>2N), which were independent factors associated with poorer survival in our previous meta-analysis,16 were more frequent in acute ATLL cases (65% and 73%, respectively) than in large Japanese studies (up 50% and 60%, respectively).8,12 A relative poorer survival has been reported in Afro-Caribbean patients in other US studies as compared with Japanese patients.7,20 While limited health care access to immigrants from poor resource settings could ultimately influence patient outcome, the above mentioned clinical findings suggest Afro-Caribbean patients encountered in the United States present with more aggressive ATLL.
In our retrospective study, patients with acute ATLL who received chemotherapy as first line had tendency toward higher ORR (acute 71% [CR 35%]) as compared with those treated with AZT-IFN up-front (55% [CR 24%]). Acute type patients treated with AZT-IFN had lower CR and OS rates than those reported in our previous meta-analysis.16 When used as second-line treatment, response to AZT-IFN was low; however, 2 acute type patients treated within 3-6 weeks after chemotherapy initiation with kinetic failure had prolonged responses (5.9 years and 2.2 years) demonstrating some patients may benefit from AZT-IFN after failing chemotherapy. Remarkably, patients with acute ATLL who achieved CR after AZT-IFN had a significantly longer PFS as compared with those who achieved CR after chemotherapy alone (40 months, vs 8.2 months respectively) (Figure 3D). In fact, some patients treated with AZT-IFN remained progression-free and chemotherapy naive for several years (Figure 3D). Notably, 7 patients with unfavorable chronic ATLL treated with AZT-IFN had a relatively high ORR (86%) and long-term disease control with 4-year PFS of 83% and OS not reached, which compares favorably over the outcome reported in Japan (4-year OS 36% for chronic ATLL).12 This positive outcome was similar to our previous meta-analysis16 and suggests that AZT-IFN should be considered the first-line option for patients with unfavorable chronic ATLL. A common disease feature shared by all patients who achieved a CR by AZT-IFN in our study was lymph node size of ≤3 cm and lymphomatous mass lesions ≤3.6 cm, suggesting that this therapy may only be effective in acute ATLL presenting mainly with leukemia in the setting of nonbulky adenopathy or tumors. The absence of a plateau phase in long-term PFS after CR with AZT-IFN confirms this therapy is suppressive, but not curative, which is evidenced by the persistence of minimal residual blood circulating malignant clones in serially analyzed long-term responders during progression-free periods.18 Overall, these data support AZT-IFN as up-front therapy whenever feasible in patients with aggressive nonbulky nonlymphomatous ATLL; this approach would be particularly beneficial in patients who are not suitable for intensive chemotherapy or allo-HSCT.
In this study, a superior outcome was observed in patients with lymphomatous-type ATLL treated with chemotherapy, providing further evidence that combination chemotherapy should remain the standard first-line treatment of this subtype, as was observed in our prior meta-analysis.16,17 A randomized study from Japan demonstrated a superior outcome using the LSG-15 regimen (VCAP-AMP-VECP) over intensive CHOP in terms of CR rate, and a trend toward better survival in patients with aggressive ATLL, at the expense of higher toxicities.21 The modified intensive regimen VCAP-AP-VECP resulted in a higher CR rate (41%) than EPOCH and CHOP-based chemotherapy (24% and 35%, respectively), but there was no statistical survival difference, which could be related to sample size (supplemental Figure 1). Therefore, it would be reasonable to use VCAP-AMP-VECP, or a similar regimen, in patients with aggressive ATLL who are good candidates for high-dose chemotherapy and allo-HSCT. In our limited experience, 2 of 5 patients with lymphomatous ATLL who underwent allo-HSCT had PFS of 24 and 28 months.
Regarding CNS involvement by ATLL, only 7% of patients had evidence of lymphomatous CNS spread at diagnosis (supplemental Table 1). Higher frequencies of CNS involvement (28% to 31%) were found in other US cohorts.7,20 At our center, CNS imaging and/or cerebrospinal fluid analysis was only performed in patients who had neurologic symptoms; lumbar puncture was commonly avoided in asymptomatic patients with leukemia to prevent seeding in the CNS, which is prudent when dealing with a highly chemotherapy resistant disease. Further, the great majority of patients did not receive IT chemotherapy in the absence of CNS symptoms. In our current practice, prophylactic IT chemotherapy prophylaxis is administered in patients with lymphomatous type, and in aggressive types treated with systemic chemotherapy after clearance of blood circulating ATLL cells.
Lastly, several patients in this study were treated with newly available targeted biological or single chemotherapy agents known to be active against other peripheral T-cell lymphomas (Table 2). In general, these agents only had a temporary stabilizing effect in ATLL, except in 2 patients who remained progression-free for >2 years, including heavily pretreated unfavorable chronic ATLL with prolonged PR after alemtuzumab and a CD30+ relapsed lymphomatous type who achieved a prolonged CR after brentuximab. The analysis of ATLL patients treated with newly available drugs is currently ongoing and beyond the scope of this study.
In conclusion, ATLL patients encountered in the United States continue to have a poor outcome with currently available therapies. Our study is limited because of its retrospective nature, but it revealed that AZT-IFN can be effective in the long-term in patients with aggressive leukemic ATLL and superior to chemotherapy in those who achieve CR. Altogether, our experience suggests AZT-IFN is a reasonable first-line option in patients with nonlymphomatous and nonbulky aggressive ATLL and may be the best option for patients with unfavorable chronic-type ATLL. Chemotherapy remains the preferred choice for lymphomatous or acute ATLL with bulky disease, with consideration of allo-HSCT with curative intent in suitable candidates.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the Sylvester Comprehensive Cancer Center (PO1 Program Project Developmental Grant) (J.C.R and G.N.B.) and the National Institutes of Health, National Cancer Institute (grant 1R01CA223232-01) (J.C.R.).
Authorship
Contribution: L.M., A.P., and I.M.R. made the figures and tables; J.C.R. designed the research; and L.M., A.P., I.M.R., E.G., L.L., K.K., T.H., G.N.B., and J.C.R. analyzed results and/or wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan C. Ramos, University of Miami, Sylvester Comprehensive Cancer Center, Division of Hematology and Oncology, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: jramos2@med.miami.edu.
References
Author notes
L.M. and A.P. are joint first authors.


![Figure 3. PFS in ATLL. (A-B) PFS in acute (A) and lymphomatous ATLL (B) cases according to first-line treatment (AI [AZT-IFN] vs chemotherapy alone). (C) PFS in 51 aggressive ATLL cases who achieved CR altogether (28 acute, 2 UC, and 21 lymphomatous). (D) PFS in 27 acute ATLL cases who achieved CR. Survival estimates were calculated by Kaplan-Meier method and compared using the log-rank test.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/6/10.1182_bloodadvances.2017011106/4/m_advances011106f3.jpeg?Expires=1767964431&Signature=Dxzhv14fq1~6ZTlxzTi1Pu~b~qsZOkCFxehhLXh2c97WkxJRBPjYTH4zutL1yfhOou~l7QtKZelx3n8KMg0cr3jNdg1dwMPp-hqG3~TiY4rVrwYaOgRrGllqq6XrtXg3zx3Cm3KmGdRd5-2sDDWbV7mV6wkrQX3JSJz3nCsU9~Jop0K6fHxAt7scnNArtc15OJmKIlo8VV44cNfOmh2rKMdOpQRMVUjjp8D10k-KS3oTr8SLpPX5EalLMIFu4JykLk--lvTQRTOmSYiNOvM1kaK3JmCAGg8L40uKjsJ3Af8NQ0fhpRrXQQltSgICE-QGkJKn5xCaJWg8iAL0D~ackg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
