Key Points
Lenalidomide (vs no) maintenance therapy post-ASCT improved survival outcomes in patients with NDMM.
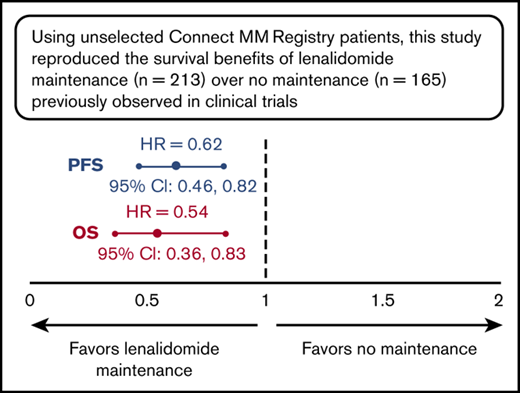
Using unselected registry patients, this study reproduced the survival benefits of lenalidomide maintenance observed in clinical trials.
Abstract
Autologous stem cell transplantation (ASCT) followed by lenalidomide maintenance therapy is the standard of care for transplant-eligible patients with newly diagnosed multiple myeloma (NDMM). Clinical trials show progression-free survival (PFS) benefits, with some studies (Cancer and Leukemia Group [CALGB] trial and meta-analysis) also showing overall survival (OS) benefits, but applicability to real-world clinical settings is unclear. Using data from Connect MM, the largest US-based observational registry of NDMM patients, we analyzed effects of maintenance therapy on long-term outcomes in 1450 treated patients enrolled from 2009 to 2011. Patients who received induction therapy and ASCT (n = 432) were analyzed from 100 days post-ASCT (data cut 7 January 2016): 267 received maintenance (80% lenalidomide-based [of whom 88% received lenalidomide monotherapy]); 165 did not. Lenalidomide maintenance improved median PFS and 3-year PFS rate vs no maintenance (50.3 vs 30.8 months [hazard ratio (HR), 0.62; 95% confidence interval (CI), 0.46-0.82; P < .001] and 56% vs 42%, respectively). Improvements in median OS and 3-year OS rate were associated with lenalidomide maintenance vs no maintenance (not reached in either group [HR, 0.54; 95% CI, 0.36-0.83; P = .005] and 85% vs 70%, respectively). Five hematologic serious adverse events were reported with lenalidomide maintenance (pancytopenia [n = 2], febrile neutropenia, anemia, and thrombocytopenia [n = 1 each]) and 1 with no maintenance (thrombocytopenia). Second primary malignancies occurred at rates of 1.38 and 2.19 events per patient-year in lenalidomide maintenance and no maintenance groups, respectively. Survival benefits associated with lenalidomide maintenance previously demonstrated in clinical trials were observed in this community-based Connect MM Registry.
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by the accumulation of mature, clonal plasma cells in the bone marrow, leading to osteolytic bone lesions, impaired hematopoiesis, presence of serum/urine monoclonal immunoglobulin, renal disease, and immunodeficiency.1,2 The standard of care for eligible patients with newly diagnosed MM (NDMM) is autologous stem cell transplantation (ASCT) followed by lenalidomide maintenance.3-5
Multiple phase 3 clinical trials have demonstrated the benefits of lenalidomide maintenance therapy in patients with NDMM for outcomes including progression-free survival (PFS) and overall survival (OS). In 1 of these studies, lenalidomide maintenance therapy significantly extended median PFS compared with no maintenance therapy (41.9 vs 21.6 months; hazard ratio [HR], 0.47; P < .001).3 In another, patients who received lenalidomide vs placebo maintenance therapy had improved median PFS (41 vs 23 months; HR, 0.50; P < .001) and a higher 3-year PFS rate (59% vs 35%).4 An interim analysis of a third study in patients who had undergone induction therapy and ASCT showed longer median time to progression for lenalidomide maintenance therapy vs placebo (46 vs 27 months; P < .001), a higher 3-year PFS rate (66% vs 39%), and significantly improved OS (88% vs 80%; HR, 0.62 [95% confidence interval (CI), 0.40-0.95]).6 In the Myeloma XI study, lenalidomide maintenance therapy 100 days post-ASCT improved median PFS compared with no maintenance (60 vs 28 months; HR, 0.46 [95% CI, 0.36-0.58]; P < .001) in patients with NDMM.7 A recent meta-analysis in patients with NDMM confirmed the PFS benefit and the significant OS benefit of post-ASCT lenalidomide maintenance therapy when compared with placebo or observation (not reached [NR] vs 86.0 months, respectively; HR, 0.75 [95% CI, 0.63-0.90]; P = .001).8 However, the impact of lenalidomide maintenance on survival outcomes has not been assessed thoroughly in the community setting, in which greater representation of unselected patients (typically including those who are older and sicker) would be expected compared with clinical trials.
Connect MM is the largest noninterventional, US-based prospective registry of patients with NDMM, enrolling >3000 patients at 250 academic-, government-, and community-based centers; 84% of enrolled patients were from community sites. The registry was designed to examine diagnostic patterns, treatment choices and sequencing, clinical outcomes, and quality of life in patients with NDMM.9,10 Here, data from the Connect MM Registry were used to investigate the impact of maintenance therapy on long-term outcomes in ASCT-eligible patients with NDMM who were treated from 2009 to 2011.
Methods
Study design
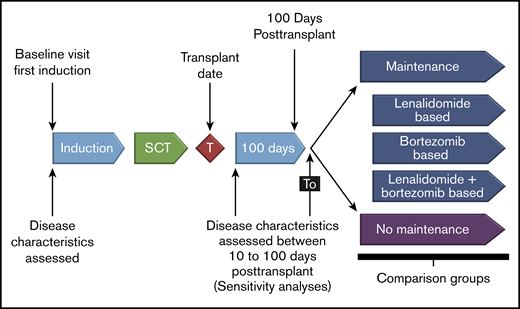
Details of the study design and patient population of Connect MM have been previously described.10 The registry comprises 2 cohorts: cohort 1 (n = 1493) includes patients enrolled from September 2009 to December 2011, and cohort 2 (n = 1518) includes patients enrolled from December 2012 to April 2016. Patients are treated with therapies (including maintenance) at physician discretion. Patients were enrolled within 60 days of diagnosis (median, 25 days), and were followed quarterly for treatment and outcomes until study end or early discontinuation (eg, due to death, withdrawing consent, or lost to follow-up). All participants gave written informed consent upon enrollment, and study sites obtained approvals from their local or central institutional review boards or ethics committees.9,11 For this analysis, patients from cohort 1 (N = 1493) who received induction therapy and ASCT were included. The date of data cutoff was 7 January 2016. Analysis was by the following first-line post-ASCT maintenance therapy groups (Figure 1): no maintenance, lenalidomide-based maintenance, bortezomib-based maintenance, and lenalidomide combined with bortezomib maintenance. The latter 3 groups were mutually exclusive.
Study design. Patients in cohort 1 who received induction therapy and ASCT were included and analyzed by first-line post-ASCT maintenance therapy. The exploratory analysis was performed on groups with adequate sample sizes (lenalidomide maintenance therapy and no maintenance therapy). SCT, stem cell transplantation.
Study design. Patients in cohort 1 who received induction therapy and ASCT were included and analyzed by first-line post-ASCT maintenance therapy. The exploratory analysis was performed on groups with adequate sample sizes (lenalidomide maintenance therapy and no maintenance therapy). SCT, stem cell transplantation.
Analysis
Patients in the maintenance and no maintenance groups were followed starting from 100 days after receiving ASCT until progressive disease, death, discontinuation, or data cutoff. The median duration of follow-up time for all patients (including those who were ongoing, had discontinued, or had died) was 39.3 months. Patients who received allogeneic, tandem, or unknown types of transplant were excluded from the analysis (intended to reduce potential bias). To exclude patients who had extreme delays in the start of maintenance therapy, the top 5% of patients with maintenance start furthest from transplant were excluded from analysis. The primary end point was PFS. Secondary end points included second PFS (time from start of second-line therapy until progression), time to next treatment,12 OS, and safety. Safety was assessed from start of maintenance or from 100 days after ASCT (for those with no maintenance). Serious adverse events (SAEs) were collected throughout the study as reported by physicians; from 2012 onward, adverse events of interest were also collected (collected by grade). Due to limitations in collection of response data (eg, no mandatory clinic visits, no formal criteria for response assessment), response rates are not presented. An exploratory analysis of the impact of baseline characteristics on survival outcomes was performed. Survival analyses were adjusted for Eastern Cooperative Oncology Group (ECOG) performance status, serum creatinine, and use of a novel agent, lenalidomide treatment, bortezomib treatment, and triplet treatment in the first-line setting; stratified survival curves adjusting for these covariates were generated using previously published methods.13
The frequencies and exposure-adjusted incidence rates of second primary malignancy (SPM) were analyzed. Exposure was calculated for each patient without SPM as the time from 100 days post-ASCT to death, discontinuation or the cutoff date, whichever occurred earliest. For patients with SPM, exposure was the total exposure prior to the invasive malignancy. Patient-years of exposure is the sum of exposure of all ASCT patients.
Results
Disposition
A total of 1450 patients were treated in cohort 1 of the Connect MM Registry, 81% (n = 1173) in a community setting. The breakdown of patient disposition is provided in the supplemental Figure. Of the 432 patients who received induction therapy and ASCT, the most common first induction regimens were lenalidomide-bortezomib-dexamethasone (RVd; n = 147), Vd (n = 78), Rd (n = 51), and Vd plus an alkylating agent (n = 49). Of those 432 patients, 267 received first-line post-ASCT maintenance therapy: 213 (80%) with lenalidomide, 30 (11%) with bortezomib, 16 (6%) with lenalidomide and bortezomib combined, and 8 (3%) with other maintenance therapies. Among the 213 patients treated with lenalidomide, 188 (88%) received lenalidomide monotherapy and 25 (12%) received lenalidomide in combination with another agent (primarily dexamethasone [22 of 25 patients (88%)]). Lenalidomide dosing was administered using a 28/28-day schedule (50%), a 21/28-day schedule (39%), or other schedules (11%). Median time from ASCT to start of maintenance therapy was 118 days (quarter 1-quarter 3, 93-159; maximum, 470). A total of 165 patients did not receive maintenance therapy. The median treatment duration for patients receiving lenalidomide maintenance therapy vs observation without maintenance was 35.2 vs 26.1 months, respectively. Following ASCT, 11 patients received consolidation treatment. Because of small sample sizes in other groups, data from only the lenalidomide maintenance and no maintenance groups are presented.
Patient characteristics
Baseline characteristics were generally similar for patients receiving lenalidomide maintenance or no maintenance therapy (Table 1). The median age in both groups at study entry was 60 years (range, 24-78 years), 60% were men, and 85% were white. Patients receiving lenalidomide maintenance were more likely to have received triplet induction therapy (64.8% vs 51.5%) compared with the group receiving no maintenance therapy. Novel agents (bortezomib or lenalidomide) were used in the first regimen in all patients who received lenalidomide maintenance and in 97% of patients who did not receive maintenance therapy.
Survival outcomes
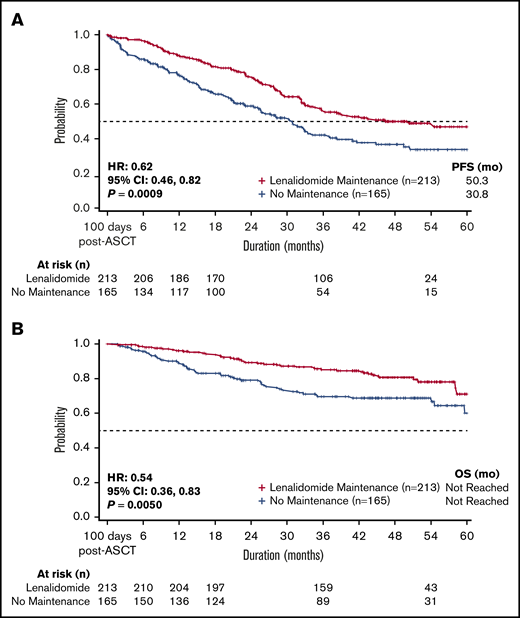
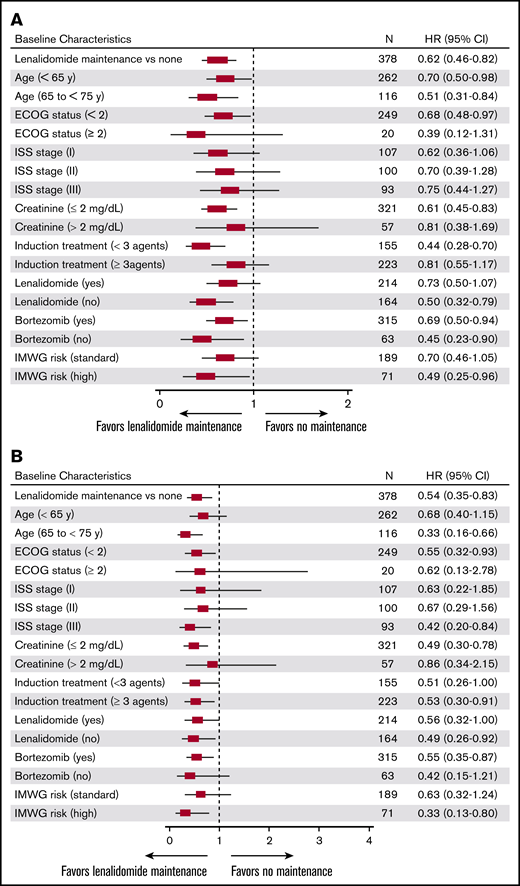
Median PFS, measured from 100 days post-ASCT, was significantly longer for patients treated with lenalidomide as first-line post-ASCT maintenance vs no maintenance therapy (50.3 vs 30.8 months; hazard ratio [HR], 0.62; 95% CI, 0.46 to 0.82; P < .001; Figure 2). The 3-year PFS rate was 56% for lenalidomide maintenance vs 42% for no maintenance therapy. Median PFS for patients receiving lenalidomide monotherapy maintenance (n = 188) was 54.5 months. Overall, PFS improvements were similar across subgroups (based on baseline characteristics) in patients treated with lenalidomide maintenance vs no maintenance therapy (Figure 3).
Survival for patients treated with lenalidomide vs no maintenance therapy. Patients receiving post-ASCT lenalidomide maintenance therapy had improved (A) PFS (P < .001) and (B) OS (P = .005) compared with those who did not receive maintenance therapy. Analysis was adjusted for covariates of ECOG performance status, serum creatinine level, treatment with a novel agent, treatment with lenalidomide, treatment with bortezomib, and triplet treatment during first-line induction therapy.
Survival for patients treated with lenalidomide vs no maintenance therapy. Patients receiving post-ASCT lenalidomide maintenance therapy had improved (A) PFS (P < .001) and (B) OS (P = .005) compared with those who did not receive maintenance therapy. Analysis was adjusted for covariates of ECOG performance status, serum creatinine level, treatment with a novel agent, treatment with lenalidomide, treatment with bortezomib, and triplet treatment during first-line induction therapy.
Exploratory survival subgroup analysis for lenalidomide vs no maintenance therapy. Improvements in (A) PFS and (B) OS observed with lenalidomide maintenance therapy were generally similar across all subgroups.
Exploratory survival subgroup analysis for lenalidomide vs no maintenance therapy. Improvements in (A) PFS and (B) OS observed with lenalidomide maintenance therapy were generally similar across all subgroups.
Significant improvement in OS was observed for patients treated with lenalidomide maintenance vs no maintenance therapy (median OS NR in either group; HR, 0.54; 95% CI, 0.36 to 0.83; P = .005; Figure 2). The 3-year OS rate was 85% for lenalidomide maintenance vs 70% for no maintenance therapy, and was 85% in patients receiving lenalidomide monotherapy maintenance (n = 188). Similar to improvements in PFS, improvements in OS with lenalidomide maintenance therapy were also similar across baseline subgroups (Figure 3).
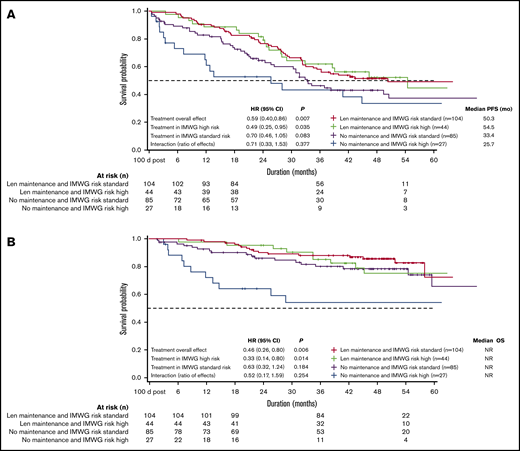
Patients with International Myeloma Working Group (IMWG) high-risk and standard-risk disease receiving lenalidomide maintenance therapy had similar median PFS (54.5 and 50.3 months) and OS (NR; Figure 4). Patients with high-risk disease who received lenalidomide maintenance had significantly longer PFS vs those who did not (median 54.5 vs 25.7 months; P=.035); this difference did not reach significance for patients with standard-risk disease (median 50.3 vs 33.4 months; P = .083). High-risk patients who did not receive maintenance therapy had poor PFS and OS (median 25.7 months and NR, respectively).
PFS (unadjusted) and OS (unadjusted) by IMWG risk criteria. (A) PFS and (B) OS were similar among IMWG high- and standard-risk patients receiving lenalidomide (Len) maintenance therapy, but poorest in high-risk patients with no maintenance therapy.
PFS (unadjusted) and OS (unadjusted) by IMWG risk criteria. (A) PFS and (B) OS were similar among IMWG high- and standard-risk patients receiving lenalidomide (Len) maintenance therapy, but poorest in high-risk patients with no maintenance therapy.
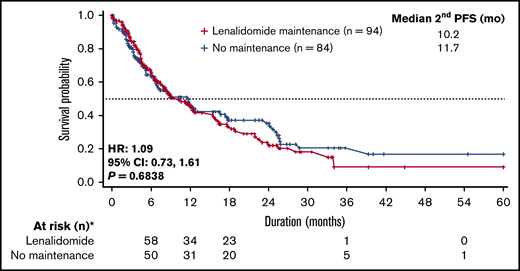
Second PFS was similar between patients in the lenalidomide and no maintenance groups (10.2 vs 11.7 months; HR, 1.09; 95% CI, 0.73-1.61; P = .6838; Figure 5). However, patients who received first-line lenalidomide maintenance had significantly longer median time from start of maintenance to start of second-line treatment than patients receiving no maintenance therapy (NR vs 33.4 months; HR, 0.58; 95% CI, 0.43-0.79; P < .001). Furthermore, a higher percentage of patients who received lenalidomide maintenance had not initiated second-line therapy at 3 years’ postmaintenance compared with patients receiving no maintenance therapy (66% vs 48%, respectively).
Second PFS for lenalidomide vs no maintenance therapy. Median second PFS was similar between the 2 groups. Only patients who had first progression were considered for this analysis.
Second PFS for lenalidomide vs no maintenance therapy. Median second PFS was similar between the 2 groups. Only patients who had first progression were considered for this analysis.
Safety
At the data cutoff, 30% of patients receiving lenalidomide-based maintenance were continuing treatment and 19% had discontinued therapy due to adverse events (Table 2). A total of 5 hematologic SAEs were reported in the lenalidomide maintenance group: pancytopenia (n = 2) and febrile neutropenia, anemia, and thrombocytopenia (n = 1 each). One patient in the no maintenance group experienced an SAE of thrombocytopenia. Hematologic SAEs led to treatment discontinuation in 2 (of 41) patients receiving lenalidomide maintenance, including pancytopenia (n = 1), anemia (n = 1), and thrombocytopenia (n = 1).
SPMs were uncommon in both groups, with low incidence in patients who received lenalidomide maintenance and patients who received no maintenance therapy (4.7% and 6.1% had invasive malignancies, respectively, including 2.8% and 0.6% with hematologic malignancies and 2.3% and 5.5% with solid tumor malignancies; 1.9% and 0.6% had nonmelanoma skin cancer [NMSC]). When adjusted for exposure, SPMs occurred at rates of 1.38 and 2.19 events per patient-year (E/PY) for invasive malignancies in lenalidomide maintenance and no maintenance therapy groups, respectively, including 0.82 and 0.21 E/PY for hematologic malignancies and 0.69 and 1.97 E/PY for solid tumor malignancies; there were 0.55 and 0.21 E/PY for NMSC.
Discussion
The survival benefits of post-ASCT lenalidomide maintenance therapy in ASCT-eligible patients observed in our analysis of data from patients with NDMM enrolled in the largely community-based Connect MM Registry are the first to confirm the benefits previously observed in phase 3 randomized clinical trials.3,4,6,7
Patients receiving lenalidomide-based maintenance therapy, 88% of whom received lenalidomide monotherapy, had significant improvements in PFS and OS (which were generally consistent across analyzed subgroups) compared with patients who received no maintenance therapy. The observed rates of PFS and OS with lenalidomide maintenance therapy were comparable with those found in clinical studies using more selected populations.3,4,6,7 Analyses of non-lenalidomide-maintenance were not included herein, as small patient numbers made drawing meaningful conclusions difficult. Similar to this observational study, the Cancer and Leukemia Group (CALGB) clinical trial (median follow-up, 34 months) demonstrated PFS and OS benefits (HR, 0.48 and 0.62, respectively) with lenalidomide maintenance vs placebo.6
Notably, lenalidomide maintenance benefited patients regardless of IMWG risk status. High-risk patients had a statistically significant increase in PFS and OS, whereas there was a similar but nonsignificant trend in standard-risk patients. Thus, lenalidomide maintenance may provide substantial clinical benefits in high-risk patients who tend to have poor prognoses.14 Our study used a physician assessment of risk, without a centralized reference laboratory.
Preliminary analysis of the effect of post-ASCT lenalidomide maintenance on second PFS suggests there is no impact on the efficacy of second-line therapy, consistent with other findings demonstrating second PFS benefits of lenalidomide maintenance vs no maintenance therapy.8 No new safety signals were observed, although collection of registry safety data is not as comprehensive as in clinical studies.
The frequencies of SPM were low among the lenalidomide maintenance and no maintenance groups, similar to observations in a previous analysis of Connect MM cohort 1 patients receiving lenalidomide maintenance, which had a shorter median follow-up (33.5 months).9 Although some clinical trials have shown increased risk of SPM with post-ASCT lenalidomide maintenance,15,16 our analysis showed that the frequency of invasive SPM was lower with lenalidomide maintenance vs no maintenance (4.7% vs 6.1%). This might suggest that the detection of SPM is enhanced in populations from clinical trials compared with those from a mostly community-based setting. Despite limitations inherent to registry studies (eg, data reported by sites), SPM data from Connect MM supports clinical data. Notably, clinical consensus assesses the risk of SPM as being outweighed by the risk of disease progression without lenalidomide maintenance treatment.3,8,15,16 Other potential limitations of registries should be acknowledged, including the nonrandomized nature of the study, the lack of mandate for specific treatments (investigator selection) or response assessments, limitations in collection of AE data (lack of information on low grade events), and variations in treatment duration and intensity. As in any observational study, there is also potential for missing and erroneous data. However, a strength of this registry is the ability to query sites for more information on questionable data. Furthermore, by applying multiple imputation methods in the analyses, the impact of missingness should be substantially mitigated.
Despite limitations, the Connect MM Registry allows examination of clinical outcomes in patients with NDMM in a mostly community-based setting, which better reflects real-world populations and clinical practice compared with clinical trials. Here, we demonstrate that the beneficial impact of lenalidomide maintenance on PFS and OS in clinical trials can be reproduced in unselected ASCT-eligible patients with NDMM, with no new safety concerns.
Acknowledgments
Ahmed YoussefAgha and Lihua Yue provided statistical support and Rick Soong provided programming support.
The Connect MM Registry is supported by Celgene Corporation. This study was supported by research funding from Celgene Corporation to all authors. Maryann Obiorah provided drafts and editorial assistance to the authors during preparation of this manuscript, supported by funding from Celgene Corporation.
Authorship
Contribution: All authors have contributed to the acquisition, analysis, or interpretation of data for this article and drafts of the article, revised the manuscript critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the article.
Conflict-of-interest disclosure: S.J. provided consulting services to Celgene, Bristol-Myers Squibb, Novartis, and Merck, and was on speakers’ bureaus for MMRF and Medicom. R.A. received research funding from Celgene, Takeda, and Prothena. B.G.M.D. provided consulting services to Takeda and Janssen. M.N. provided consultancy services to Celgene, and was on speakers’ bureaus for Celgene and Janssen. H.R.T. provided consulting services to Celgene and was on speakers’ bureaus for Janssen, Takeda, and Pharmacyclics LLC, an AbbVie Company. C.J.G. provided consulting services to Celgene, Takeda, and Janssen; received research funding from Celgene; and received reimbursement for travel expenses from Celgene and Janssen. K.T. provided consulting services to Celgene; was on a speakers’ bureau for Myriad Genetics; and received reimbursement for travel expenses from Dava Oncology. J.W.H. provided consulting services to Celgene. L.W. provided consulting services to EveryFit, Gilead, and Janssen. A.A., E.D.F., S.S., A.K., and M.S. are employed by, and have stock in, Celgene. R.M.R. provided consulting services to Amgen, Boehringer Ingelheim, Celgene, EMD Serono, Sandoz, and Takeda, and has stock in McKesson.
Correspondence: Sundar Jagannath, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Pl, New York, NY 10029; e-mail: sundar.jagannath@mountsinai.org.
References
Author notes
The full-text version of this article contains a data supplement.