Key Points
Met69Thr/Ser substitutions conferred blood group A glycosyltransferase with weak FS activity to produce FORS1 antigen of the FORS system.
Met69Thr and LeuGlyGly266-268GlyGlyAla substitutions synergistically increase the acquired FS activity of A glycosyltransferase.
Abstract
Blood group A/B glycosyltransferases (AT/BTs) and Forssman glycolipid synthase (FS) are encoded by the evolutionarily related ABO (A/B alleles) and GBGT1 genes, respectively. AT/BT and FS catalyze the biosynthesis of A/B and Forssman (FORS1) oligosaccharide antigens that are responsible for the distinct blood group systems of ABO and FORS. Using genetic engineering, DNA transfection, and immunocytochemistry and immunocytometry, we have previously shown that the eukaryotic expression construct encoding human AT, whose LeuGlyGly tripeptide at codons 266 to 268 was replaced with FS-specific GlyGlyAla tripeptide, induced weak appearance of FORS1 antigen. Recently, we have shown that the human AT complementary DNA constructs deleting exons 3 or 4, but not exons 2 or 5, induced moderate expression of FORS1 antigen. The constructs containing both the GlyGlyAla substitution and the exon 3 or 4 deletion exhibited an increased FS activity. Here, we report another molecular mechanism in which an amino acid substitution at codon 69 from methionine to threonine or serine (Met69Thr/Ser) also modified enzymatic specificity and permitted FORS1 biosynthesis. Considering that codon 69 is the first amino acid of exon 5 and that the cointroduction of Met69Thr and GlyGlyAla substitutions also enhanced FS activity, the methionine substitutions may affect enzyme structure in a mode similar to the exon 3 or 4 deletion but distinct from the GlyGlyAla substitution.
Introduction
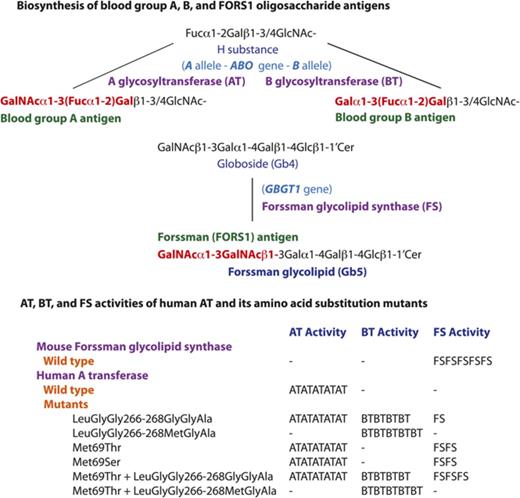
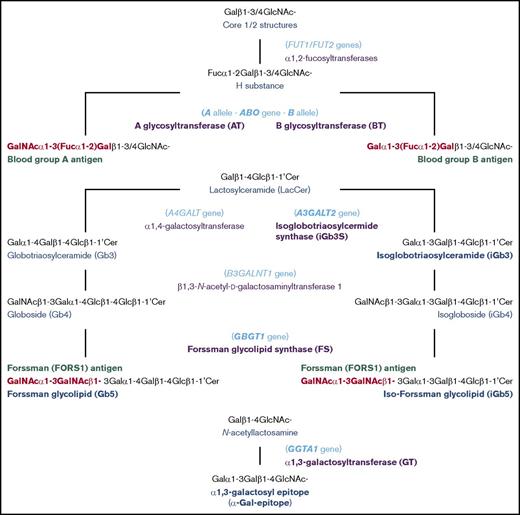
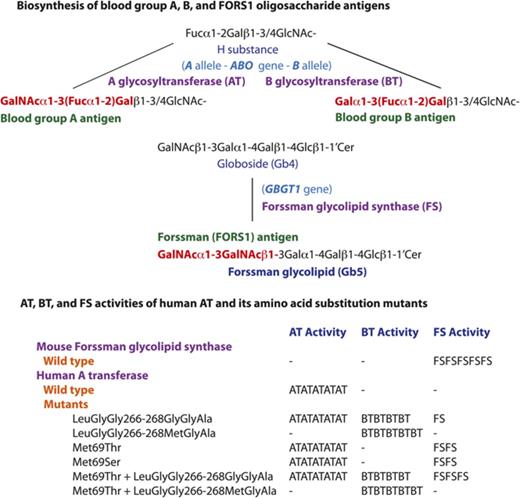
Human individuals can be classified into 4 blood groups, A, B, AB and O, depending on whether one, both, or neither of blood group A and B antigens are present on red blood cells (RBCs). The biochemistry and genetics knowledge underlying this ABO system has been well established. The A and B antigens are oligosaccharide antigens, whose chemical structures are GalNAcα1-3(Fucα1-2)Gal- and Galα1-3(Fucα1-2)Gal-, respectively. They are synthesized from the common precursor substrate H substance (Fucα1-2Gal-).1,2 Their biosynthetic pathways and the structurally related oligosaccharide antigens are represented schematically in Figure 1. The functional A and B alleles at the ABO genetic locus encode A and B glycosyltransferases (AT and BT, respectively), which catalyze the last biosynthetic step to form A and B oligosaccharides. Group A individuals have 1 or 2 A alleles encoding AT that synthesize A antigen. Group B individuals have 1 or 2 B alleles encoding BT that synthesize B antigen. Group AB individuals have A and B alleles and express both AT and BT. On the other hand, group O individuals have two non-functional O alleles but no A or B allele. Without modification to A or B antigen, type O RBCs express H substance. In addition to A and B antigens, anti-A and anti-B antibodies are also important components of the ABO system, and the presence or absence of these antibodies is reciprocally specified by the ABO alleles. For instance, group A individuals possess anti-B antibodies, whereas group B individuals possess anti-A antibodies. In other words, individuals possess antibodies against the A and/or B antigens they do not express (Landsteiner law). Consequentially, transfusions of ABO mismatched RBCs may induce potentially fatal, acute immune reactions in the recipient.
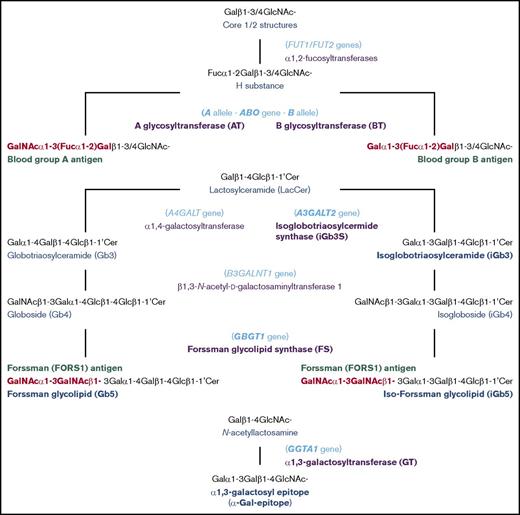
Schematic representation of biosynthesis of blood group A, B, FORS1, and related antigens. The biosynthetic pathways of blood group A, B, FORS1, and related glycan antigens are schematically shown. The gene names, glycan names, and transferase names are shown in light blue, dark blue, and purple, respectively. Important genes/transferases/glycans are shown in bold. The immunodominant epitopes of A, B, and FORS1 are shown in red and bold.
Schematic representation of biosynthesis of blood group A, B, FORS1, and related antigens. The biosynthetic pathways of blood group A, B, FORS1, and related glycan antigens are schematically shown. The gene names, glycan names, and transferase names are shown in light blue, dark blue, and purple, respectively. Important genes/transferases/glycans are shown in bold. The immunodominant epitopes of A, B, and FORS1 are shown in red and bold.
In 1990, we cloned the human A, B, and O allelic complementary DNAs (cDNAs) and determined the nucleotides characterizing those 3 major alleles. We also identified amino acids differentiating A and B transferases with different sugar specificities of GalNAc and galactose, respectively.3,4 Furthermore, we correlated the nucleotide and deduced amino acid sequences with A and/or B antigen expression and experimentally demonstrated the central dogma of ABO: A and B alleles encode A and B transferases, and these enzymes catalyze the biosyntheses of A and B oligosaccharides antigens, respectively. Human AT and BT are both 354-amino-acid proteins but differ by 4 amino acids (arginine [Arg], glycine [Gly], leucine [Leu], and Gly in AT and Gly and serine [Ser], methionine [Met], and alanine [Ala] in BT) at codons 176, 235, 266, and 268, respectively. We prepared 14 AT-BT chimeras that were different at those 4 positions, having either amino acid of AT or BT. We then examined their activity and sugar specificity by DNA transfection into human cancer cells of the uterus (HeLa cell line), which express cell surface H substance.5 We found that the amino acids at codons 266 and 268 are critical in determining the GalNAc/galactose specificity, whereas the amino acids at codons 176 and 235 exhibited no and slight effects, respectively. The importance of codons 266 and 268 has also been demonstrated from sugar specificity analyses of additional AT/BT derivatives of ours6,7 and of others,8,9 as well as the determination of 3-dimensional structures of AT/BT by X-ray crystallography10,11 and the structural modeling of AT/BT.12
The International Society of Blood Transfusion recognizes a total of 36 RBC blood group systems, the ABO system being just one of these. It should be noted that many of the blood group antigens, especially oligosaccharide antigens, may also be expressed on cell types other than RBCs. Therefore, they may be more accurately referred to as histo–blood group antigens. In the case of A and B antigens, for instance, those antigens are also presented on epithelial cells of the gastrointestinal tract and endothelial cells lining blood capillaries, depending on the individual’s ABO group. This is the reason why ABO matching is vital for not only RBC transfusion but also cell, tissue, and organ transplantations. Those antigens may also be expressed on proteins in secretion. The genetically well-defined and conserved A/B antigen expression has been widely used in forensic science. The ABO types of biological specimens, such as blood, skin, saliva, and seminal fluid, found at crime scenes are used as essential markers in identifying or distinguishing suspects in crimes.
In recent years, we have been interested in another blood group system: Forssman (FORS).13-16 The Forssman antigen (FORS1) was initially recognized as an antigen in guinea pig and sheep, but not in rabbit.17 Using antibodies against this antigen, species were categorized into FORS1 antigen-positive and antigen-negative species.18 Mice (Mus musculus) belong to the former and humans (Homo sapiens) to the latter. However, rare individuals express FORS1 antigens on RBCs. Initially, those individuals were incorrectly categorized into a novel A subgroup, Apae. This was based on serological characterization of RBCs showing strong reaction with Helix pomatia lectin and weak reaction with polyclonal anti-A reagents but no reaction with monoclonal anti-A antibodies.19 Glycolipid analysis later showed this to be a misnomer, because the antigen proved to be FORS1 antigen and not a blood group A antigen.20,21 The immunodominant FORS1 epitope has the structure of GalNAcα1-3GalNAcβ1-, and it is carried on glycolipids such as pentasaccharide Forssman glycolipid (Gb5: GalNAcα1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1′Cer). This is in contrast to A and B antigens, which may be carried on either glycoproteins or glycolipids. FORS was recognized as a novel blood group system21 because it fulfills the following criteria: (1) polymorphic FORS system is specified by functional and nonfunctional alleles at the genetic locus GBGT1, which is different from other blood group genes; (2) FORS1 is chemically distinct from other blood group antigens; and (3) there are individuals possessing anti-FORS1 antibodies.
The GBGT1 gene is evolutionarily related to the ABO gene, and the functional GBGT1 allele encodes Forssman glycolipid synthase (FS), which catalyzes the last biosynthetic step of Forssman glycolipid from its precursor globoside (Gb4: GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1’Cer) by transferring GalNAc.22 While we were comparing the deduced amino acid sequences of FS proteins between FORS1 antigen-positive and antigen-negative species, we found many amino acids conserved within a group, but not between the 2 groups.13 Therefore, we prepared structural chimeric expression constructs between functional mouse FS and nonfunctional human FS proteins, performed DNA transfection, immunologically detected FORS1 antigen, and identified human GBGT1 gene cDNA fragments annulling FS activity. We then prepared constructs containing potentially inactivating amino acid substitutions and finally showed that 2 substitutions, c.688G>A [p.Gly230Ser] and c.887A>G [p.Gln(glutamine)296Arg], are responsible for the FS inactivity of the human protein. We also showed that the reversion of either of the 2 substitutions conferred the human FS protein with weak FS activity and that the reversion of both endowed strong FS activity. The c.887G>A[p.Arg296Gln] was later discovered to be a dominant, activating mutation in the GBGT1 gene of the Apae individuals.21
There have been additional advances in our FORS/GBGT1/FS/FORS1 studies. While analyzing species evolution of the ABO and GBGT1 genes, we realized that the GlyGlyAla tripeptide sequence is conserved in the GBGT1 gene–encoded FSs at codons corresponding to codons 266 to 268 of human AT/BT.7 We also found that mouse ABO gene–encoded cis-AB transferase possesses the same tripeptide sequence.23 Therefore, we prepared the expression construct, transfected DNA to appropriate cells, and examined whether the murine cis-AB transferase exhibits FS activity.14 We found FORS1 antigen expression on some cells. Furthermore, we observed weak FORS1 expression when cells were transfected with the human AT construct containing the GlyGlyAla substitution. Because this substitution did not confer full FS activity, we searched for additional molecular mechanisms that might allow ABO gene–encoded AT to biosynthesize FORS1. Surprisingly, we discovered that the deletion of exons 3 or 4 of human AT cDNA, but not of exons 2 or 5, granted the encoded proteins with moderate FS activity.16 This crosstalk between ABO and GBGT1 genes may explain, at least partially, why some FORS1-negative individuals express FORS1 in malignant cells/tissues as alterations in messenger RNA (mRNA) splicing are frequent in cancer.24-26 We also observed an enhanced FS activity by cointroducing the GlyGlyAla substitution with the deletion of exons 3 or 4.
We have expanded the search for other molecular mechanisms. While we were studying the activity and subcellular localization of the ABO gene–encoded ATs with various N termini,27 we prepared and examined a series of methionine (Met) to threonine (Thr) substitution constructs of human AT, including H_ABO-A(Met1,20,26,43,53,69Thr), which contained the methionine-threonine substitutions at codons 1, 20, 26, 43, 53, and 69. Because those substitution constructs were available, we included them for the FS activity analysis. Unexpectedly, we observed weak FS activity with a couple of the constructs. Further studies have revealed that single amino acid substitutions at codon 69 from methionine to threonine or serine endow human AT with FS capability.
Methods
Preparation of amino acid substitution constructs by in vitro mutagenesis
Human AT and derivatives having GlyGlyAla or MetGlyAla tripeptide, mouse cis-AB transferase, and mouse FS constructs H_ABO-A, H_ABO-A(GlyGlyAla), H_ABO-A(MetGlyAla), M_ABO-AB, and M_GBGT1, respectively, were prepared in the pSG5 eukaryotic expression plasmid vector as previously described.7,14,16 Additional amino acid substitution constructs were prepared by in vitro mutagenesis, employing the 2-round primer-mediated polymerase chain reaction strategy using primers with nucleotide substitutions.7,16
DNA transfection
HeLa(FUT2) cells were previously created from HeLa cells to achieve enhanced detection sensitivity of AT and BT activities by retrovirally transducing FUT2 gene encoding α1,2-fucosyltransferase that catalyzes the last biosynthetic step of H substance, the acceptor substrate for AT and BT.27,28 COS1(B3GALNT1) cells were similarly generated from African green monkey kidney COS1 cells by the modular expression of B3GALNT1 gene–encoded β1,3-N-acetyl-d-galactosaminyltransferase 1 to produce increased Gb4, the acceptor substrate for FS.14 Following the manufacturer’s protocols, Lipofectamine 2000 and 3000 reagents (Thermo Fisher Scientific) were used for DNA transfection for immunocytometry and immunocytochemistry, respectively. DNA from pEGFP plasmid vector expressing enhanced green fluorescent protein (GFP) or pLL3.7-mRFP vector expressing monomeric red fluorescent protein (mRFP) was co-transfected to estimate transfection efficiency.
Immunological detection of FORS1 and A and B antigens
DNA-transfected COS1(B3GALNT1) cells were immunostained with clone FOM-1 rat immunoglobulin M (IgM) monoclonal antibody against FORS1 (BMA Biomedicals). For immunocytochemistry, GFP-expressing cells were counted under fluorescence microscopy 2 days after DNA transfection. The following day cells were fixed on culture plates with 4% paraformaldehyde and thoroughly washed with phosphate-buffered saline. The cells were then treated first with the primary antibody and next with a biotinylated goat anti-rat IgG+IgM(H+L) secondary antibody (Jackson ImmunoResearch Laboratories). After washing, cells were incubated with the Vectastain ABC reagents (Vector Laboratories). For peroxidase-mediated color development, 3,3′-diaminobenzidine tetrahydrochloride from the same company was used. Positive cells were counted under the microscope and normalized using numbers of cells positively expressing cotransfected GFP. The percentage values were calculated using the mouse FS construct (M_GBGT1) value of 100.
For immunocytometry of DNA-transfected COS1(B3GALNT1) cells, cells were detached from culture plates with 5 mM EDTA in phosphate-buffered saline 1 day after DNA transfection, washed, and subjected to immunostaining, first with the primary antibody and then with Alexa Fluor 488 (AF488)-conjugated goat anti-rat IgM (µ-chain specific) secondary antibody (Thermo Fisher Scientific). A BD LSRFortessa Analyzer (BD Biosciences) was used for fluorescence-activated cell sorting (FACS) analyses, employing the same FACS detection settings for a single set of experiments. Viable single cells were gated based on their forward and side scattering values. Fluorescence was measured at 575 nm to identify cotransfected mRFP expression and determine the transfection efficiency. FORS1 expression (AF488 fluorescence) was detected at 530 nm. FORS1-positive cell percentages and their B530-A median fluorescence intensity (MFI) values were determined. For immunocytometry of DNA-transfected HeLa(FUT2) cells, detached cells were incubated first with the mixture of anti-A murine monoclonal antibodies or the mixture of anti-B murine monoclonal antibodies (Ortho Clinical Diagnostics) and then with Alexa Fluor 647–conjugated goat anti-mouse IgM (µ chain specific) secondary antibody (Thermo Fisher Scientific). The immunostained cells were FACS analyzed as described above with COS1(B3GALNT1) cells. The A or B antigen-positive cell percentages and MFI were determined by Alexa Fluor 647 fluorescence at 660 nm and GFP expression at 530 nm.
Results
Methionine-threonine substitution at codon 69, but not codon 1, 20, 26, 43, or 53, confers human AT with FS activity
In an attempt to search for additional molecular mechanisms that might confer human AT with FS activity, we analyzed a couple dozen AT derivatives, including H_ABO-A(Met1,20,26,43,53,69Thr). We found that some COS1(B3GALNT1) cells transfected with H_ABO-A(Met1,20,26,43,53,69Thr) expressed FORS1 antigen weakly. Because H_ABO-A(Met1,20,26,43,53,69Thr) contained 6 methionine-threonine substitutions, we wondered which substitutions might be responsible for FS activity. Several intermediate constructs were prepared during the H_ABO-A(Met1,20,26,43,53,69Thr) construction through sequential introduction of substitutions, which included H_ABO-A(Met1,69Thr), H_ABO-A(Met20,26Thr), H_ABO-A(Met1,20,26Thr), H_ABO-A(Met1,20,26,43,69Thr) and H_ABO-A(Met1,20,26,53,69Thr). We also prepared the constructs afresh with a single substitution, H_ABO-A(Met1Thr), H_ABO-A(Met20Thr), H_ABO-A(Met26Thr), H_ABO-A(Met43Thr), H_ABO-A(Met53Thr), H_ABO-A(Met69Thr), and H_ABO-A(Met142Thr) containing Met1Thr, Met20Thr, Met26Thr, Met43Thr, Met53Thr, Met69Thr, or Met142Thr, respectively. Codons 1, 20, and 26 are located in the cytoplasmic domain of human AT, whereas codons 43 and 53 are in the transmembrane (TM) region, and codons 69 and 142 in the lumen of Golgi apparatus.27
Together with positive controls of mouse GBGT1 gene construct (M_GBGT1) and mouse ABO gene construct (M_ABO-AB) and a negative control of human AT construct (H_ABO-A), we examined the FS activity of H_ABO-A(Met1,20,26,43,53,69Thr) and 12 other methionine-threonine substitution constructs. Results are shown in Table 1.
We observed cell-surface FORS1 antigen expression on some cells transfected with human ATs with Met1,69Thr, Met1,20,26,43,69Thr, Met1,20,26,53,69Thr, or Met1,20,26,43,53,69Thr. As the Met69Thr substitution was shared among those constructs, this substitution was presumed to be responsible for FS activity. The positive result obtained with H_ABO-A(Met69Thr), having a single substitution at codon 69, confirmed this prediction. None of the other single methionine-to-threonine substitutions at codons 1, 20, 26, 43, or 53 induced FORS1 antigen expression, suggesting that they were ineffective, although they did not inhibit Met69Thr-induced FS activity of human AT.
Methionine-serine substitution at codon 69 (Met69Ser) also confers human AT with weak FS activity, and cointroduction of Met69Thr and GlyGlyAla substitutions enable strong FS activity
In order to examine whether the threonine residue at codon 69 is essential, we prepared H_ABO-A(Met69Ser) with serine rather than threonine at codon 69. In addition, we prepared H_ABO-A(Met69Thr&GlyGlyAla) and H_ABO-A(Met69Thr&MetGlyAla) with both Met69Thr and FS-specific LeuGlyGly266-268GlyGlyAla or BT-specific LeuGlyGly266-268MetGlyAla substitutions. DNA from those constructs was also transfected, and FS activity was examined (Table 1). Human AT with Met69Ser also exhibited weak FS activity. The combination of Met69Thr with GlyGlyAla enhanced FS activity, suggesting that the molecular mechanisms to confer FS activity are different and synergistic between those 2 modifications. On the other hand, the combination with MetGlyAla eliminated FS activity, indicating that the negative effect of MetGlyAla is dominant over the FS activity of human AT induced by Met69Thr.
FACS immunocytometry confirmed the immunocytochemistry results
Immunocytochemistry presented semiquantitative results due to cell detachment during immunostaining and difficult microscopical observation of weakly stained cells. To better quantify FS activity, we performed FACS analyses, which permitted more accurate measurements without enzyme-mediated signal amplification. We repeated transfection experiments using several important constructs. We selected human AT and their derivative constructs containing Met43Thr, Met53Thr, Met69Thr, Met69Ser, or Met142Thr substitution and GlyGlyAla or MetGlyAla tripeptide alone or in combination with Met69Thr. They were examined together with M_GBGT1 and M_ABO-AB as positive controls, and H_ABO-A and the empty vector as negative controls. DNA from those 13 constructs was separately transfected into COS1(B3GALNT1) cells, together with DNA from pLL3.7-mRFP vector. After transfection, cells were detached and incubated with anti-FORS1 monoclonal antibody followed by AF488-conjugated secondary antibody. The cells were then analyzed by FACS. Results were compared among constructs and are shown in Table 2. In addition to M_GBGT1 and M_ABO-AB, FORS1 expression above background levels was observed for H_ABO-A(GlyGlyAla), H_ABO-A(Met69Thr), H_ABO-A(Met69Ser), and H_ABO-A(Met69Thr&GlyGlyAla). The FACS results presented a similar tendency in positive cell percentages with the immunocytochemistry results.
Met69Thr/Ser substitutions confer FS activity without affecting AT and BT activity
In addition to COS1(B3GALNT1) cells, the same set of constructs were transfected to HeLa(FUT2) cells, and AT and BT activity was examined by FACS. A antigen–positive and B antigen–positive HeLa(FUT2) cell percentages and MFI values are also shown in Table 2. A antigen expression was observed in HeLa(FUT2) cells transfected with M_ABO-AB and H_ABO-A constructs with LeuGlyGly or GlyGlyAla irrespective of the presence or absence of Met69Thr/Ser. B antigen expression was observed in cells transfected with M_ABO-AB and H_ABO-A constructs with GlyGlyAla or MetGlyAla irrespective of Met69Thr. Some fluctuations were observed, but Met69Thr/Ser did not greatly affect the expression of A and/or B antigens.
Met69Thr/Ser substitutions may affect human AT in a manner similar to exon 3 or 4 deletion of human AT mRNA
We performed additional FACS analyses of COS1(B3GALNT1) cells transfected with selected constructs. In addition to H_ABO-A(Met69Thr), H_ABO-A(Met69Ser), H_ABO-A(GlyGlyAla), H_ABO-A(Met69Thr&GlyGlyAla), and H_ABO-A(Met69Thr&MetGlyAla), the exon deletion constructs were also included for comparison.
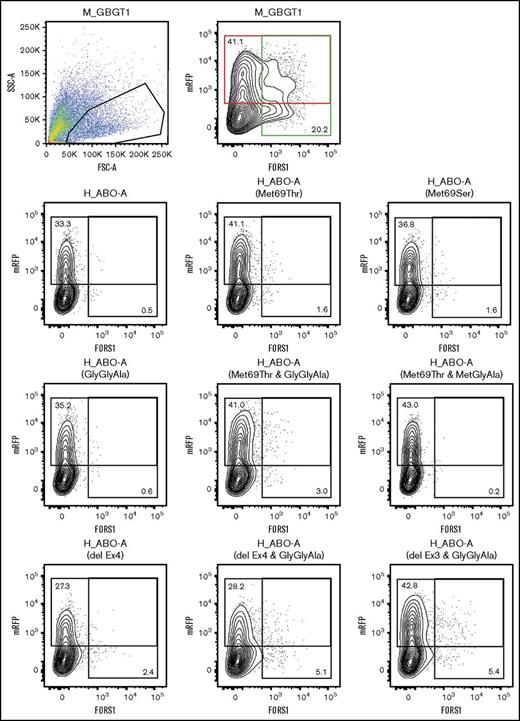
Representative cytometry results are shown in Figure 2. The results clearly show FORS1 appearance after the substitution of the methionine residue with threonine or serine at codon 69 of human AT. We also observe that the double mutants containing Met69Thr and LeuGlyGly266-268GlyGlyAla exhibited larger number of FORS1-positive cells than the single-substitution constructs containing either Met69Thr or LeuGlyGly266-268GlyGlyAla.
Cytometry analysis of COS1(B3GALNT1) cells transfected with M_GBGT1, H-ABO-A, and 8 representative human A transferase derivative constructs. Cells were cotransfected with DNA from selected expression constructs together with a vector expressing mRFP. Cells were then detached and stained with anti-FORS1 antibody followed by AF488-conjugated secondary antibody. The first 2 panels correspond to M_GBGT1 transfection. On the left, the selection of single viable cell population by forward (FSC-A) and side (SSC-A) scattering amplitudes is depicted. Events are plotted in pseudocolor, with red representing higher frequency and dark blue the lowest. This selection was maintained for all the other samples. The right panel shows a contour plot of the mRFP fluorescence intensity, used as transfection control, on the y-axis, and the fluorescence corresponding to FORS1 on the x-axis. Both axes are on logarithmic scales. The upper red rectangle represents mRFP-positive cells, and the percentage of mRFP-positive cells is also indicated at the upper left corner. The rightmost green rectangle corresponds to AF488 fluorescence, and the percentage of FORS1-positive cells is indicated at the lower right corner. The other panels show the graph for the other constructs, using the same gate, second row: H_ABO-A, H_ABO-A(Met69Thr), and H_ABO-A(Met69Ser); third row: H-ABO-A(GlyGlyAla), H_ABO-A(Met69Thr&GlyGlyAla), and H_ABO-A(Met69Thr&MetGlyAla); and fourth row: H_ABO-A(delEx4), H_ABO-A(delEx4&GlyGlyAla), and H_ABO-A(delEx3&GlyGlyAla).
Cytometry analysis of COS1(B3GALNT1) cells transfected with M_GBGT1, H-ABO-A, and 8 representative human A transferase derivative constructs. Cells were cotransfected with DNA from selected expression constructs together with a vector expressing mRFP. Cells were then detached and stained with anti-FORS1 antibody followed by AF488-conjugated secondary antibody. The first 2 panels correspond to M_GBGT1 transfection. On the left, the selection of single viable cell population by forward (FSC-A) and side (SSC-A) scattering amplitudes is depicted. Events are plotted in pseudocolor, with red representing higher frequency and dark blue the lowest. This selection was maintained for all the other samples. The right panel shows a contour plot of the mRFP fluorescence intensity, used as transfection control, on the y-axis, and the fluorescence corresponding to FORS1 on the x-axis. Both axes are on logarithmic scales. The upper red rectangle represents mRFP-positive cells, and the percentage of mRFP-positive cells is also indicated at the upper left corner. The rightmost green rectangle corresponds to AF488 fluorescence, and the percentage of FORS1-positive cells is indicated at the lower right corner. The other panels show the graph for the other constructs, using the same gate, second row: H_ABO-A, H_ABO-A(Met69Thr), and H_ABO-A(Met69Ser); third row: H-ABO-A(GlyGlyAla), H_ABO-A(Met69Thr&GlyGlyAla), and H_ABO-A(Met69Thr&MetGlyAla); and fourth row: H_ABO-A(delEx4), H_ABO-A(delEx4&GlyGlyAla), and H_ABO-A(delEx3&GlyGlyAla).
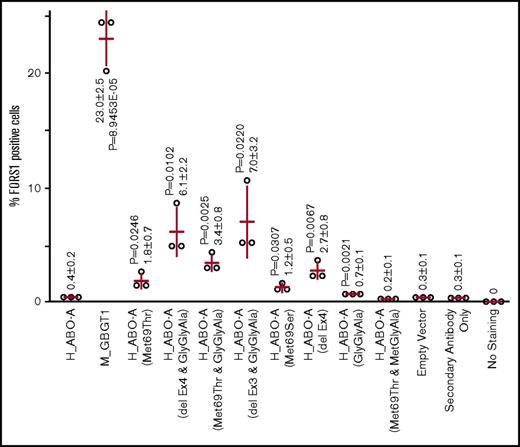
FORS1 antigen–positive cell percentages were determined from the cytometry experiments, which were repeated in triplicate. Results are shown in a scatterplot in Figure 3. It was observed that FS activity takes place in human AT with Met69Thr/Ser. FS activity was enhanced by adding the GlyGlyAla substitution, as has been reported of the combinations between exon 3 or 4 deletions and GlyGlyAla substitution. On the other hand, H_ABO-A(Met69Thr&MetGlyAla) did not induce FORS1 antigen expression. Rather, MetGlyAla substitution nullified the FS activity of human AT induced by Met69Thr.
Expression of FORS1 antigen after DNA transfection of the selected expression constructs into COS1(B3GALNT1) cells analyzed by FACS cytometry. After DNA transfection of COS1(B3GALNT1) with a variety of expression constructs and a vector expressing mRFP, cells were immunostained using anti-FORS1 antibody, followed by FACS analysis. The percentages of FORS1-positive cells for each construct and for all 3 independent experiments are depicted as open circles. Their mean ± standard deviation is written in numbers and represented on the graph as red bars (horizontal for the mean and vertical for the standard). The construct names are shown under the x-axis. Two-tailed, homoscedastic Student t tests comparing each set of data to the empty vector set were performed, and P < .05 is shown.
Expression of FORS1 antigen after DNA transfection of the selected expression constructs into COS1(B3GALNT1) cells analyzed by FACS cytometry. After DNA transfection of COS1(B3GALNT1) with a variety of expression constructs and a vector expressing mRFP, cells were immunostained using anti-FORS1 antibody, followed by FACS analysis. The percentages of FORS1-positive cells for each construct and for all 3 independent experiments are depicted as open circles. Their mean ± standard deviation is written in numbers and represented on the graph as red bars (horizontal for the mean and vertical for the standard). The construct names are shown under the x-axis. Two-tailed, homoscedastic Student t tests comparing each set of data to the empty vector set were performed, and P < .05 is shown.
Discussion
In living cells and organisms, a variety of enzymes catalyze biological reactions in a specific and efficient manner at ambient pH and temperature. This wide enzymatic repertoire has resulted from vertically transmitted gene duplications, exon shuffling, and divergence. Enzyme-encoding genes may also have been transmitted horizontally from other organisms. Catalytic activities and substrate specificities may have been modified from existing enzymes or created anew from nonfunctional proteins by mutations. In addition, alterations in intracellular localization and protein conformation may have influenced enzymatic activity due to reduced or enhanced substrate availability or utility. Linkages to different promoters, enhancers, or insulators may have triggered gene expression changes.
The evolutionarily related ABO and GBGT1 genes belong to the α1,3-Gal(NAc) transferase gene family. The other members include A3GALT2 gene encoding isoglobotriaosylceramide synthase (iGb3S) involved in the biosynthesis of isoglobotriaosylceramide (iGb3: Galα1-3Galβ1-4Glcβ1-1′Cer) and GGTA1 gene encoding α1,3-galactosyltransferase (GT) involved in the biosynthesis of α1,3-galactosyl epitope (α-Gal-epitope: Galα1-3Galβ1-4GlcNAc-).29 In 2014, we illustrated the α1,3-Gal(NAc) transferase gene evolution.7 The ancestral gene underwent repeated gene duplications, followed by separations into different gene lineages. Gene absence in some species indicates that they are dispensable for species survival. In addition to species variations, polymorphisms may also exist. Therefore, those genes are also dispensable for individual survival. However, interspecies variations and intraspecies polymorphisms may have affected survival, considering that cell-surface glycans play a pivotal role as receptors for bacteria, viruses, and toxins.30,31
While studying the activity and subcellular localization of ATs with various N-terminal deletions, we prepared H_ABO-A(Met1,20,26,43,53,69Thr).27 This construct encoded AT protein, whose methionine residues in the N-terminal cytoplasmic, TM, and stem regions were all replaced by threonines. We hypothesized that truncated enzymes translated from internal methionines propelled A antigen synthesis in those alleles lacking a proper translation initiation codon.32 However, the encoded protein exhibited AT activity and induced A antigen expression on HeLa(FUT2) cells after DNA transfection. The N-terminal amino acid sequencing of the encoded protein revealed that the threonine residue at codon 1 was actually the translation initiation codon, demonstrating that codons other than AUG may start protein synthesis. The current study showed that this construct also exhibited weak FS activity and that the Met69Thr substitution was responsible for the FS activity. Together with our previous finding that the GlyGlyAla substitution at codons 266 to 268 of human AT and the deletion of exon 3 or 4 of AT mRNAs also endowed FS activity,14,16 it is evident that multiple molecular mechanisms exist allowing the crosstalk between ABO and FORS systems. The fact that FS activity was enhanced by the cointroduction of GlyGlyAla tripeptide and Met69Thr substitutions suggests 2 different synergistic mechanisms. The TM region of human AT/BT spans over codons 33 to 54, which coincides well with codons 33 to 52 contained in exon 3. Codons 53 to 68, present in exon 4, and codon 69, the first amino acid of exon 5, are located in the stem region of the enzymes between the TM region and catalytic domain. Accordingly, the molecular mechanisms may be similar between the deletion of exons 3 or 4 and the Met69Thr/Ser substitution, potentially affecting intra-Golgi localization and/or protein folding.
In addition to H_ABO-A(Met69Thr&GlyGlyAla), we also analyzed the FS activity of H_ABO-A(Met69Thr&MetGlyAla). No FORS1 was detected. The LeuGlyGly266-268MetGlyAla substitution of human AT converts the sugar specificity from GalNAc to galactose, producing B antigens in place of A antigens.5,6 We could not immunologically detect such an epitope, because antibodies that recognize the Galα1-3GalNAcβ1- structure are not available. Therefore, a possibility exists that AT with Met69Thr and MetGlyAla still utilizes globoside/isogloboside substrates but transfers galactose in place of GalNAc. We have observed that AT and BT activities were not very affected by the Met69Thr/Ser substitution, although weak FS activity was acquired (Table 2). However, the study did not analyze qualitative changes. Therefore, it is possible that carrier molecule preference may have been altered by those amino substitutions.
In vitro mutagenesis has also been employed to characterize the substrate specificity of other glycosyltransferases that synthesize blood group antigens and otherwise biologically important sugars. For example, Dupuy et al found that a single amino acid at codon 111 in the hypervariable stem domain of vertebrate α1,3/1,4-fucosyltransferases determines H-type 1 or H-type 2 transfer specificity.33 FUT3-encoded Lewis α1,3/1,4-fucosyltransferases and FUT5-encoded α1,3/1,4-fucosyltransferases, which can transfer fucose in an α1,4-linkage, have tryptophan (Trp) at codon 111, whereas it is Arg in other α1,3-fucosyltransferases. They showed that the Trp111Arg substitution of Lewis α1,3/1,4-fucosyltransferase changed the specificity from H-type 1 to H-type 2 acceptors. Kaczmarek et al34 studied human A4GALT gene-encoded α1,4-galactosyltransferase. Codon 211 is Gln in the majority of individuals, whereas it is glutamic acid (Glu) in rare individuals carrying RBCs expressing terminal disaccharide of the NOR antigen. It was shown that consensus human α1,4-galactosyltransferase may synthesize the Pk and P1 blood group antigens and that its Gln211Glu variant is able to synthesize all the 3 P1PK blood group antigens (Pk, P1, and NOR). The specificity of β1,4-galactosyltransferase that synthesize Galβ1-4GlcNAc- disaccharide unit of glycoconjugates was also studied by in vitro mutagenesis.35 Although the normal enzyme transfers galactose and not GalNAc, the Tyr(tyrosine)289Leu substitution was found to change the donor nucleotide-sugar specificity and to transfer GalNAc to the acceptor GlcNAc. Our finding of the crosstalk between AT and FS is unique in the sense that the acceptor specificities were modified across the gene and the blood group boundary between ABO and GBGT1 and ABO and FORS. However, it is obvious that the modifications in substrate specificities have been frequent in the evolution of 200-plus glycosyltransferases.
The FORS1 antigen has been detected in human cancer.36-42 We have recently proposed that the appearance of genetically inappropriate heterophile FORS1 antigen in cancer cells/tissues may result from the exon 3 or 4 deletion of human AT mRNA,16 because altered splicing is one of the hallmarks of cancer.24-26 In contrast, Met69Thr/Ser may not be responsible for this FORS1 antigen expression in human cancer. We have examined the SNP database, but no SNPs have been found corresponding to the Met69Thr/Ser substitutions in the human ABO gene. We also searched the Catalogue of Somatic Mutations in Cancer database for somatic mutations resulting in the Met69Thr/Ser missense mutations, but none were found. However, it should be noted that other amino acid substitutions at codon 69 or neighboring codons of human AT might also confer the FS activity. Together with the conversion issue of sugar specificity discussed above, further studies will be needed to clarify this possibility.
Acknowledgments
The authors thank Marco Antonio Fernández Sanmartín and Gerard Requena Fernández at the Cytometry Core Facility of the Institut d’Investigació en Ciències de la Salut Germans Trias i Pujol for their help with FACS analyses, Alba Garcia and Albert Perez for their technical assistance, and Michael Maher for editing the manuscript. The work was performed in part at the now-defunct Institut de Medicina Predictiva i Personalitzada del Càncer and in part at the Josep Carreras Leukaemia Research Institute (IJC).
The study was supported by the Spanish Health Research Foundation (Instituto de Salud Carlos III) (PI11/00454), the fund from Agència de Gestió d’Ajuts Universitaris i de Recerca (2014 SGR 1269 and 2017 SGR 529), and institutional start-up funds from Institut de Medicina Predictiva i Personalitzada del Càncer and IJC–“La Caixa” Foundation (F.Y.). IJC and Institut d’Investigació en Ciències de la Salut Germans Trias i Pujol are CERCA centers supported by CERCA Programme/Generalitat de Catalunya. IJC is also supported by the Josep Carreras Foundation.
Authorship
Contribution: M.Y. and F.Y. conceived the experiments; M.Y., E.C., and F.Y. designed the experiments; M.Y. prepared expression constructs and performed DNA transfection and immunocytochemistry; E.C. performed DNA transfection and FACS immunocytometry; and F.Y. wrote the manuscript with contributions from the other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fumiichiro Yamamoto, Josep Carreras Leukaemia Research Institute, Can Ruti Campus, Ctra de Can Ruti, Camí de les Escoles s/n, 08916 Badalona, Barcelona, Spain; e-mail: fyamamoto@carrerasresearch.org.
References
Author notes
E.C. and M.Y. contributed equally to this study.