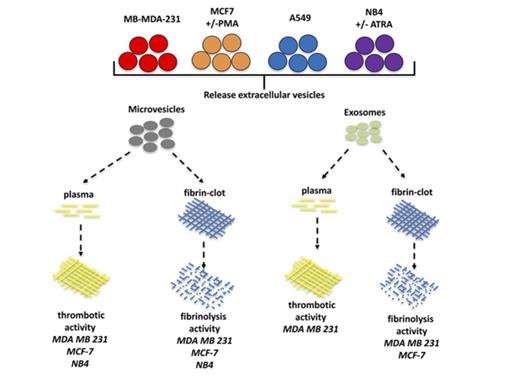
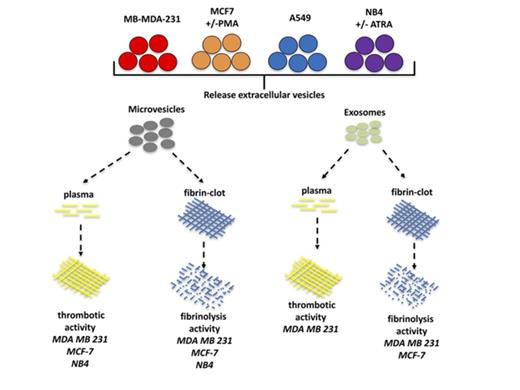
Key Points
Microvesicles, but not exosomes, from tumor cells have thrombotic activity.
Tumor derived–exosomes can confer increased plasmin-generating capacity to a recipient cell.
Abstract
Exosomes and microvesicles (MVs) are small extracellular vesicles secreted by tumor cells and are suggested to contribute to the thrombotic events that commonly occur in patients with advanced malignancies. Paradoxically, these vesicles have been reported to also possess fibrinolytic activity. To determine whether thrombotic or fibrinolytic activity is a predominant characteristic of these extracellular vesicles, we prepared exosomes and MVs from 2 breast cancer cell lines (MDA-MB-231 and MCF7), a lung cancer cell line (A549), and a leukemia cell line (NB4) and assayed their thrombotic and fibrinolytic activities. We observed that thrombotic activity was a common feature of MVs but not exosomes. Exosomes and/or MVs from several cell lines, with the exception of the A549 cell line, displayed fibrinolytic activity toward a pure fibrin clot, but only exosomes from MDA-MB-231 cells could degrade a fibrin clot formed in plasma. Increasing the malignant potential of MCF7 cells decreased the thrombotic activity of their MVs but did not alter their fibrinolytic activity. Decreasing the malignant potential of NB4 cells did not alter the thrombotic or fibrinolytic activity of their MVs or exosomes. Finally, the incubation of MDA-MB-231 cell–derived exosomes with A549 cells increased plasmin generation by these cells. Our data indicate that MVs generally have thrombotic activity, whereas thrombotic activity is not commonly observed for exosomes. Furthermore, exosomes and MVs generally do not display fibrinolytic activity under physiological conditions.
Introduction
Extracellular vesicles (EVs) are lipid bilayer–surrounded particles that are released by many mammalian cell types and include exosomes and microvesicles (MVs). Exosomes and MVs are nanoparticles with a diameter of 30-100 and 100-1000 nm, respectively,1 although the size of MVs has also been reported to be <100 nm in some studies.2,3 A variety of cell types has been shown to produce these EVs, including platelets, epithelial cells, dendritic cells, B cells, T cells, mast cells, and tumor cells.4-10 They are present in biologic fluids, such as urine,11 blood,12 ascites,13 saliva,14 and breast milk.15 Exosomes are released from multivesicular endosomes,16 whereas MVs are formed from the plasma membrane.17
EVs express bioactive lipids, proteins, and nucleic acids, including microribonucleic acid.18 The dramatic increase in the risk for developing venous thrombosis in cancer patients has been suggested to be linked to the release of tumor-derived EVs.19 For example, tissue factor released in tumor cell exosomes20 and MVs21 is known to initiate thrombotic activity.
Interestingly, in addition to possessing thrombotic activity, exosomes and MVs have been suggested to possess fibrinolytic activity.22,23 Plasmin, the chief fibrinolytic enzyme, is generated by the cleavage of plasminogen by the activators, tissue plasminogen activator (tPA) and the urokinase plasminogen activator. The rate of activation is exceedingly slow but is greatly accelerated by the presence of plasminogen receptors on the surface of cells. Multiple plasminogen receptors have been identified as being associated with EVs, including S100A10,24 S100A4,25 Plg-rkt,26 enolase,27 and cytokeratin 8.28
Although several studies have investigated the potential thrombotic or fibrinolytic activity of EVs, a systematic study of these activities within a given vesicle population has not been investigated. Therefore, in this study, we have investigated the thrombotic and fibrinolytic activity of tumor cell–derived exosomes and MVs in vitro.
Materials and methods
Cells
Human breast cancer cell lines (MDA-MB-231 and MCF7; American Type Culture Collection, Manassas, VA) and a human lung cancer cell line (A549; American Type Culture Collection) were maintained in DMEM/High glucose medium (GE Healthcare Life Sciences, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO) and 1× penicillin-streptomycin (GE Healthcare Life Sciences) at 37°C, in a 5% CO2 atmosphere. A human acute promyelocytic leukemia cell line (NB4; DSMZ) was maintained in RPMI 1640 medium (GE Healthcare Life Sciences) supplemented with 10% FBS and 1× penicillin-streptomycin at 37°C, in a 5% CO2 atmosphere.
Antibodies
The following primary antibodies were used for western blot: TSG101 (clone sc7964; Santa Cruz Biotechnology), CD81 (clone sc166029; Santa Cruz Biotechnology), and actinin-4 (GTX101669; GeneTex, Irvine, CA). We used infrared DyLight–conjugated antimouse and antirabbit secondary antibodies (Thermo Scientific, Burlington, ON, Canada).
Exosome and MV isolation
Human cells (MDA-MB-231, MCF7, and A549) were seeded at 4-5 million cells in 150-mm plates and were cultured for 5-6 days before isolating the EVs. The FBS used was centrifuged at 100 000g for 2 hours to eliminate the exosomes and MVs. For the human NB4 cell line, 100 nM all-trans retinoic acid (ATRA; Sigma) or 0.04% dimethyl sulfoxide (DMSO) was added to the medium daily. For the MCF7 cell line, 100 nM phorbol 12-myristate 13-acetate (PMA) or 0.04% DMSO was added to the medium daily.
To isolate exosomes, the conditioned media were collected and centrifuged at 500, 2000, and 10 000g for 10, 10, and 30 minutes, respectively, to eliminate cells, cell debris, and large particles, respectively. Then, the supernatant was collected and centrifuged at 100 000g for 2 hours. The pellet obtained was washed with phosphate-buffered saline (PBS) and resuspended in PBS. This fraction was used as a source of exosomes.
To isolate MVs, the conditioned media were collected and centrifuged at 500 and 2000g for 10 minutes to eliminate cells and cell debris, respectively. Then, the supernatant was collected and centrifuged at 20 000g for 2 hours. The pellet obtained was washed and resuspended in PBS. This fraction was used as a source of MVs.
Transmission electron microscopy
An aliquot of each exosome and MV sample was coated on grids for 10 minutes and treated with 2% uranyl oxalate for 20 seconds, and the samples were observed by transmission electron microscopy (TEM). For MVs, we took 5 measurements per photograph; 5 photographs of MB-MDA-231 cells and 5 photographs of MCF7 cells were used. These 25 measurements from MB-MDA-231 cells and 25 measurements from MCF7 cells were used to calculate the mean size. For exosomes, we took 5 measurements per photograph using 4 photographs of MB-MDA-231 cells, 3 photographs of MCF7 cells, and 3 photographs of A549 cells. These 20 measurements from MD-MBA-231 cells, 15 measurements from MCF7 cells, and 15 measurements from A549 cells were used to calculate the mean size.
Western blot analysis
Exosomes and MVs, suspended in PBS, were used directly or stored at −80°C prior to use. Cell lysates (MDA-MB-231, MCF7, A549, and NB4) were prepared by washing cells with PBS and preparing lysates with radioimmunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate) supplemented with 1 mM phenylmethylsulfonyl fluoride, 5 mM EDTA, and protease and phosphatase inhibitor. The samples (25-30 μg of protein) were separated on a 12% polyacrylamide gel under reducing conditions and transferred onto a nitrocellulose membrane. The membranes were blocked and incubated with appropriate primary and secondary antibodies, as described above. The blots were scanned using an Odyssey infrared imaging system (LI-COR Biosciences).
Clot lysis assay
The platelet-poor plasma was prepared as follows. Blood obtained from volunteers was collected in BD Vacutainer ACD tubes; after centrifugation at 2000g for 10 minutes, the plasma was collected and centrifuged again at 2000g for 10 minutes. The plasma was collected and stored at −80°C. The plasma was diluted in clot lysis buffer (50 mM Tris [pH 7.5], 100 mM NaCl, and 5 mM CaCl2) to form the plasma clot.
Fibrin clots were formed in 8-well flat-bottom strips (Evergreen, Los Angeles, CA) by incubating in a final volume of 100 μL of clot lysis buffer in the presence of 0.8 mg/mL pure fibrinogen (FIB 13142; Enzyme Research Laboratories, South Bend, IN) or human platelet-poor plasma and with 1 U/mL α-thrombin (HCT-0020; Haematologic Technologies, Essex Junction, VT) for 2-4 hours at 37°C. We added 30 μg of exosomes or MVs to the newly formed fibrin/plasma clot, followed by the addition of plasminogen (0.15 μM), in the presence or absence of inhibitor ε-aminocaproic acid (ε-AcA; 100 mM; Sigma-Aldrich) or aprotinin (Apro; 2.2 μM; Pentapharm, Basel, Switzerland). For a positive control, plasminogen (0.15 μM) and tPA (25 nM) were added to the fibrin/plasma clot instead of the exosomes and MVs. The optical density (OD) was measured at 350 nm every 20 minutes for 18 hours at 37°C using a spectrophotometer (Spectra Max M3; Molecular Devices, Sunnyvale, CA).
The percentage of clot retention was calculated using the following equation: 100 − [(OD at 0 hours) − (OD at 18 hours)/(OD at 0 hours) − (OD buffer/plasma) × 100]. OD buffer/plasma refers to the control containing only buffer or human plasma. The wells were also photographed at the end of the experiment.
Thrombotic activity
Thrombotic activity was determined by measuring the ability of exosomes or MVs to form a fibrin clot from pure fibrinogen or platelet-poor plasma. Initially, 100 μl of fibrinogen (0.8 mg/mL) or 100 μl of human platelet-poor plasma was incubated in 8-well flat-bottom strips with 50 mM Tris (pH 7.5), 100 mM NaCl, and 5 mM CaCl2, followed by addition of exosomes or MVs (30 μg) or 1 U/mL α-thrombin (positive control). The turbidity was measured at 350 nm every 2 minutes for 1 hour at 37°C with a SpectraMax M3 spectrophotometer. The wells were also photographed at the end of the experiment.
Incubation of exosomes with tumor cells
Exosomes from MDA-MB-231 cells were prepared from conditioned media, as described. About 150-200 μg of proteins of exosomes was added to 1 million MCF7 or A549 cells and incubated for 2 hours at room temperature with rotation. The exosome-treated cells were seeded at 50 000 cells per well in a 96-well plate and incubated for 3 days at 37°C, in a 5% CO2 atmosphere.
Plasmin generation
Cells were plated in 96-well plates at 50 000 cells per well and cultured for 3 days. The cells were washed with Hank’s balanced salt solution (Thermo Scientific), incubated with plasminogen (0.5 μM final concentration) for 10 minutes at 37°C, followed by addition of the chromogenic substrate S2251 (0.5 mM final concentration; Chromogenix; Diapharma), and plasmin generation was measured at 405 and 600 nm every 4 minutes for 4 hours at 37°C using a spectrophotometer (SpectraMax M3).
Statistics
Results were analyzed using GraphPad Prism 6.0. Statistical significance was determined by the Student t test (1 tailed) or ANOVA in combination with the Bonferroni post test. The threshold for significance was set at P = .05.
Results
Isolation and characterization of exosomes and MVs from tumor cells
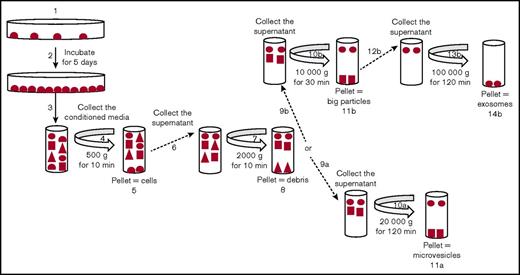
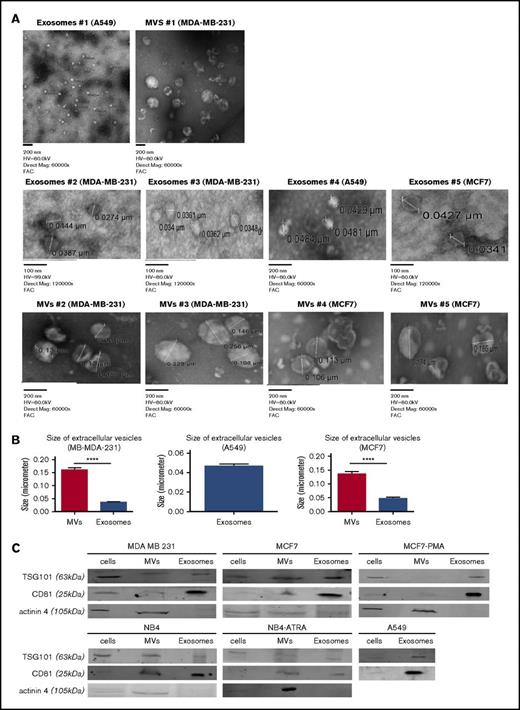
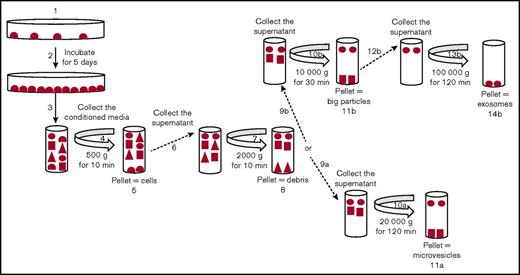
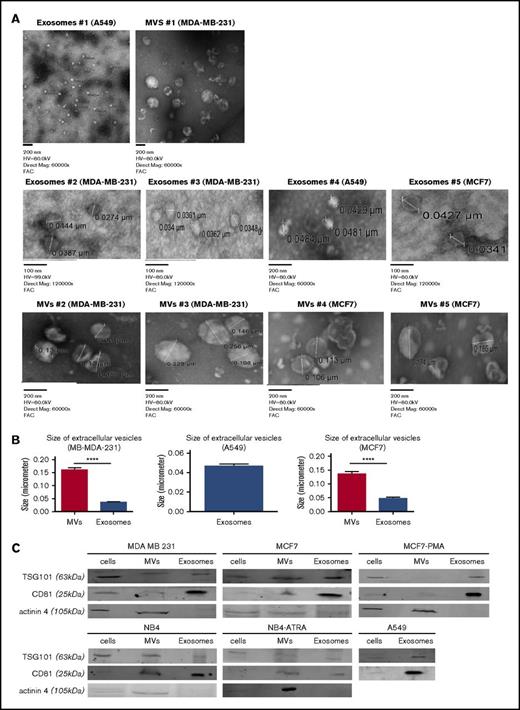
Exosomes and MVs were isolated by differential centrifugation (Figure 1). Cancer cells (MDA-MB-231, MCF7, A549, and NB4) were seeded and incubated for 5 days. Trypan blue–exclusion analysis suggested that, after 5 days of culture, the plated cells were ∼99% viable. ATRA or DMSO was added daily to the media for the NB4 cell line, and PMA or DMSO was added daily to culture media for the MCF7 cell line. The conditioned media were collected and centrifuged at 500g to eliminate cells (4 and 5 in Figure 1) and at 2000g to remove cell debris (7 and 8 in Figure 1). MVs were collected by centrifugation at 20 000g (10a and 11a in Figure 1). For exosomes, the supernatant was centrifuged at 10 000g to eliminate larger particles (10b and 11b in Figure 1) and then collected after centrifugation at 100 000g (13b and 14b in Figure 1). After isolation, the purity of the exosomes and MVs was characterized by TEM and western blot analysis (Figure 2). In the TEM analysis, we observed the presence of circular particles with an average size of 35.74 ± 2.9 nm (n = 20), 47.01 ± 4.1 nm (n = 15), and 46.7 ± 1.9 nm (n = 15) for exosomes from the MD-MBA-231, MCF7, and A549 cell lines, respectively, and an average size of 160.9 ± 8.1 nm (n = 25) and 135.7 ± 8.9 nm (n = 25) for MVs from the MDA-MB-231 and MCF7 cell lines, respectively (Figure 2A-B).
Purification of exosomes and MVs from tumor cells. Representative schema for the purification of exosomes and MVs by differential centrifugation. Tumor cells were seeded in dishes (1) and cultured for 5 days (2). The conditioned media were collected (3) and centrifuged at 500g (4) to eliminate the cells (5), and the supernatant was collected (6) and centrifuged at 2000g (7) to eliminate the cell debris (8). To isolate MVs, the supernatant was collected (9a) and centrifuged at 20 000g (10a). This pellet was used as a source of “MVs” (11a). To isolate the exosomes, the supernatant was collected (9b) and centrifuged at 10 000g (10b) to eliminate the large particles (11b). Then, the supernatant was collected (12b) and centrifuged at 100 000g (13b). The pellet was used as a source of “exosomes” (14b).
Purification of exosomes and MVs from tumor cells. Representative schema for the purification of exosomes and MVs by differential centrifugation. Tumor cells were seeded in dishes (1) and cultured for 5 days (2). The conditioned media were collected (3) and centrifuged at 500g (4) to eliminate the cells (5), and the supernatant was collected (6) and centrifuged at 2000g (7) to eliminate the cell debris (8). To isolate MVs, the supernatant was collected (9a) and centrifuged at 20 000g (10a). This pellet was used as a source of “MVs” (11a). To isolate the exosomes, the supernatant was collected (9b) and centrifuged at 10 000g (10b) to eliminate the large particles (11b). Then, the supernatant was collected (12b) and centrifuged at 100 000g (13b). The pellet was used as a source of “exosomes” (14b).
Characterization of exosomes and MVs from tumor cells. Exosomes and MVs, collected by differential centrifugation, were characterized by TEM (A) and western blot (C). The size of MVs was determined by TEM on 25 measurements from MB-MDA-231 cells and 25 measurements from MCF7 cells. (B) The size of exosomes was determined from 20 measurements from MD-MBA-231 cells, 15 measurements from MCF7 cells, and 15 measurements from A549 cells. Results are presented as mean ± SEM. (C) Cell lysates, exosomes, and MVs (30 μg) from each cell line were analyzed by western blotting for TSG101, CD81, and actinin-4. One representative blot is shown for each cell line. Each blot was repeated 3 times with different samples. ****P ≤ .0001, Mann-Whitney U test (1 tailed).
Characterization of exosomes and MVs from tumor cells. Exosomes and MVs, collected by differential centrifugation, were characterized by TEM (A) and western blot (C). The size of MVs was determined by TEM on 25 measurements from MB-MDA-231 cells and 25 measurements from MCF7 cells. (B) The size of exosomes was determined from 20 measurements from MD-MBA-231 cells, 15 measurements from MCF7 cells, and 15 measurements from A549 cells. Results are presented as mean ± SEM. (C) Cell lysates, exosomes, and MVs (30 μg) from each cell line were analyzed by western blotting for TSG101, CD81, and actinin-4. One representative blot is shown for each cell line. Each blot was repeated 3 times with different samples. ****P ≤ .0001, Mann-Whitney U test (1 tailed).
TSG101 and CD81 have been suggested to be markers for exosomes,29-31 and comparative analysis has suggested that CD81 is ∼10-fold enriched in exosomes compared with MVs, whereas TSG101 is about sevenfold enriched in the exosome fraction.21 As shown in Figure 2C, we detected the presence of TSG101 and CD81 in all 3 fractions (cell lysates, exosomes, and MVs), but, as expected, the exosome preparation was enriched in these markers. In contrast, the MV marker actinin-432 was primarily detected in the MV fraction. Exosomes and MVs were isolated from the conditioned media of all cells that we examined, with the exception of A549 cells, which only yielded EVs in the exosome fraction.
Thrombotic activity of exosomes and MVs
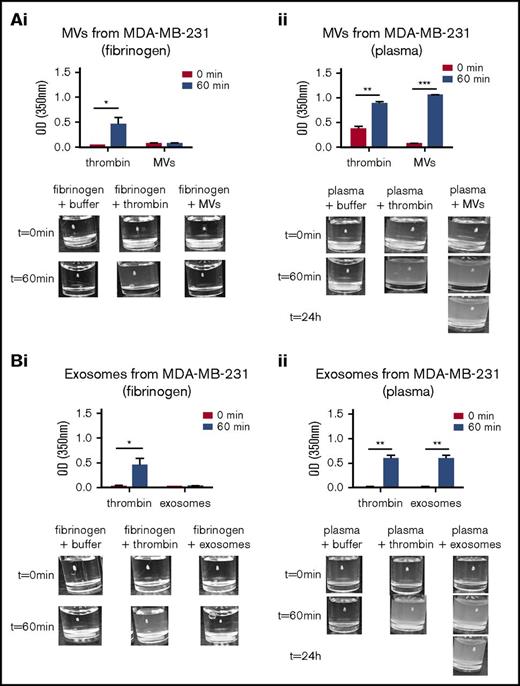
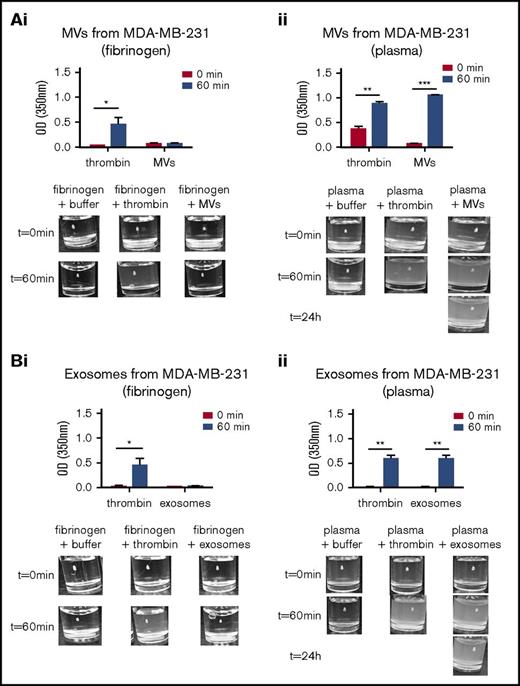
Exosomes and MVs from tumor cells have been reported to have procoagulant activity.21 To examine the universality of this observation, we examined the thrombotic activity of exosomes and MVs from the MDA-MB-231, MCF7, NB4, and A549 cell lines (Figure 3; supplemental Figure 1). The thrombotic activity was determined by measuring the formation of a fibrin clot using pure fibrinogen or human platelet-poor plasma, which contains fibrinogen. As observed in Figure 3Ai and Bi, the addition of thrombin to pure fibrinogen resulted in fibrin clot formation. Exosomes and MVs from MDA-MB-231 cells did not exhibit measurable thrombotic activity when pure fibrinogen was used as substrate (Figure 3Ai,Bi). When thrombin was added to platelet poor-plasma, a fibrin clot was formed. However, when human platelet-poor plasma was used as a source of fibrinogen, the exosomes and MVs from MDA-MB-231 cells stimulated clot formation, and the clot persisted even after 24 hours (Figure 3Aii,Bii). However, the exosomes isolated from MCF7, NB4, and A549 cells did not exhibit thrombotic activity (supplemental Figure 1B,D-E).
Thrombotic activity of exosomes and MVs from MDA-MB-231 cell line. The thrombotic activity of exosomes and MVs was tested using pure fibrin clots or clots produced in platelet-poor plasma. A total of 30 μg of exosomes or MVs was added to pure fibrinogen or platelet-poor plasma. The OD was measured at 350 nm every 2 minutes for 1 hour at 37°C. α-Thrombin was added to the fibrinogen or platelet-poor plasma as a positive control. Thrombotic activity in the presence of fibrinogen is shown for MVs (Ai) and exosomes (Bi) from MDA-MD-231 cells. The thrombotic activity with human plasma is shown for MVs (Aii) and exosomes (Bii) from MDA-MD-231 cells. Representative photographs of the experiment are presented in the lower panels. *P ≤ .05, **P ≤ .01, ***P ≤ .001, Mann-Whitney U test (1 tailed).
Thrombotic activity of exosomes and MVs from MDA-MB-231 cell line. The thrombotic activity of exosomes and MVs was tested using pure fibrin clots or clots produced in platelet-poor plasma. A total of 30 μg of exosomes or MVs was added to pure fibrinogen or platelet-poor plasma. The OD was measured at 350 nm every 2 minutes for 1 hour at 37°C. α-Thrombin was added to the fibrinogen or platelet-poor plasma as a positive control. Thrombotic activity in the presence of fibrinogen is shown for MVs (Ai) and exosomes (Bi) from MDA-MD-231 cells. The thrombotic activity with human plasma is shown for MVs (Aii) and exosomes (Bii) from MDA-MD-231 cells. Representative photographs of the experiment are presented in the lower panels. *P ≤ .05, **P ≤ .01, ***P ≤ .001, Mann-Whitney U test (1 tailed).
MCF7 cells are breast cancer cells of low malignant potential that become more malignant after incubation with PMA.33,34 Exosomes from MCF7 cells or MCF7 cells treated with PMA to increase their malignant potential failed to display thrombotic activity with fibrinogen or human platelet-poor plasma (supplemental Figure 1B). However, thrombotic activity was detected with MVs from MCF7 cells with fibrinogen or human platelet-poor plasma (supplemental Figure 1A), whereas MVs from PMA-treated MCF7 cells displayed thrombotic activity only in the presence of fibrinogen (supplemental Figure 1A).
The treatment of the malignant cell line NB4 with ATRA induces differentiation and a decrease in the malignant phenotype.35 MVs isolated from NB4 cells or ATRA-treated NB4 cells displayed thrombotic activity with human platelet-poor plasma (supplemental Figure 1C2) but not with fibrinogen (supplemental Figure 1C1). In contrast, exosomes isolated from these cells failed to display thrombotic activity (supplemental Figure 1D). Furthermore, exosomes isolated from A549 cells also failed to display thrombotic activity with fibrinogen (supplemental Figure 1E1) or human platelet-poor plasma (supplemental Figure 1E2). Table 1 summarizes the thrombotic activity of exosomes and MVs.
Fibrinolytic activity of exosomes and MVs
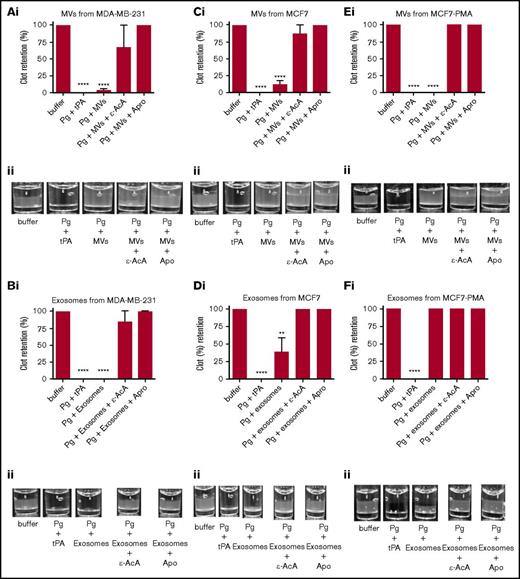
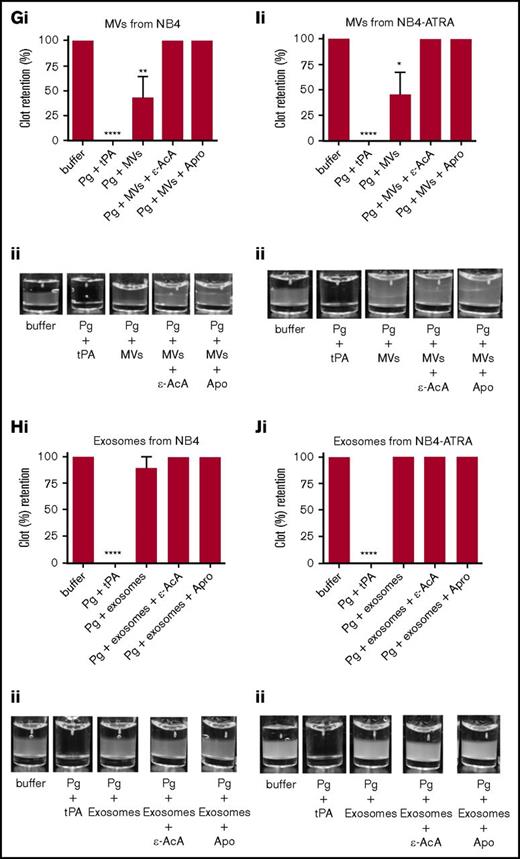
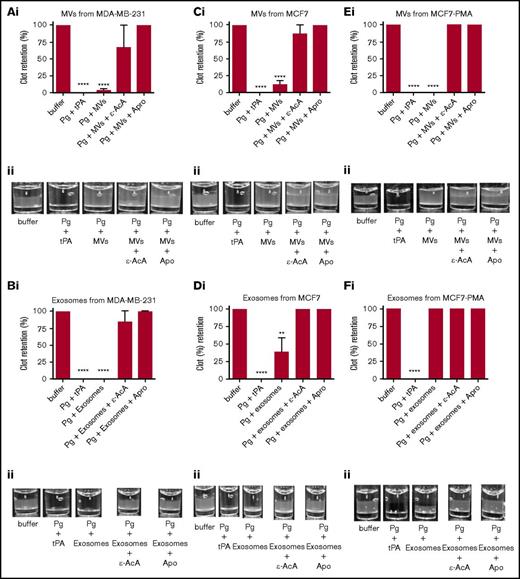
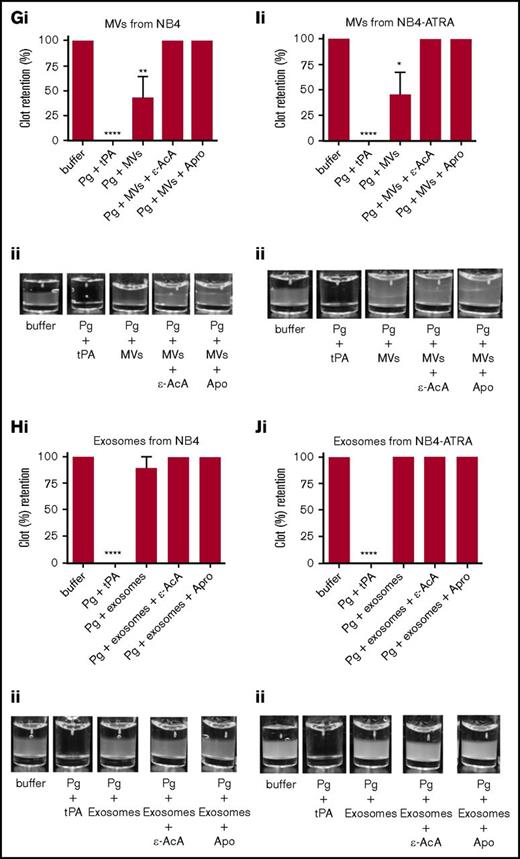
It has been reported that MVs derived from endothelial cells can convert plasminogen into plasmin.36-38 Because we have observed that exosomes and MVs from some tumor cells expressed plasminogen receptors and activators (supplemental Figure 2), it was reasonable to suspect that these vesicles might also display fibrinolytic activity. To measure the fibrinolytic activity in vitro, we measured the ability of the exosomes and MVs to digest a pure fibrin clot that was formed by incubating fibrinogen with thrombin (Figure 4; supplemental Figure 3) or a plasma clot that was formed by incubating human platelet-poor plasma with thrombin (Figure 5; supplemental Figure 4). As a positive control for 100% clot digestion, tPA was added in place of EVs. Most plasminogen receptors use a carboxyl-terminal lysine residue to bind to the lysine-binding domains of plasminogen and tPA.39,40 Therefore, we have used 2 potent antifibrinolytic agents to characterize the fibrinolytic activity of the EVs: ε-AcA, which blocks plasminogen activation by blocking the interaction of the carboxyl-terminal lysine of fibrin and plasminogen receptors with the lysine-binding kringle domains of plasminogen, and aprotinin (Apro), a potent inhibitor of the plasmin active site. Exosomes (Figure 4B) and MVs (Figure 4A) from the MDA-MB-231 cell line were placed on the fibrin clot, followed by the addition of plasminogen. Robust fibrinolytic activity was observed for the exosomes and MVs, which was dramatically inhibited by ε-AcA and Apro. For the MCF7 cell line, the fibrinolytic activity of MVs (Figure 4C) was greater than that of the exosomes (Figure 4D), with >80% of the clot lysed by the MVs. Also, ε-AcA and Apro completely inhibited the fibrinolytic activity of these exosomes and MVs. MVs isolated from MCF7 cells possessed robust fibrinolytic activity that was unaffected by incubation with PMA (Figure 4E), whereas the fibrinolytic activity of the exosomes was decreased after PMA preincubation (Figure 4F). For NB4 cells incubated in the presence or absence of ATRA, we observed that MVs (Figure 4G,I) possessed fibrinolytic activity that was unaffected by ATRA pretreatment, whereas the exosomes (Figure 4H,J) did not possess fibrinolytic activity, and this was unaffected by ATRA pretreatment. These results suggested that changes in malignant potential did not affect the fibrinolytic activity of MVs or exosomes from NB4 cells. Interestingly, exosomes from the malignant cell line A549 did not display fibrinolytic activity (supplemental Figure 3). These results are summarized in Table 2.
Fibrinolytic activity of exosomes and MVs from cancer cells on a fibrin clot. The analysis was performed on MDA-MB-231 cells (A-B), MCF7 cells (C-F), and NB4 cells (G-J) cell lines. Each experiment was repeated with 3 independent EV isolations. A fibrin clot was formed by the incubation of thrombin with pure fibrinogen. Exosomes and MVs (30 μg of protein) were added to the top of clot, followed by the addition of plasminogen (0.15 μM), with or without inhibitor (100 mM ε-AcA or 2.2 μM Apro). For a positive control, plasminogen (0.15 μM) and tPA (25 nM) were added to the fibrin clot. OD was measured at 350 nm every 20 minutes for 18 hours at 37°C. Results are represented as the mean ± SEM (n = 3 independent samples). The percentage of clot retention is shown for MVs (Ai) and exosomes (Bi) from MDA-MB-231 cells, MVs (Ci) and exosomes (Di) from MCF7 cells, MVs (Ei) and exosomes (Fi) from MCF7-PMA cells, MVs (Gi) and exosomes (Hi) from NB4 cells, and MVs (Ii) and exosomes (Ji) from NB4-ATRA cells. A representative photograph of the experiment is shown for MVs (Aii) and exosomes (Bii) from MDA-MB-231 cells, MVs (Cii) and exosomes (Dii) from MCF7 cells, MVs (Eii) and exosomes (Fii) from MCF7-PMA cells, MVs (Gii) and exosomes (Hii) from NB4 cells, and MVs (Iii) and exosomes (Jii) from NB4-ATRA cells. *P ≤ .05, **P ≤ .01, ****P ≤ .0001, Mann-Whitney U test (1 tailed).
Fibrinolytic activity of exosomes and MVs from cancer cells on a fibrin clot. The analysis was performed on MDA-MB-231 cells (A-B), MCF7 cells (C-F), and NB4 cells (G-J) cell lines. Each experiment was repeated with 3 independent EV isolations. A fibrin clot was formed by the incubation of thrombin with pure fibrinogen. Exosomes and MVs (30 μg of protein) were added to the top of clot, followed by the addition of plasminogen (0.15 μM), with or without inhibitor (100 mM ε-AcA or 2.2 μM Apro). For a positive control, plasminogen (0.15 μM) and tPA (25 nM) were added to the fibrin clot. OD was measured at 350 nm every 20 minutes for 18 hours at 37°C. Results are represented as the mean ± SEM (n = 3 independent samples). The percentage of clot retention is shown for MVs (Ai) and exosomes (Bi) from MDA-MB-231 cells, MVs (Ci) and exosomes (Di) from MCF7 cells, MVs (Ei) and exosomes (Fi) from MCF7-PMA cells, MVs (Gi) and exosomes (Hi) from NB4 cells, and MVs (Ii) and exosomes (Ji) from NB4-ATRA cells. A representative photograph of the experiment is shown for MVs (Aii) and exosomes (Bii) from MDA-MB-231 cells, MVs (Cii) and exosomes (Dii) from MCF7 cells, MVs (Eii) and exosomes (Fii) from MCF7-PMA cells, MVs (Gii) and exosomes (Hii) from NB4 cells, and MVs (Iii) and exosomes (Jii) from NB4-ATRA cells. *P ≤ .05, **P ≤ .01, ****P ≤ .0001, Mann-Whitney U test (1 tailed).
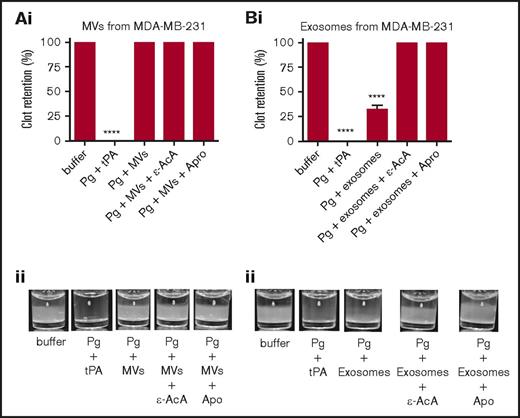
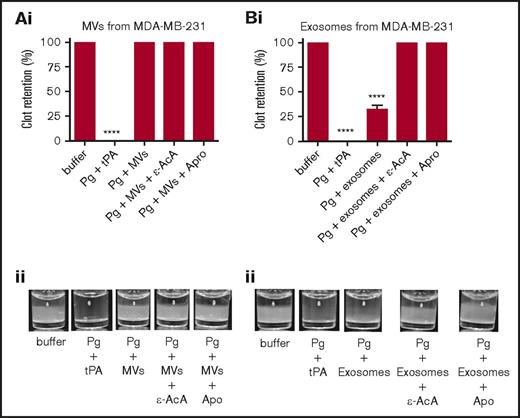
Fibrinolytic activity of exosomes and MVs on a plasma clot. The analysis was performed on MVs from MDA-MB-231 cells (A) and exosomes from MDA-MB-231 cells (B). Each experiment was repeated 3 times with different samples. The plasma clot was formed by incubating human platelet-poor plasma with thrombin. Then, exosomes and MVs (30 μg of protein) were added to the top of clot, followed by plasminogen (0.15 μM), with or without inhibitor (ε-AcA at 100 mM or Apro at 2.2 μM). A positive control was included, in which plasminogen (0.15 μM) and tPA (25 nM) were added directly to the freshly formed clot. The OD was measured at 350 nm every 20 minutes for 18 hours at 37°C. Results are presented as mean ± SEM (n = 3 independent samples). The percentage of clot retention is shown for MVs (Ai) and exosomes (Bi) from MDA-MD-231 cells. A representative photograph of the experiment is shown for MVs (Aii) and exosomes (Bii) from MDA-MD-231 cells. ****P ≤ .0001, Mann-Whitney U test (1 tailed).
Fibrinolytic activity of exosomes and MVs on a plasma clot. The analysis was performed on MVs from MDA-MB-231 cells (A) and exosomes from MDA-MB-231 cells (B). Each experiment was repeated 3 times with different samples. The plasma clot was formed by incubating human platelet-poor plasma with thrombin. Then, exosomes and MVs (30 μg of protein) were added to the top of clot, followed by plasminogen (0.15 μM), with or without inhibitor (ε-AcA at 100 mM or Apro at 2.2 μM). A positive control was included, in which plasminogen (0.15 μM) and tPA (25 nM) were added directly to the freshly formed clot. The OD was measured at 350 nm every 20 minutes for 18 hours at 37°C. Results are presented as mean ± SEM (n = 3 independent samples). The percentage of clot retention is shown for MVs (Ai) and exosomes (Bi) from MDA-MD-231 cells. A representative photograph of the experiment is shown for MVs (Aii) and exosomes (Bii) from MDA-MD-231 cells. ****P ≤ .0001, Mann-Whitney U test (1 tailed).
Fibrin clot architecture is a key determinant of the efficiency of clot lysis, as is the milieu of regulatory proteins and enzymes that form with and surround the clot. To examine fibrinolysis in a more physiological setting, we prepared plasma clots and tested the fibrinolytic activity of the exosomes and MVs against these clots by measuring the ability of EVs to digest the clot. We observed that exosomes (Figure 5B), but not MVs (Figure 5A), from MDA-MB-231 cells digested the plasma clot, and this activity was completely inhibited by ε-AcA and Apro. However, exosomes and MVs from control and PMA-treated MCF7 cells (supplemental Figure 4A-D) and from control and ATRA-treated NB4 cells (supplemental Figure 4E-H), as well as exosomes from A549 cells (supplemental Figure 4I), did not demonstrate fibrinolytic activity against the plasma clot. Results are summarized in Table 2.
We also measured the fibrinolytic activity of the exosomes and MVs using fibrin zymography (supplemental Figure 5). Regions of lysis were observed at molecular weights ∼ 70 and 55 kDa for exosomes and MVs from MDA-MB-231 cells. However, exosomes and MVs from MCF7 cells and exosomes from A549 cells did not demonstrate fibrinolytic activity with the fibrin gel.
Exosomes from the MDA-MB-231 cell line can increase plasmin generation by tumor cells
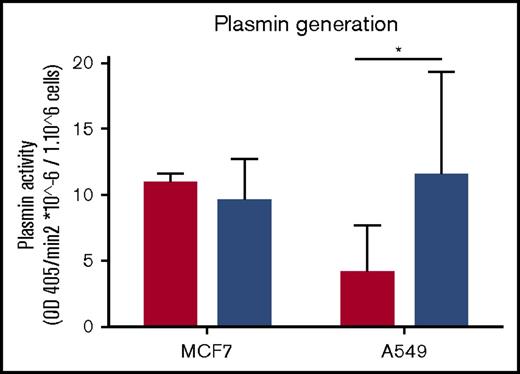
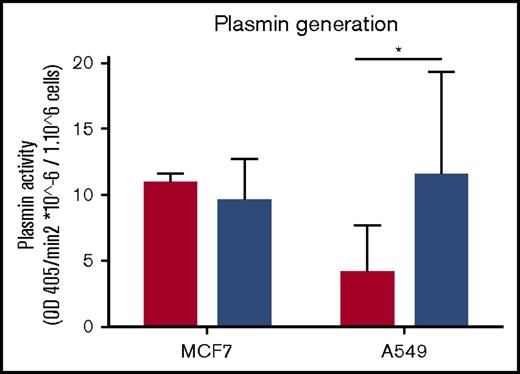
We have observed that exosomes from MDA-MB-231 cells have robust fibrinolytic activity against fibrin and plasma clots (Figures 4B and 5B). We then investigated the possibility that EVs from these cells could influence the fibrinolytic activity of recipient cells. Exosomes from MDA-MB-231 cells were incubated with MCF7 and A549 cells. After 3 days, we compared the plasmin-generating activity of these cells. We observed an increase in plasmin generation when the A549 cell line, but not MCF7 cells, was incubated with exosomes (Figure 6).
Exosomes from MDA-MB-231 cells can increase the plasmin generation of tumor cells. Exosomes were collected from MDA-MB-231 cells and incubated with MCF7 and A549 cells for 2 hours. Subsequently, tumor cells were seeded at 50 000 cells per well for 3 days. Cells were tested for plasmin generation with plasminogen at 0.5 μM final concentration and the substrate S2251 at 0.5 μM final concentration. OD was read at 405 and 600 nm for 4 hours every 4 minutes at 37°C. The experiment was repeated 3 times in triplicate. Results show the rate of plasmin generation per 1 million cells. Cells with no incubation of exosomes are represented by the red bars, and cells with incubation of exosomes are represented by blue bars. *P < .05, Mann-Whitney U test (1 tailed).
Exosomes from MDA-MB-231 cells can increase the plasmin generation of tumor cells. Exosomes were collected from MDA-MB-231 cells and incubated with MCF7 and A549 cells for 2 hours. Subsequently, tumor cells were seeded at 50 000 cells per well for 3 days. Cells were tested for plasmin generation with plasminogen at 0.5 μM final concentration and the substrate S2251 at 0.5 μM final concentration. OD was read at 405 and 600 nm for 4 hours every 4 minutes at 37°C. The experiment was repeated 3 times in triplicate. Results show the rate of plasmin generation per 1 million cells. Cells with no incubation of exosomes are represented by the red bars, and cells with incubation of exosomes are represented by blue bars. *P < .05, Mann-Whitney U test (1 tailed).
Discussion
Cancer is associated with a hypercoagulable state, and this elevated risk for thrombosis is the second leading cause of death in these patients. Tumor cells are known to release EVs into the circulation, which may contribute to the hypercoagulable state. In addition to possessing thrombotic activity, EVs, such as exosomes, possess fibrinolytic activity and contain proteins associated with the regulation of fibrinolytic activity, such as urokinase plasminogen activator.41 However, no study has systematically characterized the thrombotic and fibrinolytic activity of tumor cell–derived exosomes and MVs. Furthermore, many of the reported studies tend to characterize a population of EVs from a single cell line; therefore, it has been difficult to evaluate the universality of these data. In this study, we compared the thrombotic and fibrinolytic activity of exosomes and MVs from several tumor cell lines. Our approach was to examine the ability of these EVs to directly form a fibrin clot from pure fibrinogen or platelet-poor plasma (thrombotic activity) or to lyse a fibrin clot made by incubating pure fibrinogen or platelet-poor plasma with α-thrombin (fibrinolytic activity). We reasoned that a comparison of thrombotic activity in the presence of pure components would reveal whether the EVs were capable of directly converting fibrinogen to fibrin (ie, possessed thrombin activity) or if incubated with plasma could stimulate the conversion of prothrombin, present in the plasma, to active thrombin. Furthermore, fibrinolytic activity against pure fibrin, but not against a plasma clot, would indicate that the fibrinolytic activity measured in vitro might not be active if the MVs were released into the blood. We observed that thrombotic activity was a more common feature of these EVs than fibrinolytic activity. Although thrombotic activity was displayed by MVs, in general, we were unable to detect this activity in the exosomes prepared from the same cancer cells. It has been reported that the biomolecules contained within the EVs from donor cells could influence the growth,42 mobility,23 and metastasis4,41,43 of recipient cells. We have also shown that exosomes from MDA-MB-231 donor cells could enhance the fibrinolytic activity of A549 recipient cells; however, the same donor exosomes failed to alter the fibrinolytic activity of recipient MCF7 cells, suggesting that this may not be a universal phenomena.
Shao et al44 concluded that ultracentrifugation was the most common and most reliable method for the isolation of exosomes and MVs; however, in some cases, density gradient centrifugation was necessary for the removal of large protein aggregates that associated with the exosomes. In our study, we used differential centrifugation to isolate exosomes and MVs. We did not use density gradient centrifugation, because our electron microscopic analysis showed few aggregates associated with the exosomes. Electron microscopic analysis revealed an average size of 43.15 nm for the exosome preparation and 148.3 nm for the MVs. These sizes were similar to what has been described in the literature.1 In addition, we have characterized these EVs by western blot analysis of 3 marker proteins.29-32 We observed that TSG101 and CD81 were enriched in the exosomal fractions, whereas actinin-4 was enriched in the MV fraction, consistent with published studies. However, some reports have suggested that some cells may produce populations of MVs of similar size to exosomes, so we cannot rule out the possibility that the exosomes might contain some contamination by MVs.
We observed that the exosomes and MVs from all cell lines examined, with the exception of A549 cells, possessed fibrinolytic activity against a pure fibrin clot, suggesting that the potential to express fibrinolytic activity is not restricted to exosomes or MVs. The observation that the fibrinolytic activity expressed by these EVs was inhibited by Apro and ε-ACA suggested that the plasmin activity promoted by the EVs was mediated by the carboxyl-terminal lysines of plasminogen receptors in a manner similar to that observed in the parent cell line.
The A549 cells failed to yield a population of MVs. Liu et al45 reported isolation of MVs from A549 cells; however, the EV population that they referred to as MVs was isolated from a 100 000g pellet, which was likely exosomes. Wysoczynski and Ratajczak46 did report on the isolation of MVs from the 20 000g pellet of conditioned media of A549 cells. It is possible that the FBS used by these investigators, which was not precleared of vesicles, was the source of these MVs. We used similar numbers of cells to generate the conditioned media used for isolation of EVs. However, because we failed to isolate MVs from the conditioned media of A549 cells, we concluded that these cells likely produce much fewer MVs than the other cell lines used in this study.
Of the cell lines tested, only the exosomes isolated from MDA-MB-231 cells displayed fibrinolytic activity against a plasma clot. Because a fibrin clot formed in plasma is different in structure from a fibrin clot formed from pure fibrinogen and is also surrounded by plasminogen activator inhibitors, such as plasminogen activator inhibitor-1,47 and plasmin inhibitors, such as α2-antiplasmin,48 it is tempting to speculate that many EVs will not display fibrinolytic activity under physiological conditions. It was also interesting that altering the malignant potential of MCF7 and NB4 cells did not affect the fibrinolytic activity of their MVs. Interestingly, the fibrinolytic activity of the exosomes from PMA-treated MCF7 cells was actually decreased, whereas the exosomes from NB4 cells lacked fibrinolytic activity, and this was unchanged after their malignant potential was decreased by ATRA pretreatment. This suggests that the fibrinolytic activity of EVs is not necessarily increased when the cancer cells become malignant.
We examined EVs for thrombotic activity using pure fibrinogen and platelet-poor plasma. Our observation that MVs from MCF7 cells could convert fibrinogen to fibrin was unexpected and suggested that these EVs possessed thrombin activity or generated thrombin activity from vesicle-associated prothrombinase. We observed that all MVs, with the exception of those isolated from PMA-treated MCF7 cells, possessed procoagulant activity in the presence of plasma. Interestingly, only exosomes from MDA-MD-231 cells possessed procoagulant activity in the presence of plasma. This suggests that, in general, MVs, and not exosomes, are typically procoagulant.
It was interesting that increasing the malignant potential of MCF7 cells resulted in a loss of the procoagulant activity of MVs. In contrast, when the malignant potential was decreased, the procoagulant activity of NB4 cells was unaffected.
Exosomes are known to carry mRNAs that increase the metastatic capacity of less malignant cells in vivo.49 We observed that when MCF7 or A549 cells were treated with exosomes from MDA-MB-231 cells, the plasmin-generating activity of the A549 cell line was increased. This suggests that the ability of exosomes to influence the biochemical parameters of a recipient cell is context dependent. Future studies will be necessary to identify the mechanism by which these exosomes increased the plasmin-generating activity of A549 cells.
In conclusion, our data suggest that, in general, MVs, but not exosomes, possess thrombotic activity. We also show that exosomes and MVs can express fibrinolytic activity against a pure fibrin clot but not against a fibrin clot formed in platelet-poor plasma. Altering the malignant potential of cancer cells does not necessarily alter their thrombotic or fibrinolytic activity. It is difficult to directly link changes in malignant potential with changes in the biochemical properties of the EVs, because any changes that occur might be indirect and not be directly related to changes in the oncogenic event. In addition, our studies show that, in certain cases, exosomes isolated from a metastatic and aggressive cancer cell line are capable of conferring plasmin-generating activity to a recipient cell.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by a grant from the Heart and Stroke Foundation of Canada. L.D. is a trainee of the Cancer Research Training Program of the Beatrice Hunter Cancer Research Institute, with funds provided jointly by the Breast Cancer Society of Canada and the QEII Foundation. A.B. is supported by a trainee award from the Beatrice Hunter Cancer Research Institute, with funds provided by the Harvey Graham Cancer Research Fund as part of The Terry Fox Strategic Health Research Training Program in Cancer Research at the Canadian Institutes of Health Research.
Authorship
Contribution: L.D. designed and performed research, analyzed data, and wrote the manuscript; A.B. designed and performed the research, analyzed data, and edited the manuscript; and D.M.W. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Waisman, Dalhousie University, Departments of Pathology and of Biochemistry & Molecular Biology, Tupper Medical Building, Room 11L2, 5850 College St, P.O. Box 15000, Halifax, NS B3H 4R2, Canada; e-mail: david.waisman@dal.ca.