Background
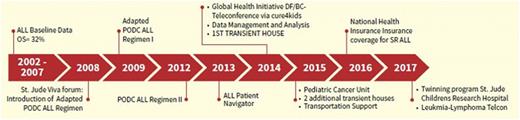
The use of adapted treatment regimens for pediatric cancers in low- and middle-income countries aims to cure as many children as possible while service delivery and infrastructure are being developed. The Philippine General Hospital (PGH) is a government tertiary referral center that sees 350 new children with cancer annually, including acute lymphoblastic leukemia (ALL) at a rate of 70 to 80 cases per year. In 2009, we uniformly adopted the International Society of Paediatric Oncology (SIOP) Paediatric Oncology in Developing Countries (PODC) graduated intensity ALL regimen,1 because our previous 1-year ALL overall survival (OS) rate on National Cancer Institute (NCI) risk-stratified conventional Children’s Cancer Group regimens was only 32%.2 At baseline, there were high rates of toxic death and failure to start/complete therapy (ie, treatment abandonment) key causes of mortality that might be mitigated by less intensive, adapted treatment. Since then, we have also implemented programs on leukemia patient navigation, blood drives, free medicine access, and data management, and we have created a dedicated pediatric cancer unit (Figure 1). In 2012, after successfully implementing the lowest-intensity regimen 1 without excess toxic deaths,3 we intensified treatment to SIOP PODC ALL regimen 2 (Table 1), including delayed intensification and radiation for high-risk (HR) patients. We report the first known prospective cohort of patients managed on this PODC regimen in a low- and middle-income country.
Methods
All newly diagnosed ALL patients seen at PGH from January 2012 to December 2016 were offered treatment with PODC regimen 2; data for the cohort was prospectively captured and retrospectively reviewed at the study endpoint in April 2017.
Results
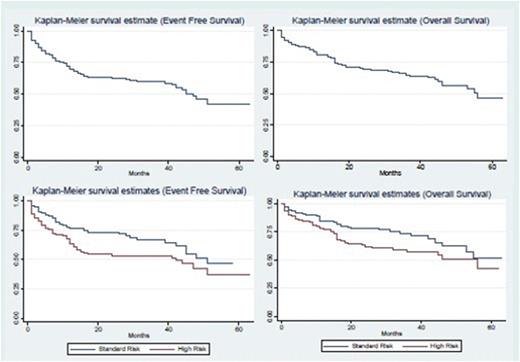
A total of 311 patients were eligible for analyses (46 patients were excluded: 19 received other treatment, 10 refused, 2 had documented transfer of care, and 15 died before the initiation of chemotherapy due to bleeding) (Table 2). Risk classification based on NCI risk stratification showed 47% of cases were standard risk (SR), and 53% were HR. During induction, 253 patients (81%) achieved remission, whereas 19 (6%) died, 30 (10%) abandoned treatment, and 9 (3%) had resistant ALL. Deaths in first complete remission occurred in 8 patients (3.2%), most with delayed intensification. The main causes of toxic death were infection and bleeding. Relapse occurred in 42 patients (67% HR, of which 76% occurred <18 months from the end of therapy): 21 bone marrow (BM), 16 central nervous system (CNS), 2 testicular, and 3 combined bone marrow/CNS relapse. The overall abandonment rate was 20% (62 patients). Of the patients who abandoned treatment, 32 (52%) returned at a mean time of 16 weeks after treatment abandonment. The reasons for abandonment cited include: financial (16%), lack of extended family support (16%), patient refusal to continue treatment (5%), chemotherapy side effects (11%), perception that child is well (7%), shifted to alternative/herbal treatment (3%), and no data (42%). Event-free survival (EFS) rates (abandonment sensitive) at 1, 2, and 4 years were 69%, 63%, and 46%, respectively, whereas OS rates at 1, 2, and 4 years were 80%, 69%, and 56%, respectively. Four-year EFS/OS rates were 51%/62% and 42%/50% for SR and HR cases, respectively (Figure 2).
Comparing the data of treatment-related events over the years, there was a marked decrease in toxic deaths from 44% to 9% and a marked decrease in upfront treatment abandonment (treatment refusal) from 46% to 3%, and there is ongoing work to target on-therapy treatment abandonment, which remains at 20% (Figure 3, Table 3).
Comparison of treatment refusal, on-therapy abandonment, and toxic death over the years.
Comparison of treatment refusal, on-therapy abandonment, and toxic death over the years.
Conclusions and future directions
The uniform adoption of the SIOP PODC ALL adapted intensity regimen 2 is feasible in a resource-limited tertiary referral setting and, coupled with improvements in supportive care, was associated with an improved OS rate and fewer toxic deaths. The leading cause of treatment failure is abandonment, which must be addressed by institutional and national programs. With the recent twinning program with St. Jude Children’s Research Hospital and bimonthly leukemia and lymphoma meetings, we hope to develop further initiatives, such as improved risk stratification to include routine cytogenetics, treatment intensification for NCI HR patients, salvage therapy for relapsed ALL, better CNS control modalities, and pediatric oncology nursing training.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joliza Patricia Caneba, Section of Hematology and Oncology, Department of Pediatrics, Philippine General Hospital, Manila, Philippines; e-mail: jolizamd@gmail.com.