Key Points
The persistence of a CEBPA mutation at the time of complete remission warrants germ line analysis.
Not all patients harboring germ line CEBPA mutations have a family history of AML.
Introduction
Familial acute myeloid leukemia (AML) with mutated CCAAT/enhancer-binding protein-α (CEBPA) is caused by germ line CEBPA mutations. These mutations are most commonly found near the N terminus and result in aberrant increased production of a short protein isoform termed p30.1-9 This isoform interferes with the full-length protein in a dominant-negative manner and promotes AML with near complete penetrance.10,11 Recently, a C-terminal germ line CEBPA mutation, resulting in loss-of-function but not dominant-negative activity, was described in a large family with multiple cases of AML.12 Penetrance of AML was lower, suggesting that although loss-of-function CEBPA mutations can predispose to AML, they might be weaker than their N-terminal dominant-negative counterparts. Here, we describe a second family with a germ line CEBPA mutation downstream of the p30 start site, in which only the index case is known to have developed AML. This strengthens the idea that CEBPA germ line mutations might not all be highly penetrant and should be screened for in select patients irrespective of family history.
Case description
The patient was a 36-year-old woman who presented at 35 weeks’ gestation with pancytopenia. She had experienced 5 weeks of concerning symptoms, including hemoptysis, dyspnea, low-grade fevers, fatigue, sore throat, gum swelling/bleeding, epistaxis, and a petechial rash. On admission, her white blood cell count was 5.5 × 109/L (absolute neutrophil count, 0.1 × 109/L); hemoglobin, 6.0 g/dL; and platelets, 4.0 × 109/L. There were 46% blasts in the peripheral blood staining positive for CD34, CD13, CD33, CD117, HLA-DR, CD123, CD15 (partial), CD7 (partial), terminal deoxynucleotidyl transferase (partial and weak), and cytoplasmic myeloperoxidase, confirming AML. Cytogenetics were normal and molecular testing revealed 2 heterozygous mutations in the CEBPA gene c.68dupC (p.H24fs*84) and c.442G>T (p.Glu148*). There was no family history of AML. The decision was made to induce labor prior to initiating AML-directed therapy and she delivered a healthy male infant 48 hours after diagnosis. Soon after delivery, the patient received induction chemotherapy with cytarabine 1500 mg/m2 IV on days 1 to 4 and idarubicin 12 mg/m2 IV on days 1 to 3. Her induction course was complicated by gram-negative sepsis with Roseomonas methylobacterium as well as hypoxemic respiratory failure due to metapneumoviral pneumonia, necessitating mechanical ventilation and transfer to the intensive care unit. After several days in the intensive care unit, her peripheral counts recovered, she was extubated, and she was transferred back to the floor. Bone marrow examination on day 29 postinduction was consistent with the achievement of complete remission (CR). Despite achievement of CR, molecular testing of bone marrow mononuclear cells revealed persistence of the c.442G>T CEBPA mutation. Notably, the c.68dupC CEBPA mutation was absent. Our patient was treated with 4 cycles of consolidation chemotherapy and remains in CR 3.5 years from diagnosis.
Methods
Preparation of skin fibroblasts and CEBPA sequencing
A 5-mm punch skin biopsy was washed in a centrifuge tube with Hanks balanced salt solution, transferred into a Petri dish, and minced. Minced tissue was digested overnight in 0.125% collagenase type 1 solution (25 mg; Sigma), and prepared in Amniomax complete medium (Invitrogen) with penicillin/streptomycin (concentration, 10 000 U/mL; Invitrogen). Dissociated cells were centrifuged at 1000 rpm, transferred into a tissue-culture flask with complete Amniomax medium to allow cell attachment, and kept at 37°C in a CO2 incubator. Genomic DNA was extracted from cultured fibroblasts using the QIAamp system (Qiagen Inc). The region around codon 442 was amplified using polymerase chain reaction (PCR) in a total volume of 12 μL with primers at a final concentration of 1 μM each, 50 μM of each deoxynucleotide triphosphate, 10% dimethyl sulfoxide, 0.75 U of HotStar Taq DNA polymerase, 1.2 μL of the 10× buffer provided by the enzyme manufacturer (Qiagen Inc), and 50 ng of genomic DNA. The gene-specific parts of the upstream and downstream primers were 5′-TTCAACGACGAGTTCCTGGCCGA-3′ and 5′-GCGGCGGCTGGTAAGGGAAGA-3′, respectively. The PCR primers were synthesized (Integrated DNA Technologies) with M13 tail sequences appended to the 5′ end to facilitate sequencing. The reactions were cycled 35 times between 95°C for 10 seconds, 64°C for 20 seconds, and 72°C for 50 seconds, preceded by 10 minutes at 95°C, and followed by 5 minutes at 72°C. The PCR products were treated with ExoSap (Amersham Biosciences) to remove the primers and deoxynucleotide triphosphates, then sequenced using the M13 tails as sequencing primers and Applied Biosystems BigDye Terminator v.3.1 chemistry. The sequencing reactions were purified using the CleanSeq system (Agencourt Bioscience) and then resolved by capillary electrophoresis on the ABI 3500XL Genetic Analyzer. Mutations were confirmed by repeat analysis starting with the PCR step. Genbank accession number NM_004364.4 was used for the reference sequence to identify mutations.
Whole-exome sequencing and analysis of sequence variations
Purified genomic DNA was quantified using the Qubit Flourometer (ThermoFisher) and quality was assessed with the gDNA Tapestation Assay (Agilent Technologies). Whole-exome libraries were generated using SureSelectXT Whole Exome v5 (+UTRs) per the manufacturer’s recommendations (Agilent Technologies). Briefly, 4 μg of good-quality genomic DNA was sheared with Covaris S2 to an average peak size of 250 bp followed by end repair, polyadenylation, Illumina adaptor ligation, and purification by AmpureXP (Beckman Coulter). Library amplification was carried out using 250 ng of purified DNA and 4 cycles of PCR followed by AmpureXP purification (Beckman Coulter). Exome probe hybridization capture was performed per the manufacturer’s protocols. Exome target-enriched samples were quantified using the Qubit Flourometer (ThermoFisher) and library sizing was assessed with the high-sensitivity Bioanalyzer 2100 DNA Assay (Agilent Technologies). Whole-exome libraries were normalized, pooled, and sequenced using 2 × 100 pair end reads configuration on a HiSeq2500 (Illumina).
Paired-end fastq files were produced using CASAVA (v.1.8.4; Illumina) and aligned to build 37 (hg19) of the human reference genome with bwa (bwa mem v0.7.15). Alignments were postprocessed using the Genome Analysis Tool Kit (GATK v3.6)13,14 best practice recommendations. Paired tumor-normal variant calling was performed using VarDictJava (v1.4.8)15 with a minimum allele frequency of 0.1 and a maximum P value of .9. Bcftools (v1.3) was used to soft-filter nonsomatic mutations and somatic mutations were filtered using strategies implemented in bcbio-nextgen (https://github.com/chapmanb/bcbio-nextgen) for VarDict: ((allele frequency × read depth < 6) && ((mean mapping quality < 55.0 && mean number of mismatches > 1.0) || (mean mapping quality < 60.0 && mean number of mismatches > 2.0) || (read depth < 10) || (alternate allele quality < 45))) and allele frequency < 0.2 && alternate allele quality < 55 && somatic variant P value > 0.06. In addition, variants were filtered if the tumor’s alternate or the normal’s reference genotype likelihood were >3.5. Passing variants were annotated with Oncotator v1.9.0.016 and prioritized if the mutation had a high impact on protein function (eg, missense, nonsense, frame shifts, and splice site variants) for genes mutated in at least 1 hematopoietic neoplasm in the Catalogue of Somatic Mutations in Cancer (COSMIC) database17 and was not found in the Exome Aggregation Consortium (ExAC) data set (v0.3.1).18 National Center for Biotechnology Information (NCBI) reference sequences used for identification of mutations were as follows: CEBPA, NM_004364.4; TET2, NM_001127208.2; KCNT2, NM_198503.3; IKBKE, NM_014002.3; KDR, NM_002253.2; KMT2C, NM_170606.2; PDZRN4, NM_001164595.1.
Results and discussion
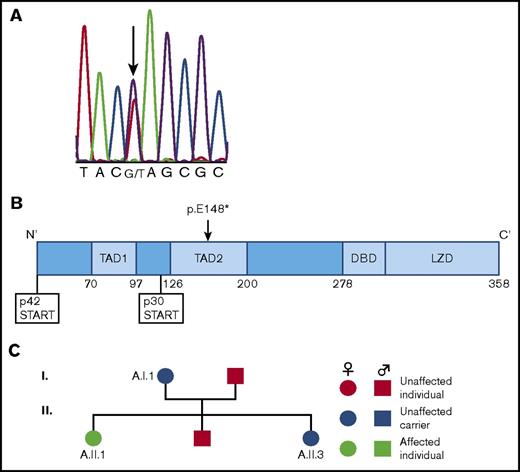
Given the persistence of CEBPA c.442G>T at the time of CR, we postulated that it was a germ line mutation. We confirmed this by conducting Sanger sequencing of the CEBPA gene in genomic DNA isolated from skin fibroblasts (Figure 1A). This is a nonsense mutation located downstream of the p30 start codon (Figure 1B), and thus unique from the majority of previously described germ line CEBPA mutations that lie upstream of this codon. Testing of the patient’s immediate family members confirmed that both her mother (aged 66 years) and sister (aged 37 years) carried the same c.442G>T CEBPA mutation, whereas her father and brother did not (Figure 1C). To date, neither the patient’s mother nor sister have developed a myeloid malignancy.
Discovery of a family with a novel CEBPA germ line mutation. (A) Sequencing chromatogram demonstrating the germ line CEBPA mutation (c.442G>T) present in our patient. Sanger sequencing was performed on genomic DNA isolated from cultured skin fibroblasts taken from our patient while she was in CR. (B) Location of the germ line CEBPA mutation (p.E148*) discovered here relative to the p30 start codon. This mutation is unusual relative to the majority of previously discovered germ line CEBPA mutations in that it is downstream of the p30 start codon. (C) Pedigree of family members showing affected patient (A.II.1) and unaffected carriers (A.I.1, A.II.3).
Discovery of a family with a novel CEBPA germ line mutation. (A) Sequencing chromatogram demonstrating the germ line CEBPA mutation (c.442G>T) present in our patient. Sanger sequencing was performed on genomic DNA isolated from cultured skin fibroblasts taken from our patient while she was in CR. (B) Location of the germ line CEBPA mutation (p.E148*) discovered here relative to the p30 start codon. This mutation is unusual relative to the majority of previously discovered germ line CEBPA mutations in that it is downstream of the p30 start codon. (C) Pedigree of family members showing affected patient (A.II.1) and unaffected carriers (A.I.1, A.II.3).
To identify somatic mutations that might have contributed to the development of AML in our patient, we conducted whole-exome sequencing (WES) of AML cells collected at diagnosis, using skin fibroblasts as a control. We focused analysis on somatic variants predicted to have a high impact on protein function in genes reported as mutated in at least 1 hematopoietic neoplasm (per the COSMIC database). In addition to CEBPA c.68dupC, this analysis identified 7 somatic variants, of which CEBPA and TET2 are well established as being relevant to AML pathogenesis19,20 (Table 1). To determine the status of these mutations posttreatment, we conducted WES of bone marrow mononuclear cells following induction and 4 cycles of consolidation; all 7 somatic variants were absent, suggesting that chemotherapy treatment had eradicated the leukemic clone.
This study highlights the importance of considering germ line testing in patients with biallelic CEBPA mutations, irrespective of family history. Recent studies suggest that germ line CEBPA mutations are not that rare; among biallelic CEBPA-mutated AML patients, their prevalence is between 7% and 11%.4,21 Although it is clear that germ line CEBPA mutations upstream of the p30 start codon cause familial AML with very high if not complete penetrance,10,11 this study and others suggest that germ line CEBPA mutations downstream of this codon are associated with a lower penetrance of AML. For example, in a study of 71 CEBPA-mutated AML patients, 3 harbored C-terminal germ line CEBPA mutations and none had a family history of AML21 ; however, family members were not tested for the presence of these mutations, limiting conclusions regarding penetrance. Moreover, in a large family harboring a C-terminal germ line CEBPA mutation, the prevalence of AML was only 46%.12 The fact that our patient’s mother has lived to 66 years of age without developing AML suggests that the germ line CEBPA mutation reported here is relatively weak; however, further follow-up of this family and others will be required to definitively determine how the structural nature of different germ line CEBPA mutations relates to AML penetrance.
Definitive clinical guidelines for the screening and management of individuals with germ line CEBPA mutations do not currently exist. Thus, our care of this patient was informed by existing literature and expert opinion in the field of AML predisposition syndromes. Given accumulating evidence that patients with familial CEBPA AML have a good prognosis with chemotherapy alone, we chose to forego allogeneic stem cell transplant in first CR.9 However, should she relapse and require an allogeneic stem cell transplant, knowledge of her family’s mutation status is critical to exclude carriers as potential stem cell donors because donor-derived AML has been reported.22 With regards to the management of unaffected family members, proposed recommendations highlight the importance of pre- and posttest genetic counseling, with formulation of a surveillance plan for those who test positively.23,24 In this case, both confirmed carriers have had genetic counseling and are being monitored with complete blood counts every 6 months, whereas our patient and her husband have declined testing for the mutation in their 3 healthy children.
Acknowledgments
The authors extend a heartfelt thank you to the patient and her family members who made this work possible.
This work was supported by startup funding from the Division of Hematology/Oncology at the University of Rochester Medical Center.
Authorship
Contribution: J.R. drafted the initial manuscript; M.B. and U.S. prepared DNA samples for WES; P.G.R. conducted Sanger sequencing of CEBPA; A.I. cultured skin fibroblasts; J.R.M., A.C., and J.M.A. conducted WES and analysis; G.F. revised the manuscript; J.H.M. conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason H. Mendler, University of Rochester Medical Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: jason_mendler@urmc.rochester.edu.