Key Points
Uptake of ADAMTS13 by macrophages is not dependent on the macrophage mannose receptor.
CD163 promotes internalization of ADAMTS13 by macrophages.
Abstract
Internalization of ADAMTS13 by macrophages may contribute to its clearance from the circulation. Here we investigated endocytic mechanisms that contribute to the uptake of ADAMTS13 by macrophages. Human monocyte-derived macrophages were used to monitor the uptake of fluorescently labeled recombinant ADAMTS13 by flow cytometry. Internalization of ADAMTS13 was blocked upon addition of the cell-permeable dynamin inhibitor dynasore. Partial blocking of ADAMTS13 uptake was observed by using mannan; however, uptake was not affected by an antibody that blocked binding to the macrophage mannose receptor CD206, which suggests that other endocytic receptors contribute to the internalization of ADAMTS13 by macrophages. A pull-down with ADAMTS13 and subsequent mass spectrometric analysis identified the class I scavenger receptor CD163 as a candidate receptor for ADAMTS13. Blocking experiments with monoclonal anti-CD163 antibody EDHu-1 resulted in decreased ADAMTS13 internalization by macrophages. Pronounced inhibition of ADAMTS13 uptake by EDHu-1 was observed in CD163 high-expressing macrophages. In agreement with these findings, CD163-expressing Chinese hamster ovary cells were capable of rapidly internalizing ADAMTS13. Surface plasmon resonance revealed binding of ADAMTS13 to scavenger receptor cysteine-rich domains 1-9 and 1-5 of CD163. Taken together, our data identify CD163 as a major endocytic receptor for ADAMTS13 on macrophages.
Introduction
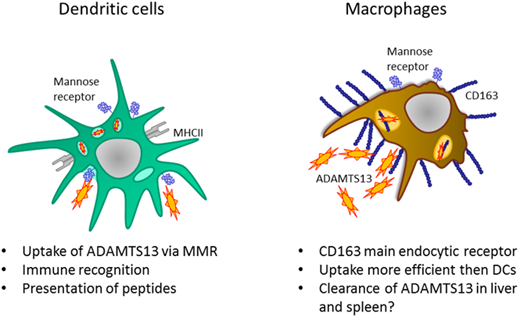
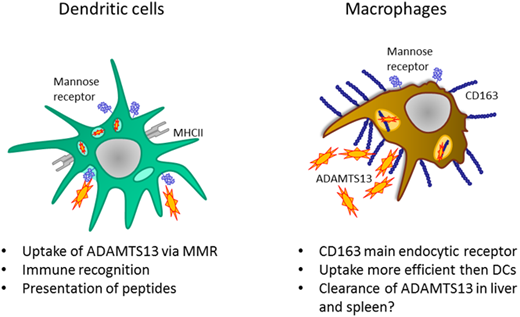
ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 repeat member 13) is a metalloproteinase that regulates the proteolysis of ultra-large von Willebrand factor multimers.1 ADAMTS13 consists of a metallo, disintegrin, cysteine-rich spacer, 2 CUB domains, and 8 thrombospondin type 1 repeats.2 ADAMTS13 is synthesized in hepatic stellate cells in the liver and in vascular endothelial cells.3-5 The plasma concentration of ADAMTS13 has been shown to range between 0.5 and 1 µg/mL.6 A genetic deficiency of ADAMTS13 results in congenital thrombotic thrombocytopenic purpura (TTP), a thrombotic microangiopathy caused by insufficient processing of ultra-large von Willebrand factor multimers.7 Development of auto-antibodies toward ADAMTS13 occurs at low frequency in previously healthy individuals and gives rise to acquired or autoimmune TTP.1 Anti-ADAMTS13 antibodies are predominantly of subclasses immunoglobulin G1 (IgG1) and IgG4,2,8-12 and the variable domains are extensively modified by somatic hypermutation.8,12,13 Isotype switching and somatic hypermutation of antibodies both depend on the presence of antigen-specific CD4+ T cells. Three independent studies have shown that HLA-DRB1*11 is a risk factor for acquired TTP, which further implicates CD4+ T cells in the pathogenesis of this disease.13-15 We have previously shown that ADAMTS13 is endocytosed by antigen-presenting cells such as dendritic cells (DCs) by the macrophage mannose receptor (MMR).16 After its intracellular processing, a restricted set of ADAMTS13-derived peptides is presented on major histocompatibility complex class II for presentation to CD4+ T cells.17 These findings reveal a critical role for DCs in the initiation of CD4+ T-cell responses to ADAMTS13 in patients with acquired TTP.
The cellular mechanisms contributing to the clearance of ADAMTS13 from the circulation have not yet been defined. The half-life of ADAMTS13 in patients with TTP who have been transfused with fresh frozen plasma ranged from 2 to 3 days.18 Macrophages can recognize both microbial structures and endogenous ligands through the expression of a large repertoire of pattern recognition receptors.19-21 Tissue-resident macrophages such as Kupffer cells residing in the liver and splenic macrophages have been implicated in clearance of a variety of plasma proteins by expression of multiligand endocytic receptors.22,23 In this study, we used human monocyte-derived macrophages (MDMs) to identify potential clearance receptors for ADAMTS13. Our findings show that macrophages very efficiently endocytose ADAMTS13 when compared with DCs. We show that endocytosis of ADAMTS13 by human MDMs does not depend on the MMR as previously described for DCs. Instead, scavenger receptor cysteine-rich (SRCR) type 1 protein M130 (CD163) was identified as a candidate receptor for ADAMTS13. We show that CD163 mediates the uptake of ADAMTS13 by macrophages, which implicates CD163 in the clearance of ADAMTS13 from the circulation.
Materials and methods
Materials
The following antibodies were used in this study: allophycocyanin-conjugated mouse monoclonal anti-CD206 (mannose receptor [MR], BD Biosciences, San Jose, CA), allophycocyanin-conjugated mouse IgG isotype control, mouse monoclonal anti-early endosome antigen (EEA1, BD Biosciences), mouse IgG isotype controls conjugated with fluorescein isothiocyanate and phycoerythrin (Dako, Glostrup, Denmark), and Alexa Fluor 488–conjugated anti-mouse antibody (Invitrogen, Carlsbad, CA). The monoclonal anti-MMR antibody clone 15.2 (BioLegend, San Diego, CA), monoclonal blocking antibody anti-CD36 (Abcam, Cambridge, United Kingdom), and monoclonal control antibody (BioLegend) were used for blocking experiments. EDHu-1, -2, -3, -4 antibodies targeting different epitopes of CD163 have been previously characterized.24 A polyclonal anti-CD163 antibody has been described previously.25
Recombinant ADAMTS13 and Alexa Fluor 488 labeling
Recombinant wild-type full-length ADAMTS13 containing a carboxy-terminal V5 epitope and polyhistadine tag was expressed in stably transfected HEK293 cells. Culture supernatant was harvested and concentrated. Subsequent purification was performed by using the polyhistadine tag at the C terminus of the protein using the ÄKTA purifier system (GE Healthcare) and an Ni-NTA column (GE Healthcare) essentially as described.16,26 The purified protein was collected and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by silver staining or immunoblotting. For flow cytometry and confocal microscopy, ADAMTS13 was directly labeled with Alexa Fluor 488 (AF488) by using the Alexa Fluor 488 Microscale Labeling Kit according to the manufacturer’s protocol (Thermo Fisher Scientific). One ADAMTS13 molecule contained on average 2.4 AF488 molecules (ADAMTS13-AF488).
Generation of immature MDMs, DCs, and uptake of ADAMTS13
Blood from healthy individuals was drawn in accordance with Dutch regulations and with approval from the Sanquin Ethical Advisory Board in accordance with the Declaration of Helsinki. Human monocytes were isolated from peripheral blood mononuclear cells from healthy individuals by using anti-CD14+ magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were differentiated into MDMs by culturing monocytes in RPMI 1640 medium (Lonza, Breda, The Netherlands), supplemented with 10% fetal calf serum for 6 days in the presence of 50 ng/mL of macrophage colony-stimulating factor (M-CSF) (Pepro Tech, London, United Kingdom). Macrophages were cultured for 4 days in RPMI-1640 supplemented with M-CSF, and on day 4, 50 ng/mL interleukin-10 (IL-10) was added to increase the expression of CD163. To generate DCs, cells were cultured in CellGro medium in the presence of 800 U/mL IL-4 and 1000 U/mL granulocyte-macrophage CSF (GM-CSF; CellGenix, Freiburg, Germany) for 6 days. For uptake experiments, 2 × 105 MDMs or DCs were incubated with 25 nM of ADAMTS13-AF488 at 37°C for 1 hour in 50 µL serum-free RPMI medium (MDMs) or CellGro (DCs). Uptake was analyzed by flow cytometry (Fortessa flow cytometer, BD Biosciences). Uptake of ADAMTS13 was also analyzed by confocal microscopy. ADAMTS13-AF488 (100 nM) was added to 0.4 × 106 MDMs that were allowed to adhere to fibronectin-coated glass slides for 2 hours in 100 µL serum-free RPMI medium, and uptake proceeded for 1 hour at 37°C. Cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in phosphate-buffered saline (PBS; Fresenius Kabi, Zeist, The Netherlands) for 15 minutes and subsequently quenched with PBS containing 50 mM NH4Cl and 0.2% saponin. Cells were stained in PBS supplemented with 0.2% gelatin, 0.02% azide, and 0.2% saponin, with antibodies against EEA1, MMR (clone 15.2), CD163 (clone GHJ/61), and subsequently with Alexa568-conjugated or Alexa633-conjugated secondary antibodies (Molecular Probes, Breda, The Netherlands). Next, coverslips were mounted with Prolong gold 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and viewed by confocal microscopy using a Zeiss LSM 510 microscope (Carl Zeiss, Heidelberg, Germany) or Leica SP8 (Leica Microsystems GmbH, Wetzlar, Germany).
Blocking experiments
To analyze the mechanisms of binding and uptake of ADAMTS13-AF488, MDMs were preincubated for 20 minutes at 37°C with 80 µM dynasore (Sigma Aldrich, Zwijndrecht, The Netherlands) and 5 mM EDTA. Uptake of ADAMTS13-AF488 was also monitored after 1 hour of preincubation of the cells with mannan (1 mg/mL or 100 μg/mL; Sigma Aldrich), d-mannose (10 mM; Sigma Aldrich), GlcNac (10 mM; Sigma Aldrich), d-galactose (10 mM; Sigma Aldrich), or with monoclonal antibody clone 15.2 (50 μg/mL) directed against MR. Blockage of uptake and/or binding was also analyzed after the cells were preincubated for 1 hour with different molecular-weight polymers of dextran sulfate (500 kDa, 9-20 kDa, 6-10 kDa, and 5 kDa were used at concentrations of 100 µg/mL to 1 mg/mL; Sigma Aldrich), heparin (1 mg/mL or 100 μg/mL; Sigma Aldrich), fucoidan (500 μg/mL; Sigma Aldrich), polyinosinic acid (poly-I); 200 μg/mL; Sigma Aldrich), and polycytidylic acid (poly-C); 200 μg/mL, Sigma Aldrich). Monoclonal antibodies EDHu-1, -2, -3, and -4 and a rabbit polyclonal α-CD163 antibody at concentrations ranging from 5 to 50 μg/mL were used to study the involvement of CD163 in ADAMTS13 uptake.
Surface plasmon resonance analysis of ADAMTS13-CD163 interaction
Binding of ADAMTS13 to CD163 was determined by using 2 recombinant constructs: CD163 SRCR domain 1-9 (SRCR1-9) and SRCR1-5.27 Surface plasmon resonance analysis was performed on a BIAcore T200 biosensor (GE Healthcare). SRCR1-9 or SRCR1-5 were immobilized to a CM5 sensor chip (∼9000 response units [RU]) in immobilization buffer (10 mM sodium acetate [pH 4.0]). Different ADAMTS13 concentrations (100-800 nM) in flow buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150 mM NaCl, 2.0 mM CaCl2, and 0.005% Tween-20 [pH 7.5]) were flowed over the sensor chip at a flow rate of 30 μL/min for 120 seconds at 25°C and a dissociation time of 600 seconds. The sensorgrams were corrected for background binding to the chip. Regeneration was performed by flowing over regeneration solution containing 40 mM EDTA and 1 M NaCl for 30 seconds.
Results
ADAMTS13 is internalized by MDMs
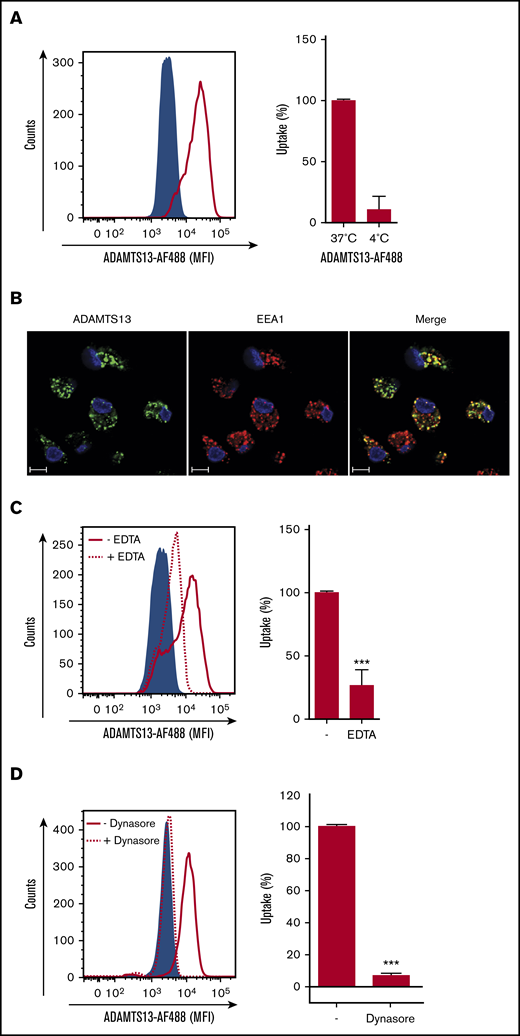
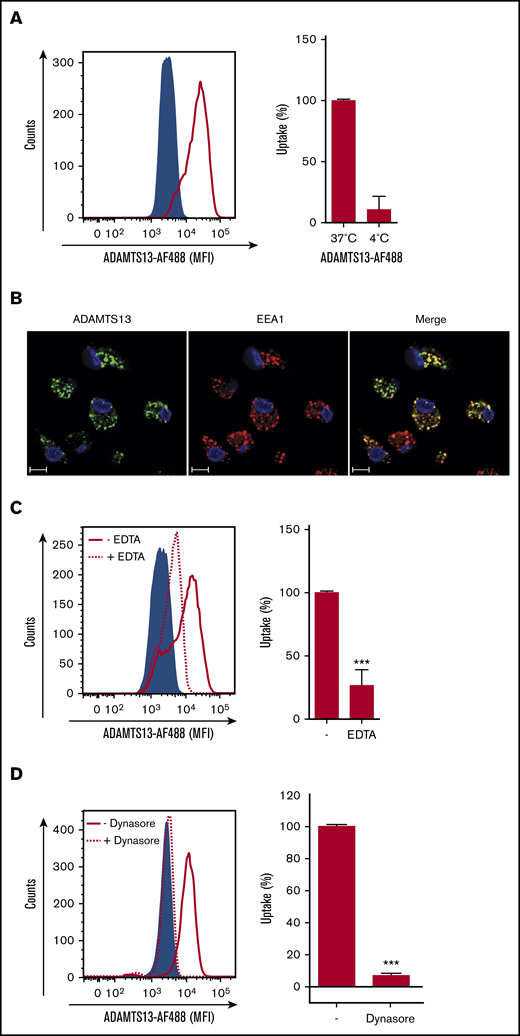
To examine the uptake of ADAMTS13 by human macrophages, monocytes from healthy donors were isolated and differentiated into macrophages. Macrophages were incubated with 25 nM of ADAMTS13-AF488 for 1 hour at 37°C or 4°C. As depicted in Figure 1A, an increase in the mean fluorescent intensity was obtained when cells were incubated at 37°C. Only limited uptake of ADAMTS13-AF488 was detected at 4°C (Figure 1A). Incubation with various concentrations of ADAMTS13-AF488, as well as a prolonged incubation time with macrophages revealed that uptake of ADAMTS13 was concentration and time dependent (supplemental Figure 1A). Endocytosis of ADAMTS13-AF488 by macrophages was confirmed by confocal microscopy. Macrophages were incubated for 30 minutes with 100 nM of ADAMTS13-AF488 at 37°C. ADAMTS13 co-localized with early endosomal marker EEA1, which indicates that ADAMTS13 is present within endocytic vesicles (Figure 1B). Preincubation with 5 mM EDTA resulted in a significant decline in uptake of ADAMTS13 (Figure 1C). Taken together, these data show that ADAMTS13 is efficiently internalized by macrophages. Previously, we showed that recombinant ADAMTS13 (rADAMTS13) is endocytosed by DCs through a receptor-mediated pathway.16 To analyze the mechanism for uptake of rADAMTS13, macrophages were preincubated for 20 minutes at 37°C with dynasore (80 μM) before the addition of ADAMTS13-AF488 (Figure 1D). Dynasore blocks the GTPases dynamin-1 and dynamin-2, which are involved in membrane fission, thereby promoting clathrin-mediated endocytosis as well as other endocytic processes.28,29 Uptake of ADAMTS13-AF488 was completely inhibited when macrophages were preincubated with dynasore (Figure 1D). These data indicate that ADAMTS13 is efficiently internalized by macrophages.
ADAMTS13 endocytosis by MDMs is receptor mediated. (A) ADAMTS13-AF488 was incubated with MDMs for 1 hour at 37°C or 4°C. Subsequently, cells were analyzed by flow cytometry. (A) Left panel shows ADAMTS13-AF488 uptake at 37°C (solid red line). The sample incubated without ADAMTS13-AF488 is shown by the blue area. Right panel displays reduced uptake of ADAMTS13-AF488 at 4°C. (B) ADAMTS13-AF488 (green) was added to MDMs for 30 minutes at 37°C. MDMs were stained for EEA1 (Alexa Fluor 568; red) and nuclei (DAPI; blue). Scale bars are 10 μm. (C) Addition of EDTA reduces the uptake of ADAMTS13-AF488 (dotted line), control cells incubated without ADAMTS13-AF488 are shown by the blue area, and ADAMTS13-AF488 uptake is shown by the solid red line. Right panel quantifies the reduced uptake of ADAMTS13-AF488 in the presence of 5 mM EDTA. (D) Preincubation with dynasore for 20 minutes reduces the uptake of ADAMTS13-AF488 by 90% (dotted line). Control cells incubated without ADAMTS13-AF488 are shown by the blue area, and ADAMTS13 uptake is shown by the red solid line. Right panel quantifies the reduced uptake of ADAMTS13-AF488 upon incubation with dynasore. Bar graphs represent data from 3 independent experiments ± standard deviation (SD). Data are expressed as percentage of mean fluorescent intensity (MFI) at 37°C; 100% corresponds to the highest MFI observed for individual experiments. ***P < .001 (Student t test).
ADAMTS13 endocytosis by MDMs is receptor mediated. (A) ADAMTS13-AF488 was incubated with MDMs for 1 hour at 37°C or 4°C. Subsequently, cells were analyzed by flow cytometry. (A) Left panel shows ADAMTS13-AF488 uptake at 37°C (solid red line). The sample incubated without ADAMTS13-AF488 is shown by the blue area. Right panel displays reduced uptake of ADAMTS13-AF488 at 4°C. (B) ADAMTS13-AF488 (green) was added to MDMs for 30 minutes at 37°C. MDMs were stained for EEA1 (Alexa Fluor 568; red) and nuclei (DAPI; blue). Scale bars are 10 μm. (C) Addition of EDTA reduces the uptake of ADAMTS13-AF488 (dotted line), control cells incubated without ADAMTS13-AF488 are shown by the blue area, and ADAMTS13-AF488 uptake is shown by the solid red line. Right panel quantifies the reduced uptake of ADAMTS13-AF488 in the presence of 5 mM EDTA. (D) Preincubation with dynasore for 20 minutes reduces the uptake of ADAMTS13-AF488 by 90% (dotted line). Control cells incubated without ADAMTS13-AF488 are shown by the blue area, and ADAMTS13 uptake is shown by the red solid line. Right panel quantifies the reduced uptake of ADAMTS13-AF488 upon incubation with dynasore. Bar graphs represent data from 3 independent experiments ± standard deviation (SD). Data are expressed as percentage of mean fluorescent intensity (MFI) at 37°C; 100% corresponds to the highest MFI observed for individual experiments. ***P < .001 (Student t test).
ADAMTS13 uptake by macrophages proceeds via a mannose receptor–independent pathway
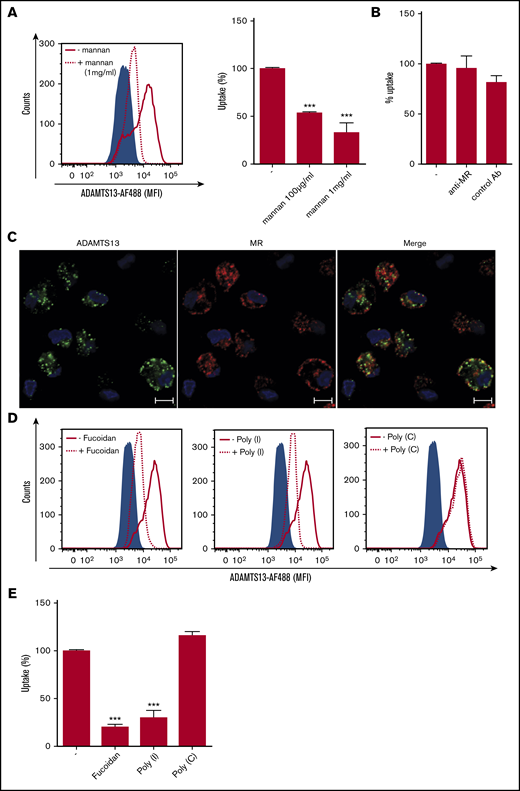
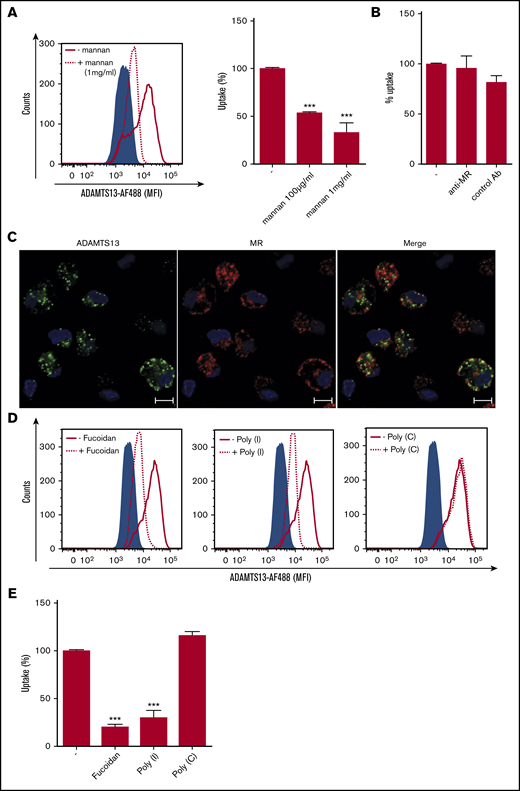
Macrophages express several C-type lectin receptors (CLRs) on the cell surface that act as pattern recognition receptors for both foreign and self-antigens. These include classical calcium-dependent sugar binding proteins such as MMR and Endo180 and also nonclassical CLRs that display structural homology with the classical CLRs but bind sugar and nonsugar ligands in a calcium-independent manner.30 To determine whether CLRs might contribute to the uptake of ADAMTS13, MDMs were preincubated with either EDTA or different concentrations of mannan. Endocytosis of ADAMTS13-AF488 by MDMs was significantly inhibited by mannan; only 30% of residual uptake of ADAMTS13-AF488 was observed when cells were incubated with mannan (Figure 2A). Preincubation of MDMs with d-mannose, GlcNAc or d-galactose did not affect uptake of ADAMTS13 (supplemental Figure 1B-C). Taken together, these results indicate that ADAMTS13 endocytosis by macrophages is mannan sensitive and metal ion dependent. We have previously shown that the MMR mediates the uptake of ADAMTS13 by DCs.16 MMR is predominantly internalized into early endosomes16 and, after release of its ligand, recycles to the plasma membrane.31 Confocal microscopy revealed that part of the endocytosed ADAMTS13-AF488 co-localized with the MMR (Figure 2C), suggesting that ADAMTS13 and MMR localize to the same endocytic compartment. To assess whether the MMR contributes to the uptake of ADAMTS13 by macrophages, we preincubated macrophages with a specific blocking antibody against the MMR. Endocytosis of 25 nM of ADAMTS13-AF488 was not reduced by the presence of anti-MMR antibody clone 15.2 (50 μg/mL) (Figure 2B). These data indicate that an MMR-independent pathway contributes to the endocytosis of ADAMTS13 by macrophages.
Polyanionic ligands block the internalization of ADAMTS13 by MDMs. (A) Preincubation with mannan (dotted line) resulted in reduced endocytosis of ADAMTS13-AF488. (B) Antibody clone 15.2 blocking the mannose receptor did not affect ADAMTS13 uptake. (C) MDMs were incubated with ADAMTS13-AF488 at 37°C for 1 hour. Cells were stained for ADAMTS13-AF488 (green), MR (Alexa Fluor 568; red), and nuclei (DAPI; blue). Scale bars are 10 μm. (D-E) MDMs were incubated with fucoidan, poly-I, or poly-C before addition of ADAMTS13-AF488. Cells were analyzed by flow cytometry. (D-E) Effect of poly-I, poly-C, and fucoidan on ADAMTS13 uptake (gray shaded area represents controls incubated in the absence of ADAMTS13). Cells incubated with ADAMTS13 are indicated by a solid black line; cells preincubated with the inhibitors are indicated by a dotted line. Data in bar graphs are representative of 3 independent experiments ± SD. ***P < .001 (Student t test). AB, antibody.
Polyanionic ligands block the internalization of ADAMTS13 by MDMs. (A) Preincubation with mannan (dotted line) resulted in reduced endocytosis of ADAMTS13-AF488. (B) Antibody clone 15.2 blocking the mannose receptor did not affect ADAMTS13 uptake. (C) MDMs were incubated with ADAMTS13-AF488 at 37°C for 1 hour. Cells were stained for ADAMTS13-AF488 (green), MR (Alexa Fluor 568; red), and nuclei (DAPI; blue). Scale bars are 10 μm. (D-E) MDMs were incubated with fucoidan, poly-I, or poly-C before addition of ADAMTS13-AF488. Cells were analyzed by flow cytometry. (D-E) Effect of poly-I, poly-C, and fucoidan on ADAMTS13 uptake (gray shaded area represents controls incubated in the absence of ADAMTS13). Cells incubated with ADAMTS13 are indicated by a solid black line; cells preincubated with the inhibitors are indicated by a dotted line. Data in bar graphs are representative of 3 independent experiments ± SD. ***P < .001 (Student t test). AB, antibody.
Polyanionic ligands block the internalization of ADAMTS13 by macrophages
Scavenger receptors (SRs) belong to a large family of immune-surveillance receptors that are highly expressed on macrophages.32 These receptors are known to bind and internalize self- and non–self-molecules and participate in cellular functions such as antigen presentation, inflammation, and clearance.32,33 We evaluated the possible role of SRs in ADAMTS13 uptake by pretreating macrophages with polyanionic compounds such as dextran sulfate and heparin. ADAMTS13 uptake was significantly reduced by both compounds (supplemental Figure 1D-E). To further evaluate whether SRs might be involved in uptake of ADAMTS13, macrophages were preincubated with fucoidan and poly-I, which have previously been shown to block the binding of Clostridium sordellii to the class A receptor macrophage receptor with collagenous structure (MARCO).34-37 Poly-C was used as a control for these experiments. Macrophages were preincubated with fucoidan, poly-I, and poly-C for 20 minutes at 37°C before the addition of ADAMTS13-AF488. Both fucoidan and poly-I blocked endocytosis of ADAMTS13 whereas the control compound poly-C did not affect internalization of ADAMTS13 (Figure 2D-E). Taken together, these data show that polyanionic ligands block the internalization of ADAMTS13 by macrophages.
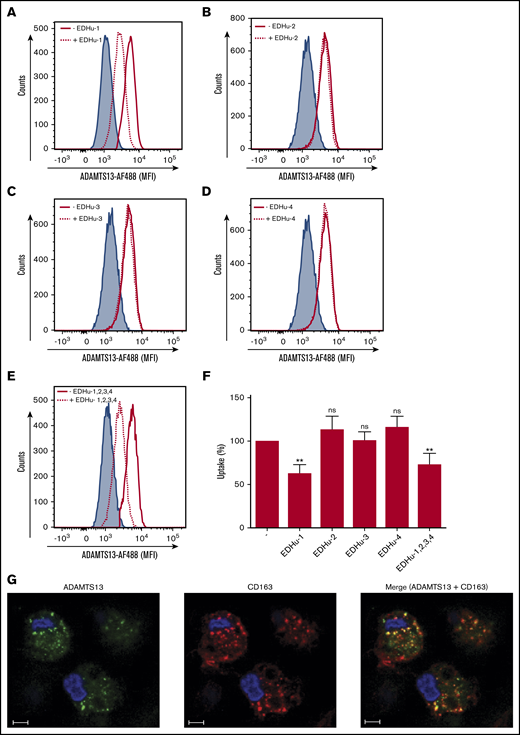
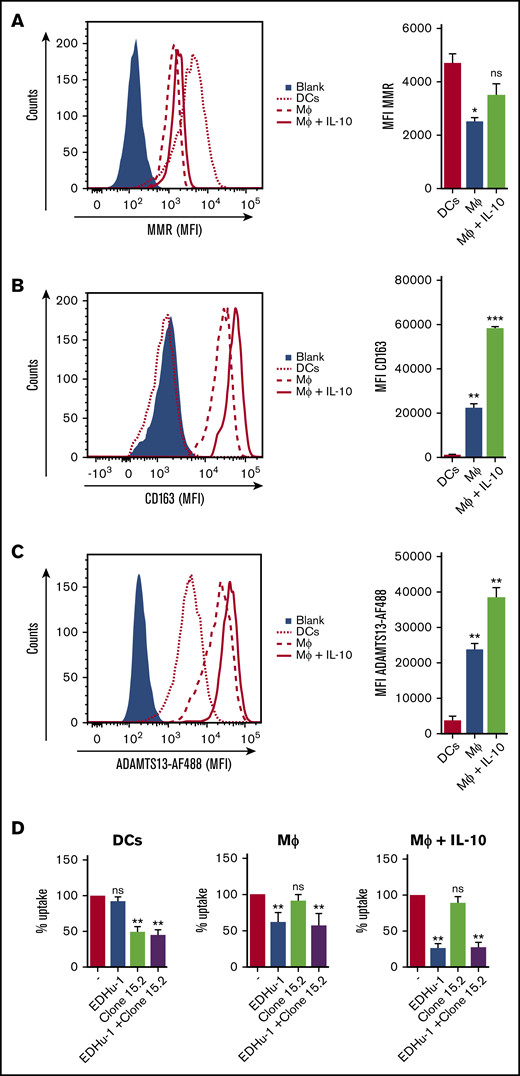
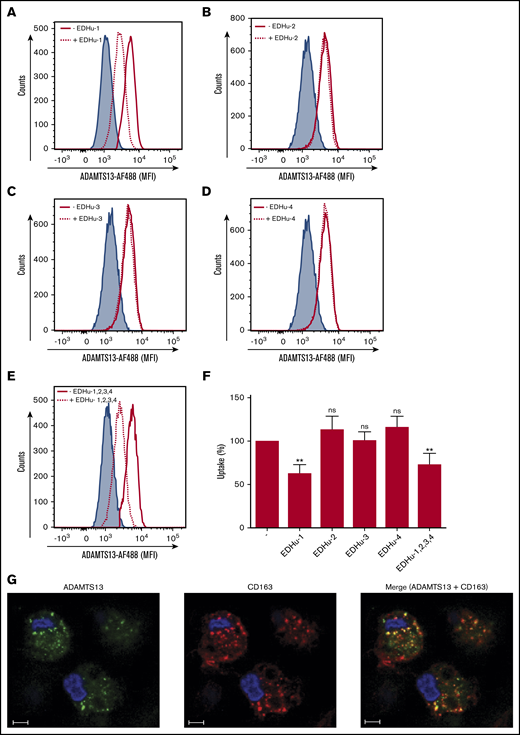
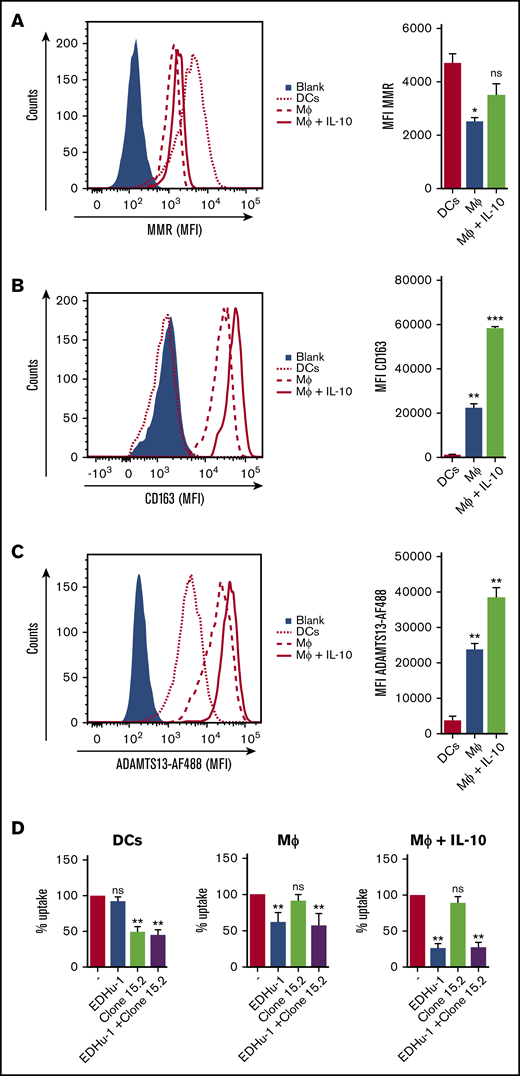
To identify the putative receptor involved in binding and endocytosis of ADAMTS13, macrophages were incubated with or without ADAMTS13 at 4°C for 2 hours. ADAMTS13 was then cross-linked to the macrophages using dimethyl pimelimidate, a membrane-permeable crosslinker that contains two amine-reactive imidoester groups. Subsequently, cells were lysed, and ADAMTS13 was purified by using anti-V5 antibody–coated magnetic beads. Proteins bound to the V5 antibody–coated magnetic beads were digested by chymotrypsin using an on-bead digestion protocol and were subsequently subjected to analysis by mass spectrometry. Thirty-one ADAMTS13-derived peptides were identified in the sample from MDMs that were incubated with ADAMTS13; No ADAMTS13-derived peptides were identified in the sample derived from the control-treated MDMs (supplemental Figure 2A). Cell surface receptors were identified in both the sample derived from the ADAMTS13-treated macrophages and the control macrophages (supplemental Figure 2A). The high-affinity immunoglobulin FcγRI receptor (FcγRI_HUMAN) was identified in both ADAMTS13-treated samples and control samples (supplemental Figure 2A). Recovery of FcγRI-derived peptides in both samples is most likely caused by binding of FcγRI to the anti-V5 monoclonal antibody used for these experiments. Screening for pattern-recognition receptors in the ADAMTS13-treated sample yielded the type I SR CD163 as a candidate (supplemental Figure 2A). A single peptide with amino acid sequence 246HNCDHAEDAGVICSK260 originating from SRCR2 was identified in the ADAMTS13 sample, but this peptide was not identified in the control sample (supplemental Figure 2A). The spectrum corresponding to this peptide is provided in supplemental Figure 2B. This finding positioned CD163 as a candidate receptor for ADAMTS13. The primary function of CD163 is the clearance of free haptoglobin-hemoglobin (Hp-Hb) complexes from the circulation.25,38-40 Ligand binding to this receptor is dependent on clathrin, dynamin, and Ca2+.41 The potential involvement of CD163 in the internalization of ADAMTS13 by macrophages was studied by using a panel of monoclonal antibodies that also included EDHu-1, which was previously shown to block the binding of Hp-Hb complexes to CD163.27 Preincubation of macrophages with monoclonal anti-CD163 blocking antibody EDHu-1 resulted in inhibition of ADAMTS13 uptake (Figure 3A), in contrast to EDHu-2, -3, and -4 (Figure 3B-D). EDHu-1 is reported to bind to SRCR3, thereby inhibiting the binding and uptake of Hp-Hb complexes.27 EDHu-2 and -3 (Figure 3B-C) bind a different epitope of CD163, and these antibodies primarily block the binding and uptake of bacteria.24 EDHu-4 (Figure 3D) does not inhibit the uptake of Hp-Hb complexes or inhibit the recognition of bacteria. A combination of EDHu-1, -2, -3, and 4 (Figure 3E) did not further decrease the uptake of ADAMTS13 by macrophages when compared with EDHu-1 only (Figure 3A). The uptake of ADAMTS13 depicted in Figure 3A-E is quantified in Figure 3F. Confocal microscopy revealed that CD163 and ADAMTS13 co-localize in endocytic vesicles (Figure 3G). To further explore the difference in binding between DCs and macrophages, we cultured DCs and macrophages from the same donors. We also cultured macrophages for 3 days in the presence of IL-10, which has been shown to upregulate the expression of both CD163 and MMR.42 The expression of the MMR was significantly lower on macrophages when compared with DCs and macrophages cultured in the presence of IL-10 (Figure 4A). CD163 expression was not detected on DCs; macrophages cultured in the presence of IL-10 expressed twofold more CD163 when compared with macrophages not treated with IL-10 (Figure 4B). Striking differences were observed when comparing the efficiency of uptake of ADAMTS13 by macrophages and DCs. DCs were 10 times less efficient in endocytosing ADAMTS13 when compared with macrophages. Macrophages cultured in the presence of IL-10 showed 1.6-fold more ADAMTS13 uptake when compared with macrophages not treated with IL-10 (Figure 4C). The blocking anti-MMR antibody clone 15.2 did not have any observable effect on the uptake of ADAMTS13 by macrophages (Figure 4D). In agreement with previous findings, anti-MMR antibody clone 15.2 readily inhibited internalization of ADAMTS13 by DCs (Figure 4D).
CD163 mediates endocytosis of ADAMTS13 by MDMs. MDMs were incubated for 20 minutes at 37°C with monoclonal antibodies directed against CD163; with EDHu-1, -2, -3, or -4; or with a combination of EDHu-1, -2, -3, and -4. ADAMTS13-AF488 was subsequently added to the cells, and endocytosis was analyzed by flow cytometry. (A) Macrophages incubated with EDHu-1 (dotted line) show reduction in the uptake of ADAMTS13-AF488 compared with ADAMTS13-AF488 (solid red line). Blue shaded area corresponds to nontreated cells. (B-D) Macrophages incubated with EDHu-2, -3, or -4 (dotted line) show no reduction in uptake of ADAMTS13-AF488 when compared with ADAMTS13-AF488 only (solid red line). Blue-shaded area corresponds to nontreated cells. (E) Macrophages incubated with a combination of EDHu-1, -2, -3, and -4 (dotted line) show reduction in uptake of ADAMTS13-AF488 when compared with ADAMTS13-AF488 (solid red line) or nontreated control cells (blue-shaded area). (F) Quantification of the data presented in panels A-E. Data are expressed as percentage of MFI with 100% corresponding to the mean fluorescence signal observed for ADAMTS13-AF488 uptake in the absence of antibodies. Data are representative of 3 independent experiments ± SD. (G) ADAMTS13-AF488 was added to MDMs for 1 hour at 37°C. MDMs were stained for ADAMTS13-AF488 (green), CD163 (Alexa Fluor 633; red), and nuclei (DAPI; blue). Scale bars are 10 μm. **P < .01 (Student t test). ns, not significant.
CD163 mediates endocytosis of ADAMTS13 by MDMs. MDMs were incubated for 20 minutes at 37°C with monoclonal antibodies directed against CD163; with EDHu-1, -2, -3, or -4; or with a combination of EDHu-1, -2, -3, and -4. ADAMTS13-AF488 was subsequently added to the cells, and endocytosis was analyzed by flow cytometry. (A) Macrophages incubated with EDHu-1 (dotted line) show reduction in the uptake of ADAMTS13-AF488 compared with ADAMTS13-AF488 (solid red line). Blue shaded area corresponds to nontreated cells. (B-D) Macrophages incubated with EDHu-2, -3, or -4 (dotted line) show no reduction in uptake of ADAMTS13-AF488 when compared with ADAMTS13-AF488 only (solid red line). Blue-shaded area corresponds to nontreated cells. (E) Macrophages incubated with a combination of EDHu-1, -2, -3, and -4 (dotted line) show reduction in uptake of ADAMTS13-AF488 when compared with ADAMTS13-AF488 (solid red line) or nontreated control cells (blue-shaded area). (F) Quantification of the data presented in panels A-E. Data are expressed as percentage of MFI with 100% corresponding to the mean fluorescence signal observed for ADAMTS13-AF488 uptake in the absence of antibodies. Data are representative of 3 independent experiments ± SD. (G) ADAMTS13-AF488 was added to MDMs for 1 hour at 37°C. MDMs were stained for ADAMTS13-AF488 (green), CD163 (Alexa Fluor 633; red), and nuclei (DAPI; blue). Scale bars are 10 μm. **P < .01 (Student t test). ns, not significant.
MDMs treated with IL-10 show increased CD163 expression and increased ADAMTS13 endocytosis. (A) MMR and (B) CD163 expression by DCs (dotted line), MDM (MΦ) (dashed line), and IL-10–treated MDM (MΦ + IL-10) (solid red line). Blue area represents isotype control. (C) ADAMTS13-AF488 uptake by IL-10–treated MDMs. MDMs incubated without ADAMTS13-AF488 (blue area), ADAMTS13-AF488 uptake by DCs (dotted line; red bar), MDMs (dashed line; blue bar), or IL-10-differentiated MDMs (solid red line; green bar) are shown. (D) Blocking of ADAMTS13 uptake by DCs, MDMs (MΦ), and IL-10–treated MDMs (MΦ + IL-10) by EDHu-1, clone 15.2, and a mixture of EDHu-1 and clone 15.2. Data are expressed as percentage of MFI; 100% corresponds to the mean fluorescence signal observed for control-treated MDMs in the absence of any inhibitor. *P < .05; **P < .01; ***P < .001 (Student t test).
MDMs treated with IL-10 show increased CD163 expression and increased ADAMTS13 endocytosis. (A) MMR and (B) CD163 expression by DCs (dotted line), MDM (MΦ) (dashed line), and IL-10–treated MDM (MΦ + IL-10) (solid red line). Blue area represents isotype control. (C) ADAMTS13-AF488 uptake by IL-10–treated MDMs. MDMs incubated without ADAMTS13-AF488 (blue area), ADAMTS13-AF488 uptake by DCs (dotted line; red bar), MDMs (dashed line; blue bar), or IL-10-differentiated MDMs (solid red line; green bar) are shown. (D) Blocking of ADAMTS13 uptake by DCs, MDMs (MΦ), and IL-10–treated MDMs (MΦ + IL-10) by EDHu-1, clone 15.2, and a mixture of EDHu-1 and clone 15.2. Data are expressed as percentage of MFI; 100% corresponds to the mean fluorescence signal observed for control-treated MDMs in the absence of any inhibitor. *P < .05; **P < .01; ***P < .001 (Student t test).
Anti-CD163 antibody EDHu-1 did not affect uptake of ADAMTS13 by DCs, but it strongly inhibited ADAMTS13 internalization by macrophages (Figure 4D). More pronounced EDHu-1 inhibition of ADAMTS13 uptake was observed in macrophages cultured in the presence of IL-10 (Figure 4D). Internalization of ADAMTS13 by macrophages and DCs was not further reduced upon co-incubation with clone 15.2 and EDHu-1 antibodies when compared with blocking experiments with the individual antibodies. The control anti-CD163 antibody EDHu-4 did not reduce the uptake of ADAMTS13 by macrophages or DCs (data not shown).
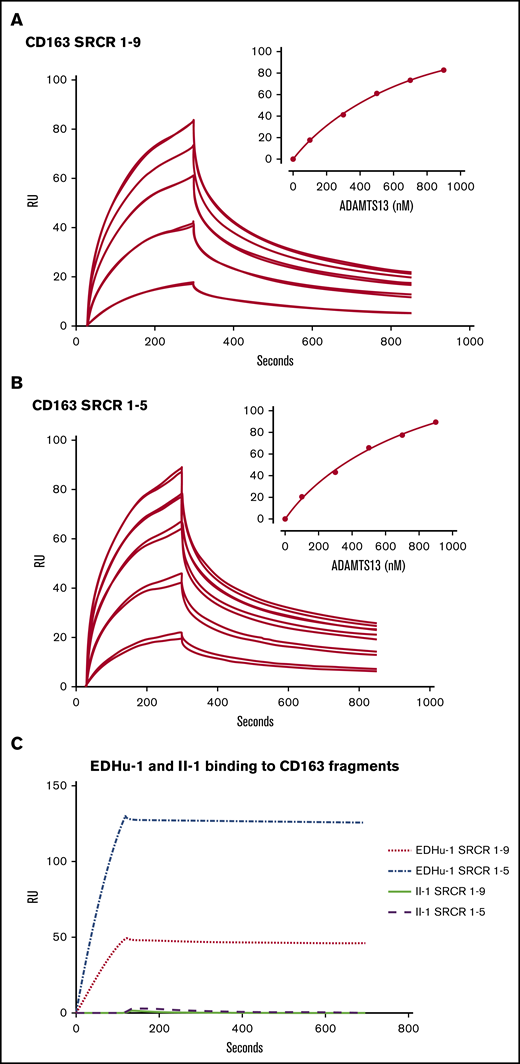
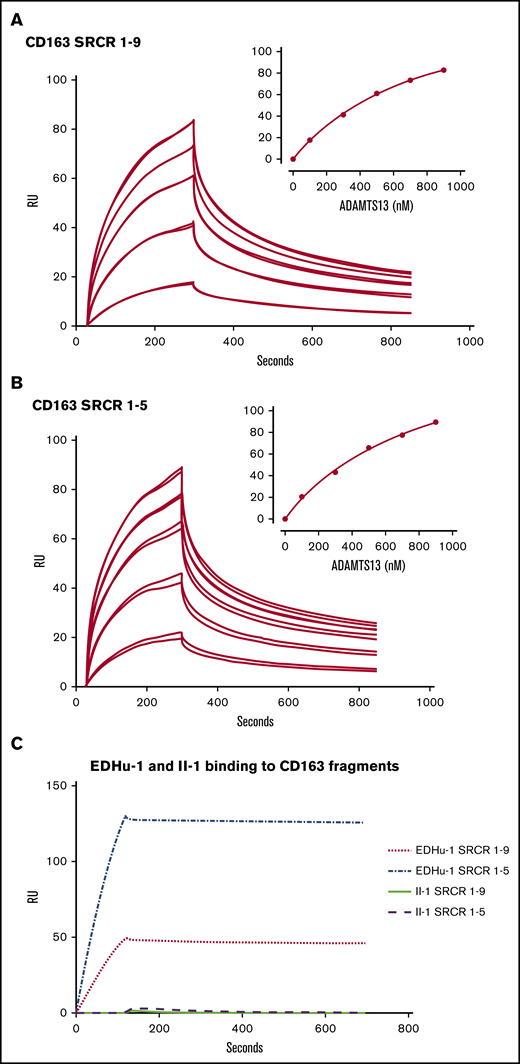
To determine whether ADAMTS13 can interact with CD163 in vitro, surface plasmon resonance experiments were performed using immobilized CD163 fragments corresponding to SRCR1-9 or SRCR1-5.27 A dose-dependent complex binding of ADAMTS13 to both SRCR1-9 and SRCR1-5 fragments was observed (Figure 5A-B). Binding of ADAMTS13 to immobilized SRCR1-9 fragments followed complex kinetics with rapid initial binding, which was then followed by a slower and more sustained binding (Figure 5A). In addition, a rapid initial dissociation was observed which was followed by a second slower rate of dissociation. The complex binding pattern obtained precluded calculation of the binding affinity of ADAMTS13 for CD163. We assessed whether EDHu-1 as a control could still bind to immobilized CD163 fragments. Binding of EDHu-1 to these fragments was observed (Figure 5C). In contrast, monoclonal antibody II-1 directed against ADAMTS13 did not bind to either SRCR1-5 or SRCR1-9 (Figure 5C).12
ADAMTS13 binds to SRCR1-5 and SRCR1-9 of CD163. Surface plasmon resonance analysis of the interaction between ADAMTS13 and CD163. (A) CD163 SRCR1-9 or (B) CD163 SRCR1-5 were immobilized on a CM5 chip. ADAMTS13 was then flowed over the chip in different concentrations. Graphs represent the binding curves using 100, 300, 500, 700, or 900 nM of ADAMTS13. Two independent experiments per concentration ADAMTS13 are displayed. Insets in panels A and B (upper right) show the concentration-dependent increase in maximal binding of ADAMTS13 to the CD163 SRCR1-9 and SRCR1-5 fragments. (C) EDHu-1 (100 nM) as a positive control was allowed to bind to immobilized SRCR1-9 and SRCR1-5 (EDHu-1 binds SRCR 3). Anti-ADAMTS13 antibody II-1 (500 nM) was used as a negative control.
ADAMTS13 binds to SRCR1-5 and SRCR1-9 of CD163. Surface plasmon resonance analysis of the interaction between ADAMTS13 and CD163. (A) CD163 SRCR1-9 or (B) CD163 SRCR1-5 were immobilized on a CM5 chip. ADAMTS13 was then flowed over the chip in different concentrations. Graphs represent the binding curves using 100, 300, 500, 700, or 900 nM of ADAMTS13. Two independent experiments per concentration ADAMTS13 are displayed. Insets in panels A and B (upper right) show the concentration-dependent increase in maximal binding of ADAMTS13 to the CD163 SRCR1-9 and SRCR1-5 fragments. (C) EDHu-1 (100 nM) as a positive control was allowed to bind to immobilized SRCR1-9 and SRCR1-5 (EDHu-1 binds SRCR 3). Anti-ADAMTS13 antibody II-1 (500 nM) was used as a negative control.
We also studied binding of recombinant ADAMTS13 to Chinese hamster ovary (CHO) cells expressing CD163.24 Confocal microscopy revealed binding of ADAMTS13-AF488 to CD163-expressing CHO cells (Figure 6A). EDHu-1 reduced binding and endocytosis of ADAMTS13-AF488 by CD163-expressing CHO cells (Figure 6B). We did not observe any binding to CHO cells that did not express CD163 (Figure 6C). Competition with a threefold excess of unlabeled ADAMTS13 reduces the uptake of ADAMTS13-AF488 (supplemental Figure 3A). To assess the effect of the earlier described inhibitors on the uptake of ADAMTS13, CD163-expressing CHO cells were preincubated with these compounds at 4°C. Subsequently, ADAMTS13-AF488 was added to the cells for 2 hours at 4°C and either directly fixed or transferred to 37°C for 45 minutes to allow for endocytosis of ADAMTS13. Binding at 4°C and endocytosis at 37°C of ADAMTS13 was blocked upon addition of EDHu-1, fucoidan, poly-I, or dextran sulfate (supplemental Figure 3B). Mannan reduced the binding and endocytosis but not as strongly as the other compounds (supplemental Figure 3B). No reduction in binding or uptake was observed when incubating the cells with EDHu-4 (supplemental Figure 3B). Dynasore did not prevent binding of ADAMTS13 to CD163-expressing CHO cells, but it did prevent endocytosis of the surface-bound protein (supplemental Figure 3B). These results show that fucoidan, poly-I, and dextran sulfate block the CD163-mediated internalization of ADAMTS13. We speculate that these negatively charged compounds interact with ADAMTS13. This was confirmed experimentally for fucoidan, which bound with high affinity to immobilized ADAMTS13 (supplemental Figure 4).
CD163 transfected CHO cells internalize ADAMTS13. Binding and uptake of Alexa Fluor 488–labeled ADAMTS13 by CD163-expressing and control CHO cells. (A) CD163-transfected CHO cells internalize ADAMTS13-AF488. (B) ADAMTS13 binding and uptake was reduced by the addition of blocking antibody EDHu-1. (C) Untransfected CHO cells stained for CD163 do not internalize ADAMTS13-AF488. Upper panels show staining for CD163–Alexa Fluor 633 (in red). Middle panels correspond to the signal obtained following internalization of ADAMTS13-AF488 (in green). The overlay of CD163 staining (red) and internalized ADAMTS13-AF488 (green) is displayed in the lower panels. ProLong Gold DAPI (blue signal) was used to stain nuclei. Scale bars are 10 μm.
CD163 transfected CHO cells internalize ADAMTS13. Binding and uptake of Alexa Fluor 488–labeled ADAMTS13 by CD163-expressing and control CHO cells. (A) CD163-transfected CHO cells internalize ADAMTS13-AF488. (B) ADAMTS13 binding and uptake was reduced by the addition of blocking antibody EDHu-1. (C) Untransfected CHO cells stained for CD163 do not internalize ADAMTS13-AF488. Upper panels show staining for CD163–Alexa Fluor 633 (in red). Middle panels correspond to the signal obtained following internalization of ADAMTS13-AF488 (in green). The overlay of CD163 staining (red) and internalized ADAMTS13-AF488 (green) is displayed in the lower panels. ProLong Gold DAPI (blue signal) was used to stain nuclei. Scale bars are 10 μm.
Discussion
In this study, we demonstrated that ADAMTS13 is endocytosed by MDMs. Blocking experiments with mannan and EDTA suggested that CLRs participate in the endocytosis of ADAMTS13 by macrophages. These receptors share at least one Ca2+/carbohydrate recognition domain and bind sugars in a calcium-dependent manner.30 We previously showed that the MMR, a mannan-sensitive CLR, mediates internalization of ADAMTS13 by DCs.16 Although mannan significantly reduced ADAMTS13 uptake by macrophages, ADAMTS13 endocytosis seems to be only partly dependent on this C-type lectin, because its uptake by macrophages is not influenced by the presence of the blocking anti-MR antibody clone 15.2 or by the addition of d-mannose or GlcNAc, which interfere with binding of ligands to mannan-sensitive CLRs.43 These data suggest that MMR does not play a major role in ADAMTS13 internalization by macrophages. We currently cannot exclude that, although it is unlikely, ADAMTS13 may bind to a site on MMR that is not blocked by clone 15.2. Other mannan-sensitive receptors (such as Endo180)44 expressed on macrophages may contribute to the uptake of ADAMTS13.
Macrophages express several pattern-recognition receptors, including CLRs, Toll-like receptors, and SRs.19 SRs comprise a large group of proteins that are divided into 10 different structural classes (classes A through J).32,33,45,46 By using a proteomic approach, CD163 was identified as a putative receptor for ADAMTS13. CD163 is an SR expressed by macrophages and then primarily by bone marrow macrophages, liver macrophages (Kupffer cells), and red pulp macrophages in the spleen42,47 ; other tissue-resident macrophages express CD163 to a lesser extent.42,47 The primary function of CD163 is to remove free Hp-Hb complexes from the circulation.25,40 Macrophage SR CD163 contains 9 SRCR domains. Domains 2, 3, 7, and 9 contain conserved Ca2+ binding domains composed of a triad of negatively charged amino acids.48 Mutagenesis studies have shown that calcium-coordinated acidic amino acid clusters contribute to SRCR2 and -3–mediated binding of Hp-Hb complexes.48 Complementary positively charged residues Arg252 and Lys262 in Hp are crucial for the binding of Hp-Hb complexes to CD163.48 EDHu-1 directed toward SCRC3 has been shown to block the binding of Hp-Hb complexes to CD163.27
In this study, we show that monoclonal antibody EDHu-1 also reduced the uptake of ADAMTS13 by macrophages, suggesting that binding of ADAMTS13 to CD163 is also mediated by the acidic amino acids triad cluster in SRCR3. The pronounced effect of the EDTA on internalization of ADAMTS13 by macrophages is consistent with the Ca2+-dependent binding of ligands to CD163. CD163 was first described as a clearance receptor for circulating Hp-Hb complexes.25,49 Hp-Hb complexes originate from lysed erythrocytes and are rapidly cleared in a CD163-dependent manner.25 No other endogenous ligands for CD163 have been identified at this point.
We also showed that ADAMTS13 is internalized by macrophages in a CD163-dependent manner, and we provided evidence for in vitro binding of ADAMTS13 to CD163, most likely via SRCR3. The half-life of infused ADAMTS13 in patients with congenital TTP upon infusion of plasma is 3 to 4 days.18 Macrophages residing in the red pulp of the spleen and the Kupffer cells in the liver express high levels of CD163.50,51 So far, the site of clearance of ADAMTS13 has not been defined. However, a prominent role for tissue-resident macrophages in clearance of circulating plasma proteins has been documented in several studies.22,52 On this basis, we propose that tissue-resident macrophages expressing CD163 contribute to the in vivo clearance of ADAMTS13.
Interestingly, we observed inhibition of ADAMTS13 uptake by macrophages using the polyanions fucoidan, dextran sulfate, and poly-I (but not poly-C) (Figure 2; supplemental Figure 1D-E). We also show a reduction in uptake of ADAMTS13 in CD163-expressing CHO cells upon incubation with these ligands (supplemental Figure 3B). These ligands have been previously shown to block the binding of C. sordellii to the class A SR MARCO (SR-A6) and binding of acetylated low-density lipoprotein to murine SR-AI.35,53 These polyanionic compounds may also interfere with binding of CD163 to its ligands, but so far, this has not been investigated. Alternatively, polyanions may bind to positively charged residues present in exosites on ADAMTS13 that mediate its binding to CD163, as has been shown for fucoidan (supplemental Figure 4). Exosites present in the disintegrin and spacer domain have been shown to be crucial for binding of ADAMTS13 to von Willibrand factor.26,54,55 Lys262 and Arg252 present in a single exposed loop of Hp are critically involved in binding of Hp-Hb complexes to CD163.48 On the basis of the observed similarities between Hp-Hb and ADAMTS13 binding to CD163, we anticipate that similar to Hp, exosites containing positively charged residues may also contribute to the binding of ADAMTS13 to CD163. Modification of these sites may help to modulate endocytosis by CD163-expressing populations of macrophages present in liver and spleen, thereby extending the circulatory half-life of ADAMTS13 for improved treatment of patients with congenital TTP.
Acknowledgments
The authors thank Robin van Bruggen for helpful discussions regarding CD163, and Simon Tol, Erik Mul, and Mark Hoogenboezem for their help with the confocal microscopy and flow cytometry.
This work was supported by a grant from the Landsteiner Foundation for Blood Transfusion Research. This project received funding from the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement 675746.
Authorship
Contribution: All authors were involved in drafting the manuscript; F.C.V., J.H., and N.S. performed the experiments and wrote the manuscript; P.H.P.K. purified recombinant ADAMTS13 and performed the BIAcore experiments; I.P. contributed to the pull-down and competition experiments; R.F. and A.t.B. provided crucial input for the project; A.B.M. and F.P.J.v.A. were responsible for analyzing the mass spectrometry data; T.K.v.d.B., J.J.H.G., and S.K.M. provided antibodies against CD163, Hb-Hp complexes, recombinant CD163 fragments, and CD163-expressing Chinese hamster ovary cells; and J.V. designed the study and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Voorberg, Sanquin Research and Landsteiner Laboratory, Academic Medical Center, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: j.voorberg@sanquin.nl.
References
Author notes
The full-text version of this article contains a data supplement.