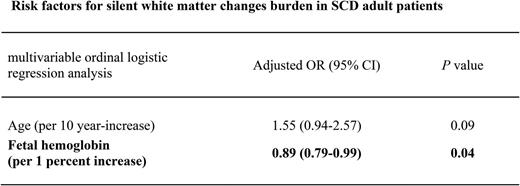
Key Points
Low %HbF is independently associated with silent WMCs on brain imaging in adults with SCD.
Our results highlight the potential use of therapeutic strategies inducing HbF expression in SCD patients with silent white matter changes.
Abstract
Silent white matter changes (WMCs) on brain imaging are common in individuals with sickle cell disease (SCD) and are associated with cognitive deficits in children. We investigated the factors predictive of WMCs in adults with homozygous SCD and no history of neurological conditions. Patients were recruited from a cohort of adults with homozygous SCD followed up at an adult sickle cell referral center for which steady-state measurements of biological parameters and magnetic resonance imaging scans of the brain were available. WMCs were rated by consensus, on a validated age-related WMC scale. The prevalence of WMCs was 49% (95% confidence interval [CI], 39%-60%) in the 83 patients without vasculopathy included. In univariable analysis, the patients who had WMCs were more likely to be older (P = .003) and to have hypertension (P = .02), a lower mean corpuscular volume (P = .005), a lower corpuscular hemoglobin concentration (P = .008), and a lower fetal hemoglobin percentage (%HbF) (P = .003). In multivariable analysis, only a lower %HbF remained associated with the presence of WMCs (odds ratio [OR] per 1% increase in %HbF, 0.84; 95% CI, 0.72-0.97; P = .021). %HbF was also associated with WMC burden (P for trend = .007). Multivariable ordinal logistic regression showed an inverse relationship between WMC burden (age-related WMC score divided into 4 strata) and HbF level (OR for 1% increase in %HbF, 0.89; 95% CI, 0.79-0.99; P = .039). Our study suggests that HbF may protect against silent WMCs, decreasing the likelihood of WMCs being present and their severity. It may therefore be beneficial to increase HbF levels in patients with WMCs.
Introduction
Stroke is one of the leading causes of death in both children1 and adults2 with sickle cell disease (SCD). During the natural course of the disease, 11% of patients with homozygous SCD have a clinically overt stroke by age 20 years, and 24% by age 45 years.3 Ischemic strokes are common in children and young adults, but the frequency of hemorrhages peaks during the third decade of life.3 Different types of ischemic stroke are observed, but the most common subtype of overt cerebral infarction is border-zone infarcts occurring between the anterior and middle cerebral artery due to a specific large-vessel vasculopathy.4 Blood exchange and transfusion therapy greatly decrease the risk of a first ischemic stroke in children with high-velocity blood flow detected on a Doppler scan.5 Furthermore, white-matter changes (WMCs) on brain imaging, in patients with no overt neurological signs or history suggestive of a cerebrovascular event, are observed in approximately one-quarter to one-third of children with homozygous SCD.6-8 Such damage is the most frequent type of permanent neurological injury in children with homozygous SCD and, probably, also in adults.9 WMCs are generally considered to be “silent lesions,” but children with SCD displaying such lesions have been found to have cognitive deficits and to be at risk of intellectual decline.10-13 Most WMCs are observed in patients with no specific vasculopathy.14 A low pain event rate, a history of seizure, high white blood cell counts, and the Senegal “SEN” β-globin haplotype have been identified as being associated with silent WMCs in children with SCD.15
It has recently been shown that regular blood transfusion therapy significantly decreases the incidence or recurrence of cerebral infarcts (silent and/or overt) in children with homozygous SCD with silent WMCs on magnetic resonance imaging (MRI) scan.16 However, the value of systematic and repeated MRI screening for such lesions in patients with SCD, and of systematic treatment with regular blood transfusion therapy, remains a matter of debate.17-19 This issue is of particular concern in the growing population of adults with SCD because of the steady increase in the prevalence of silent infarcts with age.20
The objective of this study was to identify predictors of WMCs in adults with homozygous SCD and no history of stroke or vasculopathy, focusing particularly on modifiable biological candidates.
Methods
Patients
Patients were recruited from a cohort of adults with homozygous SCD for whom steady-state biological parameters had been recorded (n = 1220), who were followed up at our adult sickle cell referral center. Patients are examined at least once every 6 months. Demographic data, vascular risk factors, and medical history were collected prospectively on a standardized electronic case report form.
Patients older than 18 years who had no history of cerebrovascular disease, had normal results on neurological examination, had steady-state biological results recorded before hydroxyurea treatment or transfusion, and had undergone brain MRI scans in our referral hospital between January 2007 and December 2013 for screening purposes were included in the study. The decision to perform an MRI scan in our referral hospital was driven mainly by close geographic location of included patients, whereas other patients had undergone MRI scan in other private or public health institutions.
Biological parameters
Laboratory data were collected during routine outpatient visits. Hematological data are expressed as 2 or 3 separate steady-state determinations. Steady state was defined as a visit at least 1 month after an acute clinical event (vaso-occlusive crisis, infection, acute chest syndrome, or any other clinical event resulting in hospitalization and/or blood transfusion) before any hydroxyurea treatment and at least 3 months after blood transfusion. All laboratory analyses were performed on site, in the clinical laboratories of Henri Mondor Hospital. Biological findings were recorded prospectively in a database. The following data were extracted: blood cell count, lactate dehydrogenase levels, bilirubin concentration, and fetal hemoglobin percentage (%HbF). The levels of hemoglobin S (HbS), HbF, and hemoglobin A2 (HbA2) were determined by cation-exchange high-performance liquid chromatography with a Variant hemoglobin analyzer (Variant Hemoglobin Testing System; BioRad, Hercules, CA). Three forms of deletional α-thalassemia (α−3.7, α−20.5, and Mediterranean type) were detected by polymerase chain reaction. Polymerase chain reaction restriction–fragment length polymorphism was used to determine β-globin gene cluster haplotype.21
MRI scans
Patients who had undergone brain MRI scans were identified from our local prospective MRI register.
All MRI scans were performed on a Magneton Verio 3-T MRI scanner (Siemens, Erlangen, Germany). Two investigators (T.T. and N.M.) trained in the analysis of WMCs and blind to clinical and biological data rated all MRI scans, specifically in the context of this study, by consensus, using the age-related WMC (ARWMC) score22 on a dedicated workstation. White matter lesions in the cerebral hemispheres and brainstem were identified as poorly defined hyperintensities of at least 5 mm in diameter on T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI scans. Changes in the basal ganglia were rated in the same manner. The extent of white matter disease was rated on a 4-point scale in different regions of the brain, on T2-weighted and FLAIR MRI scans: 0 = no lesions (including symmetric, well-defined caps or bands); 1 = focal lesions; 2 = lesions beginning to become confluent; and 3 = diffuse involvement of the entire region, with or without the involvement of U-fibers.
We assessed the 5 brain regions in the left and right hemispheres separately for each patient: the frontal area (the frontal lobe anterior to the central sulcus), the parieto-occipital area (the parietal and occipital lobes together), the temporal area (the temporal lobe, with a line from the posterior part of the Sylvian fissure to the trigones of the lateral ventricles separating this area from the parieto-occipital area), the infratentorial area (including the brainstem and cerebellum), and the basal ganglion region (including the striatum, globus pallidus, thalamus, external capsules, and insula).
The total ARWMC score was then obtained by adding the scores for each brain region. Thus, the total ARWMC score ranged from 0 to 30. In addition to rating brain lesions, both investigators assessed intracranial arteries on 3-dimensional time-of-flight MR angiography of the circle of Willis, to confirm the absence of irregularities or stenosis.
Ethics statement
In accordance with French law, this study did not require approval from an institutional review board or ethics committee, because it involved only retrospective analysis of anonymized data collected prospectively as part of routine clinical care.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range), and categorical variables are expressed as number (percentage). The relationships between the presence of WMCs (dependent variable, defined as present vs absent) and patient characteristics were assessed by calculating crude and adjusted odds ratios (ORs) with binary logistic regression models. Variables with values of P < .1 in univariable analysis were included in multivariable logistic models,23 taking potential multicollinearity into account.
We also assessed the associations between biological findings and severity of WMCs, analyzed as a discrete variable. To this end, we used Jonckheere-Terpstra tests to assess the association between each biological finding and ARWMCs. We subsequently divided ARWMC burden into 4 strata (0, 1, 2-4, and ≥5), the last 3 strata of which corresponded to the tertiles of patients in which ARWMCs were detected. We performed univariable and multivariable ordinal logistic regression analyses, using 4-strata ARWMCs as the dependent variable. Variables with values of P < .1 in univariable analysis were included in the multivariable ordinal logistic regression model. Values of P < .05 in 2-tailed tests were considered statistically significant. Statistical analysis was conducted with SPSS 23.0 (IBM Corporation, Armonk, NY) and SAS 9.4 (SAS Institute, Cary, NC).
Results
Using the database, we identified 102 patients as eligible for inclusion in our study. The included patients were similar to those for whom steady-state biological results were variable, but no MRI scan had been performed for screening purposes, in terms of age, hemoglobin levels, mean corpuscular volume, and %HbF (data not shown). Results on brain MRI scans showed motion artifacts in 5 patients, which resulted in poor image quality precluding analysis. We excluded 14 patients because of stenotic vasculopathy identified on a 3-dimensional time-of-flight MR angiographic image of the circle of Willis. The remaining 83 patients were included in the analysis. Mean age (SD) was 43.3 (9.8) years, and 50 (60%) of the patients were female. The patients originated from the West Indies (36%), West Africa (34%), Central Africa (29%), and North Africa (1%). The demographic characteristics of the population are presented in Table 1.
MRI results
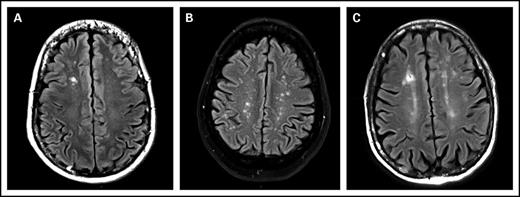
The prevalence of WMCs in the 83 included patients was 49% (95% confidence interval, 39%-60%). ARWMC score was calculated for these 41 patients. The ARWMC score was 1 in 15 patients, 2 to 4 in 12 patients, and 5 or greater in 12 patients. The lesions were focal in 49% (20/41) of patients, and the remaining patients had either lesions in several distinct brain areas or several lesions in a single area that were beginning to become confluent. None of the patients had more severe lesions. WMCs were localized to the frontal area in 32 (78%) of 41 patients, the parieto-occipital area in 19 (46%) of 41 patients, the temporal area in 3 (7%) of 41 patients, the basal ganglion region in 7 (17%) of 41 patients, and the infratentorial area in 3 (7%) of 41 patients (Figure 1).
MRI, FLAIR images, axial slices. (A) White matter lesion, type 1: focal lesion. Focal hypersignal on right frontal lobe. (B) White matter lesion, type 2: beginning confluence lesion. Multiple focal hypersignal on right and left hemispheres. (C) Another white matter lesion, type 2: beginning confluence lesion. Multiple hypersignal on right and left hemispheres with small confluence.
MRI, FLAIR images, axial slices. (A) White matter lesion, type 1: focal lesion. Focal hypersignal on right frontal lobe. (B) White matter lesion, type 2: beginning confluence lesion. Multiple focal hypersignal on right and left hemispheres. (C) Another white matter lesion, type 2: beginning confluence lesion. Multiple hypersignal on right and left hemispheres with small confluence.
Table 2 shows biological findings for the population according to the presence or absence of WMCs. β-globin gene haplotype was known for 70 patients (84%). Homozygosity for the Benin “BEN” β-globin haplotype was identified in 22 patients (31%), homozygosity for the Bantu “CAR” β-globin haplotype was found in 22 patients (31%), and homozygosity for the SEN β-globin haplotype was detected in 10 patients (14%). Other patients had heterozygous β-globin haplotypes. Status of α-globin thalassemia deletion was available for 67 patients (82%), 33 (49%) of whom had no deletion, 30 (45%) of whom had 1 α−3.7 deletion, 2 (3%) of whom had 2 α−3.7 deletions, and 2 (3%) of whom had a triplication of the α-globin gene.
Factors associated with the presence of WMCs
In univariable analysis (Table 2), patients with WMCs were more likely to be older (P = .03) and to have hypertension (P = .02), a lower mean corpuscular volume (P = .005), a lower corpuscular hemoglobin concentration (P = .008), and a lower %HbF (P = .003). Because of the strong correlation between corpuscular volume and corpuscular hemoglobin concentration (Spearman ρ = 0.94; P < .0001), we included only corpuscular volume in the multivariable model to prevent multicollinearity. In this multivariable analysis (Table 3), only a lower %HbF remained independently associated with the presence of WMCs. Mean (SD) A γ/G γ ratio, available for 56 patients (67%), was 1.1 (0.49). Median (interquartile range) %HbF was 10.0% (4.6%-16.2%) in patients homozygous for the SEN β-globin haplotype and 6.0% (2.9%-11.1%) in patients homozygous for the CAR β-globin haplotype. The association between a lower %HbF and the presence of WMCs remained significant after adjustment for β-globin haplotype in the 70 patients for whom β-globin haplotype was known. In a post hoc sensitivity analysis conducted after exclusion of the 14 patients (17%) who started receiving hydroxyurea after steady-state measurement of biological parameters but before MRI scans, the association between a lower %HbF and the presence of WMCs was similar.
Factors associated with WMC burden
The %HbF was associated with ARWMC score (Jonckheere-Terpstra test, P for trend = .007). Univariable ordinal logistic regression analysis also revealed an inverse relationship between WMC burden (ARWMC score divided into 4 strata) and %HbF (OR for 1% increase in fetal hemoglobin, 0.87; 95% CI, 0.79-0.96; P = .006). This association remained significant (OR for 1% increase in fetal hemoglobin, 0.89; 95% CI, 0.79-0.99; P = .039) after adjustment for age, hypertension, and mean corpuscular volume (Table 4).
Discussion
Our study shows that a low %HbF is associated with both the presence and burden of silent WMCs, with a 13% relative decrease in the prevalence of WMCs per 1% increase in HbF concentration among adult patients with homozygous SCD, normal results on neurological examination, no history of cerebrovascular disease, and no disease-specific vasculopathy.
The prevalence of WMCs in our cohort of adults (49%) was higher than that reported for cohorts of children,8,15,20,24-27 but it was highly consistent with the findings of a recent study reporting a prevalence of silent infarcts of 53% in a cohort of adult patients with SCD with a median age of 30 years.28 However, caution is required when studies are compared directly because of differences among studies in definitions; brain imaging techniques; and population characteristics, particularly concerning cerebrovascular history. A steady increase in the prevalence of brain lesions with age was clearly demonstrated in children, in a recent study by Bernaudin et al.24 The cumulative risk of silent cerebral infarcts was found to increase from 19.2% at age 8 years, to 32.4% at age 14 years and 39.1% at age 18 years. In our study, the most common location of WMCs was the frontal area, and half of the patients had focal lesions only, which were consistent with findings with pediatric cohorts.29 Beyond the term “silent,” which simply indicates the absence of a history of stroke, children who have silent brain lesions on brain MRI scans have been shown to have poorer cognitive function than do children who have normal brain MRI findings. Indeed, in children with SCD, such “silent” lesions have been shown to be associated with a mean decrease in full-scale IQ of 5 points,30 poor academic performance,31 and a higher risk of overt strokes.32 In this cross-sectional study, it was not possible to determine when, during the patients’ lifetime, the WMCs had appeared. It is highly likely that a significant proportion of the cases of WMCs in our population dated from childhood.
In contrast to previous studies, our study strongly suggests an association between %HbF and the presence and burden of silent WMCs. One explanation for the difference between our results and those of other studies is that previous studies in children have often taken %HbF results obtained very early in life, before the stabilization of this percentage, into account.33 HbF is known to be the major protective factor against HbS polymerization.34,35 Indeed, HbF contributes to formation of the α2βsγ hybrid, which impedes HbS polymer elongation. Many studies have shown %HbF levels to be associated with clinical and/or biological protective effects.2,36 The association between %HbF and the presence and burden of WMCs described here is highly consistent with the known antipolymerization dose-response effect of %HbF.35,37 The lack of a protective effect of the SEN haplotype on the presence of WMCs despite a higher %HbF is consistent with the lack of a protective effect of this haplotype against silent infarcts in children reported in a previous study.15
Haplotypes are of interest more because of their geographic origin than as a causal explanation. It is possible that deleterious polymorphisms of other genes in people from Senegal offset the beneficial effects of HbF, accounting for this paradoxical result.
Another difference between our findings and those from cohorts of children is the lack of association of hemoglobin concentration with WMCs in our adult population of patients with SCD. This association was also reported in patients with end-stage renal disease38 and thalassemia intermedia.39,40 In adults with SCD, anemia is closely associated with hemolysis41 and with dense, dehydrated red blood cells.42 Our findings are not consistent with an association between WMC lesions and the hemolytic phenotype.
Our study had several potential limitations. First, we could not exclude the possibility that selection bias affected the prevalence of silent WMCs obtained here. Only a few patients meeting the other inclusion criteria had undergone an MRI scan for screening purposes during the study period. However, the included patients were similar to patients for whom steady-state biological results were available but for whom no screening MRI scan had been performed, in terms of baseline characteristics and biological results. The inclusion of patients with no history of cerebrovascular disease and normal results on neurological examination only may have resulted in an underestimation of the prevalence of silent WMCs, but our results were consistent with a previous study that was conducted in adults.28 Such potential selection biases may have affected the estimated prevalence of silent WMCs, but this would be unlikely to have any major effect on the relative effects of HbF level. However, the generalizability of our results might have been affected by the proportion of patients who had undergone an MRI. Second, hypertension, a well-known predictive factor for WMCs in the general population,43 was not associated with silent WMCs in our cohort of adult patients with SCD. However, this absence of association was probably attributed to low statistical power, as suggested by the strong, but nonsignificant, relative effect of hypertension on the presence of WMCs in our study, because of the small sample size and the low prevalence of hypertension in such a cohort of SCD patients, as previously reported.44
Our study has clinical implications. Indeed, increasing HbF levels through drug treatments or gene therapy is one of the current goals of SCD research. Hydroxyurea is the most widely used drug for increasing HbF levels. Many studies have shown this drug to be effective for preventing vaso-occlusive crisis and acute chest syndrome,45 secondary cerebral vasculopathy,46,47 and death.48,49 Our study therefore suggests that treatment with hydroxyurea may be beneficial in patients with SCD in order to prevent WMCs.
This study provides new insight into the potential protective effect of fetal hemoglobin against silent WMCs in adult patients with SCD. Further studies are required to confirm this association before hydroxyurea can be considered as a suitable treatment of increasing HbF levels to protect against WMC burden and associated cognitive disorders.
Authorship
Contribution: D.C. and P.B. originated the idea for the study; D.C., T.T., N.M., and P.B. contributed to the design of the study; D.C., G.T., and P.B. analyzed the data; D.C. wrote the first draft of the manuscript; and D.C., T.T., N.M., G.T., A.H., N.A.A., L.M., F.H., M.E., F.G., and P.B. contributed to data collection, analysis, and interpretation; and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pablo Bartolucci, Unité des Maladies Génétiques du Gobule Rouge (UMGGR), CHU Henri-Mondor, 51 av du Mal de Lattre de Tassigny, 94010 Créteil Cédex, France; e-mail: pablo.bartolucci@aphp.fr.