Key Points
Etoposide addition/chemo-intensification has little role in first-line treatment of PTCL in Asian populations, regardless of subtype or age.
Upfront hematopoietic stem-cell transplantation as consolidation seems like a legitimate choice in patients with PTCL.
Abstract
Peripheral T-cell lymphomas (PTCLs) have an aggressive biological course and poor clinical outcomes. Despite producing somewhat less-than-satisfactory results, the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) remains the de facto standard in PTCL treatment. Addition of etoposide to CHOP backbone to overcome such unsatisfactory results has yielded contradictory information. We aimed to thoroughly analyze the impact of incorporating etoposide into first-line treatment. Using merged data from the Korean National Health Insurance Service and National Cancer Registry, a total of 1933 patients (median age, 58 years) were evaluated for clinical characteristics and treatment outcomes. Thirty-eight percent (n = 748) of the 1933 patients received CHOP or CHOP-like regimen, 35.1% (n = 678) received CHOP-like regimen plus etoposide, 5.9% (n = 113) received other backbone chemotherapy plus etoposide, and 20.3% (394) received other treatments in the first-line setting. When we divided the patients into 3 groups according to regimen (group 1, CHOP or CHOP-like regimen; group 2, CHOP or CHOP-like regimen plus etoposide; group 3, all others), group 1 was associated with longest progression-free survival (PFS; P < .001) and overall survival (OS; P < .001). This lack of benefit with etoposide addition was observed across different PTCL subtypes and age groups. Adding etoposide led to longer hospitalizations and cytopenias requiring more transfusion. Upfront hematopoietic stem-cell transplantation led to better OS. Addition of etoposide to CHOP-like regimens does not result in better PFS or OS for patients with PTCL. Overall, Asian patients with PTCL do not benefit from chemotherapy intensification of first-line treatment. We hereby provide crucial information on establishing standardized PTCL treatment.
Introduction
Peripheral T-cell lymphomas (PTCLs) represent a heterogeneous group of neoplasms with an aggressive biological course and poor clinical outcomes.1,2 Despite the clinical differences between B- and T-cell lymphomas,3 and despite producing somewhat less-than-satisfactory results, the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) remains the de facto standard in PTCL treatment, because evidence showing other regimens to be superior is lacking.4,5
Functioning as a phase-sensitive cytocidal drug that specifically affects cells in S or G2, etoposide offers non–cross-resistant cytotoxicity when used in combination with alkylating agents and doxorubicin. Thus, it has been integrated into multiagent protocols used in the primary treatment of various subtypes of malignant lymphomas.6 In the arena of T-cell lymphoma treatment, addition of etoposide to backbone CHOP chemotherapy has yielded contradictory results.7-12 Evidence of positive effects of etoposide addition comes from a retrospective study by Schmitz et al,12 who reported better event-free survival for patients receiving CHOP plus etoposide treatment. In contrast, the German High-Grade Non-Hodgkin Lymphoma Study Group concluded that high-dose CHOP plus etoposide for patients with mature T-cell lymphoma was no better than other high-dose regimens.7 Reflecting such controversy, current National Comprehensive Cancer Network guidelines recommend CHOP and CHOP plus etoposide with same level of evidence for most PTCL subtypes.13 Many feel that it is necessary to delineate the role of etoposide in T-cell lymphomas, considering the benefits of etoposide in their B-cell counterparts.14,15
Unfortunately, however, because of the rarity of PTCL, analyzing the impact of incorporating etoposide into first-line treatment through randomized trials with large cohorts is not a feasible strategy. In this study, we aimed to establish the role of etoposide in first-line treatment of PTCL in 1 of the largest pool of patients with systemic PTCL. We first examined results from 1 of the highest-volume hospitals from Korea, a country with a comparatively higher incidence of PTCL. With preliminary results from a single-center study suggesting etoposide addition is not associated with better outcomes, we expanded the study to a nationwide population to validate our findings.
Patients and methods
Single-center study
A retrospective, longitudinal cohort study was carried out at Seoul National University Hospital, a tertiary academic center. PTCL subtypes included in this study were PTCL not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large-cell lymphoma (ALCL), enteropathy-associated T-cell lymphoma (EATL), subcutaneous panniculitis-like T-cell lymphoma (SPTCL), and hepatosplenic T-cell lymphoma. Between 1 January 2010 and 31 December 2015, 162 patients with newly diagnosed PTCL were identified. Adult patients defined as age ≥20 years were included; those with leukemic T-cell lymphoma, cutaneous T-cell lymphoma, or secondary lymphoma were excluded. Patients not receiving any treatment and patients with HIV infection were also excluded from analyses. After excluding 31 for insufficient data or not meeting inclusion criteria, a total of 131 patients were evaluated for their demographic, laboratory, and clinical data. Patients were divided into 4 groups according to first-line chemotherapy received: (1) group 1, CHOP or CHOP-like regimen; (2) group 2, CHOP plus etoposide or CHOP-like regimen plus etoposide; (3) group 3, other regimen plus etoposide; and (4) group 4, other regimen. CHOP-like regimens included CVP (cyclophosphamide, vincristine, and prednisolone), hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alone or alternating with high-dose methotrexate and cytarabine, and COPBLAM (cyclophosphamide, vincristine, bleomycin, doxorubicin, prednisolone, and procarbazine). Data available until January 2017 were used. The study was conducted according to the Declaration of Helsinki and was approved by the institutional review board (IRB) of Seoul National University Hospital (IRB #H-1605-151-768).
Population study based on national registry data
The Ministry of Health and Welfare of Korea initiated a nationwide hospital-based cancer registry called the Korea Central Cancer Registry (KCCR) in 1988, which expanded to cover the entire Korean population under the Population-Based Regional Cancer Registry program by 1999.16 Cancer cases are classified according to the International Classification of Diseases (ICD) for Oncology, 3rd edition,17 and converted according to the ICD, 10th edition.18
On a different note, in Korea, the National Health Insurance (NHI) program operated by the Korean Ministry for Health and Welfare is the sole and mandatory insurance system that covers ∼98% of the overall Korean population.19,20 Because the Korean population itself is fairly ethnically homogenous, both the KCCR and NHI databases can be readily used for nationwide analyses. For this study, we used information extracted from the KCCR and NHI databases between January 2002 and December 2010. Using these databases, patients with newly diagnosed PTCL were retrospectively identified using morphologic codes of ICD for Oncology, 3rd edition. The code for PTCL-NOS was M97023; for AITL, M97053; for ALCL, M97143; for EATL, M97173; for SPTCL, M97023; and for hepatosplenic T-cell lymphoma, M97163. Initially, 2755 patients were identified, but after applying the same exclusion criteria used in the single-center study, 1969 patients remained. Because drugs used as part of clinical trials are not covered by NHI, we assumed that patients receiving first chemotherapy 3 months after lymphoma diagnosis were likely to have participated in clinical trials. Thus, 36 patients meeting this criterion were also excluded. A total of 1933 patients were included in final analyses. Patients were divided into the same 4 groups according to first-line chemotherapy used in our single-center study. The study protocol was approved by the IRB of the National Cancer Center, Korea (NCC2015-2017). Consort diagram is available as supplemental Data.
Definition
Comorbidity data at the time of lymphoma diagnosis were collected, and Charlson Comorbidity Index (CCI) was calculated including lymphoma.21 A high comorbidity score was defined as CCI ≥ 4. Tumor responses were assessed based on the response criteria for malignant lymphoma,22 and dose delay was defined as >5 days of delay for any subsequent cycles after cycle 1. Early mortality was defined as death occurring during first-line chemotherapy or within 4 months of lymphoma diagnosis.
Statistical analysis
The overall survival (OS) and progression-free survival (PFS) curves were estimated using the Kaplan-Meier method. OS was defined as the time from the date of first chemotherapy initiation to death resulting from any cause. PFS was derived from the date of first chemotherapy initiation to that of progression, relapse, or death resulting from any cause. If a patient survived without event, survival was censored to the latest date of follow-up when no event was confirmed. Univariate and multivariate proportional hazards regression models were used to identify independent risk factors of survival by Cox proportional hazards models. Predictors achieving P < .10 in univariate analysis were sequentially preceded to multivariate model. Differences between groups were assessed using the Student t test or 1-way analysis of variance for continuous variables and Pearson χ2 test for categorical variables, as indicated. All analyses were performed using SAS software (version 9.3; SAS Institute Inc., Cary, NC). P < .05 was considered to be statistically significant.
Results
Patient characteristics
Baseline characteristics are shown in Table 1 and supplemental Tables 1 and 2. The 3 most common subtypes of PTCL were PTCL-NOS, AITL, and ALCL. More than half of patients had International Prognostic Index scores of ≤2, and 49.6% had Prognostic Index for T-Cell Lymphomas scores of ≤1 (supplemental Table 1). Information regarding performance status and prognostic score was not available from national registry data. In the single-center study, CHOP or CHOP-like regimens comprised the majority of first-line treatment (80.9%). In comparison, in national registry data, there were similar proportions of patients in group 1 vs group 2.
Treatment response and toxicity
In the single-center study, the addition of etoposide was not associated with better treatment outcomes. When group 1 was compared with group 2 for complete remission (CR) rate, early mortality, and total mortality, there were no differences between the 2 groups (Table 2). The same analyses were performed between patients receiving CHOP or CHOP-like regimens (group 1) vs patients treated with etoposide (groups 2 and 3) to further evaluate the role of etoposide in first-line treatment of PTCL, and no benefit in terms of CR rate was found for etoposide addition (supplemental Table 3). Instead, etoposide addition was significantly associated with chemotherapy delay (group 1, 15.1% vs group 2, 47.8%; P < .001) and trend toward greater incidence of red blood cell transfusion requirement (group 1, 14.2% vs group 2, 30.4%; P = .060).
Similar results were found in national registry data. The addition of etoposide did not yield better treatment outcomes but was associated with more toxicity. Although information on CR rate was not available, when group 1 was compared with group 2 for 5-year survival rate, early morality rate, and total mortality rate, no differences were observed (Table 2). However, etoposide addition led to transfusion requirement.
OS and PFS
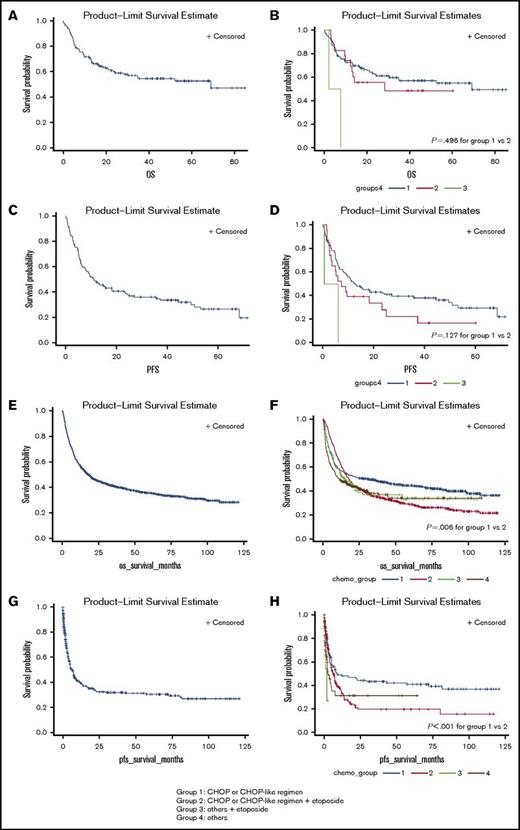
In the single-center study, during the median follow-up of 44 months, 1-year PFS rate was 47.3%, 3-year OS rate was 35.9%, and 5-year OS rate was 16.0% (Figure 1A). Among different PTCL subtypes, SPTCL was associated with the best prognosis, followed by ALCL, AITL, PTCL, and EATL (supplemental Figure 1). Evaluation of the first line of chemotherapy suggested that patients in group 1 and group 2 experienced similar OS (P = .496; Figure 1B). This pattern was observed across different PTCL subtypes (supplemental Figures 2-4). The median PFS was 11 months (Figure 1C). When PFS was analyzed according to first line of chemotherapy, there were no significant differences between group 1 compared with group 2 (P = .127; Figure 1D).
OS and PFS of all patients. (A) OS of all patients treated at SNUH. (B) OS of all patients treated at SNUH according to first-line chemotherapy. (C) PFS of all patients treated at SNUH. (D) PFS of all patients treated at SNUH according to first-line chemotherapy. (E) OS of all patients enrolled in KCCR. (F) OS of all patients enrolled in KCCR according to first-line chemotherapy. (G) PFS of all patients enrolled in KCCR. (H) PFS of all patients enrolled in KCCR according to first-line chemotherapy.
OS and PFS of all patients. (A) OS of all patients treated at SNUH. (B) OS of all patients treated at SNUH according to first-line chemotherapy. (C) PFS of all patients treated at SNUH. (D) PFS of all patients treated at SNUH according to first-line chemotherapy. (E) OS of all patients enrolled in KCCR. (F) OS of all patients enrolled in KCCR according to first-line chemotherapy. (G) PFS of all patients enrolled in KCCR. (H) PFS of all patients enrolled in KCCR according to first-line chemotherapy.
In national registry data, our findings in the single-center study were exaggerated. During the median follow-up of 65 months, 1-year PFS rate was 39.2%, 3-year OS rate was 41.3%, and 5-year OS rate was 18.9% (Figure 1). Evaluation of first-line chemotherapy showed that patients in group 1 experienced better OS (P = .006) compared with those in group 2 (Figure 1). This pattern of OS was also observed across different PTCL subtypes (supplemental Figures 2-4). Regarding PFS, patients in group 1 experienced better PFS (P = .001) compared with those in group 2.
Through multivariate analysis, age and stage were recognized as independent prognostic factors of OS and PFS in both cohorts (Table 3). Etoposide addition was not associated with improved survival outcomes.
CHOP vs augmented CHOP as first-line chemotherapy
In attempts to evaluate if intensification of chemotherapy can lead to better prognosis, patients in group 1 were further divided into 2 groups: (1) patients receiving CHOP and (2) patients receiving COPBALM or hyperCVAD (ie, augmented CHOP). Those receiving CVP were eliminated for this analysis.
In the single-center study, there were 77 patients in the CHOP group and 23 in the augmented CHOP group. The characteristics of these patients are presented in supplemental Table 4. The patients in the augmented CHOP group tended to be younger compared with those in the CHOP group (P = .025). There were no differences between the 2 groups with regard to OS (P = .917; Figure 2A). The median PFS in the CHOP group was 11 months, compared with 15 months in the augmented CHOP group, but the difference was not statistically significant (P = .740; Figure 2B).
Comparison of survival according to chemotherapy regimen among patients in group 1. (A) OS between patients receiving CHOP vs those receiving augmented CHOP based on SNUH data. (B) PFS between patients receiving CHOP vs those receiving a CHOP-like regimen based on SNUH data. (C) OS between patients receiving CHOP vs those receiving a CHOP-like regimen based on KCCR data. (D) PFS between patients receiving CHOP vs those receiving a CHOP-like regimen based on KCCR data.
Comparison of survival according to chemotherapy regimen among patients in group 1. (A) OS between patients receiving CHOP vs those receiving augmented CHOP based on SNUH data. (B) PFS between patients receiving CHOP vs those receiving a CHOP-like regimen based on SNUH data. (C) OS between patients receiving CHOP vs those receiving a CHOP-like regimen based on KCCR data. (D) PFS between patients receiving CHOP vs those receiving a CHOP-like regimen based on KCCR data.
CHOP vs CHOP plus etoposide as first-line chemotherapy
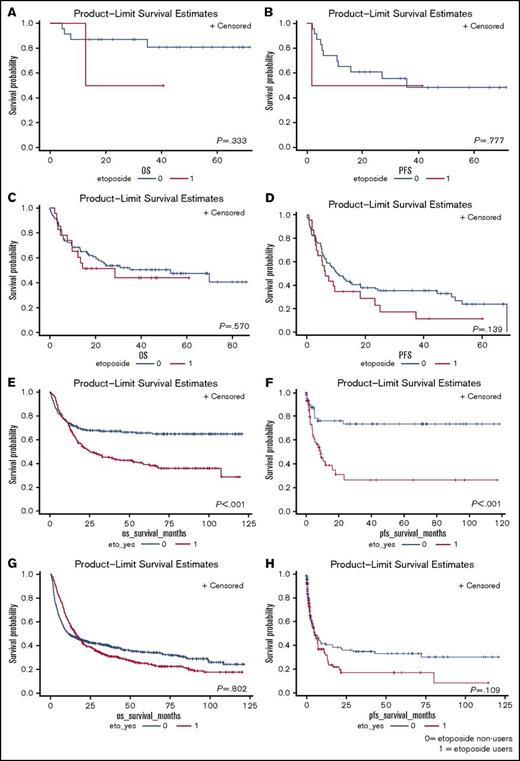
As a subgroup analysis, we compared the outcomes of patients treated solely with CHOP vs those treated with CHOP plus etoposide. Overall, similar results were seen in both cohorts in this repeated analysis. In the single-center study, there were 77 patients treated with CHOP and 20 treated with CHOP plus etoposide. Patients in both groups experienced similar OS (P = .654; supplemental Figure 5A) and PFS (P = .383; supplemental Figure 5B). Patients receiving etoposide experienced more dose delays during their treatment (supplemental Table 5).
In national registry data, there were 646 patients receiving CHOP vs 609 patients receiving CHOP plus etoposide. Patients treated solely with CHOP experienced better OS (P < .001; supplemental Figure 6A) and PFS (P < .001; supplemental Figure 6B). Etoposide addition led to greater incidence of transfusion requirement (supplemental Table 4). Through multivariate analysis, we recognized etoposide as an adverse prognostic factor in national registry data (supplemental Table 6).
Subgroup analysis according to age
The effects of intensification of chemotherapy were examined in presumably more fit patients, defined as those <45 years of age (Figure 3). In the single-center study, among 25 patients age <45 years, etoposide addition did not lead to better PFS (P = .777) or OS (P = .333). In national registry data, among 476 patients age <45 years, etoposide addition led to worse PFS (P = .003) and OS (P < .001). The possible benefits of augmented CHOP were analyzed and are shown in supplemental Figure 7.
Effect of age on survival outcomes for patients. (A) OS between etoposide users and nonusers among patients age <45 years old based on SNUH data. (B) PFS between etoposide users and nonusers among patients age <45 years based on SNUH data. (C) OS between etoposide users and nonusers among patients age ≥45 years based on SNUH data. (D) PFS between etoposide users and nonusers among patients age ≥45 years based on SNUH data. (E) OS between etoposide users and nonusers among patients age <45 years based on KCCR data. (F) PFS between etoposide users and nonusers among patients age <45 years based on KCCR data. (G) OS between etoposide users and nonusers among patients age ≥45 years based on KCCR data. (H) PFS between etoposide users and nonusers among patients age ≥45 years based on KCCR data.
Effect of age on survival outcomes for patients. (A) OS between etoposide users and nonusers among patients age <45 years old based on SNUH data. (B) PFS between etoposide users and nonusers among patients age <45 years based on SNUH data. (C) OS between etoposide users and nonusers among patients age ≥45 years based on SNUH data. (D) PFS between etoposide users and nonusers among patients age ≥45 years based on SNUH data. (E) OS between etoposide users and nonusers among patients age <45 years based on KCCR data. (F) PFS between etoposide users and nonusers among patients age <45 years based on KCCR data. (G) OS between etoposide users and nonusers among patients age ≥45 years based on KCCR data. (H) PFS between etoposide users and nonusers among patients age ≥45 years based on KCCR data.
Role of upfront consolidation HSCT
The role of upfront HSCT as consolidation was analyzed among those achieving partial response or better with first-line chemotherapy. Results showed upfront HSCT led to better OS (P = .047; supplemental Figure 8A). Because the timing of HSCT could not be exacted from national registry data, comparative effects of upfront HSCT alone could not be evaluated in the KCCR cohort.
Discussion
Despite lack of evidence that addition of etoposide to CHOP or other intensification of primary treatment improves outcomes for patients with PTCL,23 it is widely used to compensate for the less-than-satisfactory results of CHOP. However, through our study, we found that addition of etoposide was not associated with better survival; in fact, it might actually be more toxic. Patients receiving CHOP or a CHOP-like regimen plus etoposide had shorter PFS and OS compared with those receiving CHOP or a CHOP-like regimen. This trend was observed across different subtypes of PTCL and various age groups. We also observed that augmented CHOP was associated with little benefit. This report, as far as we know, is the biggest Asian population–based study on PTCL and provides important information about treatment strategies and outcomes in an unselected cohort. Also, the need for a more optimized therapeutic approach for Asian populations is highlighted.
This study was carried out in 2 parts: first, we analyzed patients with PTCL from a single tertiary academic center to get a general idea of the baseline demographics and treatment outcomes; then, we extrapolated the study to an unbiased general population for confirmation of our results. The composition of PTCL subtypes and initial treatment strategies differed between the 2 cohorts. In the single-center study, there were similar percentages of patients with PTCL-NOS and AITL, followed by ALCL. In national registry data, however, PTCL-NOS was predominantly the most common subtype of PTCL, followed by ALCL and AITL. Such a discrepancy is not surprising, because there is the inherent problem of referral bias in our single-center study. Indeed, an epidemiology study of T-cell lymphomas carried out in another tertiary referral center in Korea24 showed PTCL-NOS to be the most common subtype of PTCL at that center. However, overall, PTCL-NOS, AITL, and ALCL remained the 3 most common subtypes of PTCL, and this agrees with current knowledge of PTCL epidemiology. However, the differences in practice pattern was quite interesting. In the single-center study, CHOP or CHOP-like regimens were almost uniformly administered, and 80.9% of all enrolled patients were in group 1. In comparison, national registry data showed similar proportions of patients in group 1 and group 2. This real-world reflection of discordant practice patterns only emphasizes the importance of establishing a standard approach to PTCL treatment.
Current National Comprehensive Cancer Network guidelines for PTCL13 equally recommend CHOP plus etoposide and CHOP for at least the 3 most common types of PTCL. Augmented CHOP, namely hyperCVAD alternating with methotrexate and cytarabine, is also recommended with the same level of evidence. However, through our study, we found that etoposide addition has little benefit in treatment of PTCL, regardless of subtype. Even in multivariate analysis, etoposide addition did not have positive effects on survival; instead, it trended toward being an adverse risk factor. This finding goes against the current guidelines but is in agreement with a previous large-cohort study by the Swedish group.5 Because Ellin et al5 suggested etoposide might have a role in younger patients with PTCL, we examined the effect of etoposide addition in those age <45 years. As presented in Figure 3, we found that etoposide addition and chemotherapy intensification did not improve survival, even in fit and young patients. One quite salient difference between the single-center study and national registry data was the duration of PFS and OS. This discrepancy can be attributed to the different follow-up period. Also, the time point of data collection was different. It is likely that patients in the single-center study benefitted from improved salvage treatment and supportive care modalities, which contributed to longer survival. However, it is important to note that despite the time lapse between the 2 cohorts, the pattern of survival remained similar, and thus, difference in survival duration does not diminish the credibility of our results.
To examine the role of consolidation with autologous SCT (ASCT), survival for patients undergoing upfront HSCT were considered (supplemental Figure 5). Because there were only 8 patients undergoing upfront HSCT in the single-center study, the role of consolidation with ASCT was not evaluated per PTCL subtype. Again, because of small sample size, possible confounding factors such as baseline Eastern Cooperative Oncology Group (ECOG) performance were not considered for analysis. Unfortunately, because we could not determine the timing of HSCT or the response to chemotherapy for patients enrolled at KCCR from the available data, the role of upfront HSCT could not be analyzed in detail in the KCCR cohort. However, we did observe a median OS of 49 months for 145 patients undergoing upfront HSCT, which was significantly longer than the median OS of the total KCCR cohort (ie, 16 months; supplemental Figure 5B). As reported by previous studies, ASCT in the salvage setting is as useful in PTCL as it is in aggressive B-cell lymphomas25 and is also beneficial as consolidation after first remission in aggressive non-Hodgkin lymphoma.26 On the basis of these findings and our subgroup analysis, we suggest that HSCT has at least some positive roles in treatment of PTCL, especially as consolidation during first remission. Instead of intensification of induction chemotherapy, selective administration of consolidative transplantation can be a judicious treatment strategy for PTCL.
One of the major limitations of our study included the lack of detailed baseline characteristics in national registry data. Important prognostic factors, including International Prognostic Index/Prognostic Index for T-Cell Lymphomas score, ECOG performance status, and extent of disease, are lacking. However, at least some aspects of the prognostic scoring, such as age and stage at diagnosis, had been incorporated into the multivariate analysis. Furthermore, we included CCI score as tentative substitution for ECOG performance status for a better prognostication model. Another limitation is the lack of detailed chemotherapy response evaluation in national registry data. However, because OS ultimately represents the final treatment outcome, we believe our results remain indisputable. Finally, it should also be taken into consideration that our results are based on an Asian population, who are generally frailer and less tolerant to chemotherapy compared with white populations. The tentative age cutoff for presumably fitter patients was 45 years in our cohort compared with the 60 years used in previous studies, emphasizing the racial difference in chemotherapy safety and efficacy. As such, our study suggests there is a need for more innovative treatment approaches in Asian populations with PTCL.
In conclusion, through 1 of the biggest population studies outside of the clinical trial setting, we report that etoposide has little role in treatment of PTCL in Asian populations, regardless of subtype or age. Upfront HSCT as consolidation seems like a legitimate choice, but more investigation is required. We hereby provide crucial information on establishing a standardized PTCL treatment.
Presented in poster form at the International Conference on Malignant Lymphoma, Lugano, Switzerland, 14-17 June 2017.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all the staff members of the Hematology and Medical Oncology Departments of Seoul National University Hospital for their hard work and dedication, with special thanks to S. Park, D. S. Heo, I. Kim, T. M. Kim, B. S. Keam, and D. -Y. Shin.
This study was supported by the National Cancer Center, Korea (NCC-1632080).
Authorship
Contribution: Y.K. and J.M.B. conceived of and designed the study; K.P., D.L., D.S.K., S.-S.Y., and Y.K. provided study materials; J.M.B., Y.A.K., and G.H.B. collected and assembled data; J.M.B., Y.A.K., G.H.B., and Y.K. analyzed and interpreted data; and all authors wrote and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Youngil Koh, Department of Internal Medicine, Seoul National University Hospital, 101, Daehak-ro, Jongro-gu, Seoul 03080, Korea; e-mail go01@snu.ac.kr.
References
Author notes
Y.A.E. and J.M.B. are joint first authors and contributed equally to this work.