Key Points
In sickle cell disease, ONFH incidence accelerates in early adulthood.
Frequent hospitalizations and antecedent acute chest syndrome are independently associated with sickle cell–related ONFH.
Abstract
Osteonecrosis of the femoral head (ONFH) is a prevalent complication of sickle cell disease (SCD) that has not been well described in population-based cohort studies. Using California’s Office of Statewide Planning and Development discharge databases (1991-2013), we estimated the cumulative incidence of ONFH after accounting for the competing risk of death and used a multivariable Cox proportional hazards regression to identify factors associated with ONFH diagnosis. We also calculated rates of readmissions to the hospital or emergency department within 30 to 90 days of hip replacement surgery. Of the 6237 patients in our SCD cohort, 22% (n = 1356) developed ONFH at a median age of 27 years, and 23% (n = 308) of the patients with ONFH underwent hip replacement surgery at a median age of 36 years. The cumulative incidence of ONFH to age 30 years was higher among SCD patients with more severe disease (24%; vs 8% in less severe) and those with antecedent acute chest syndrome (ACS) (18%; vs 8% without prior history of ACS). From 2003 to 2013, SCD patients with more severe disease (hazard ratio [HR], 2.77; 95% confidence interval [CI], 2.38-3.23) or with antecedent ACS (HR, 1.61; CI, 1.35-1.91) were more likely to develop ONFH. Twenty-seven percent of post–hip surgery patients were readmitted within 30 days, mostly for painful vaso-occlusive crises. ONFH is a common SCD complication that increases with age; ongoing studies into prevention and effective nonsurgical interventions for SCD-induced osteonecrosis must remain a high research priority.
Introduction
Osteonecrosis, a common skeletal complication of sickle cell disease (SCD), presumably arises when stiff and abnormally adherent red blood cells repeatedly impair blood flow to susceptible articular surfaces, causing bone infarction at the epiphyseal plates and early onset degenerative arthritis.1,2 Although multiple joints can be simultaneously affected by osteonecrosis in SCD, the femoral head is most commonly involved because it lacks collateral blood flow and is most vulnerable to vascular insults.3-5 Osteonecrosis of the femoral head (ONFH) prevalence ranges from 10% to 30% in single-institution and collaborative research studies.6-8
ONFH may rapidly progress to femoral head collapse and intractable hip joint pain in patients with SCD.9-11 Indeed, advanced ONFH is the most common indication for total hip arthroplasty in young adults with SCD.9,12 The National Heart, Lung, and Blood Institute recommends symptomatic management with analgesics, physical therapy, and early referral for hip surgery for patients with SCD-related ONFH because no standard medical management exists.13,14 However, perioperative complications in SCD patients increase the morbidity of hip arthroplasty; surgical outcomes can be varied, with up to 75% reporting persistent hip pain postoperatively.15,16 Although emerging data support the noninferiority of less invasive alternative procedures to joint replacement surgery,17-20 there is a dearth of outcomes studies on hip arthroplasty as the gold standard of treatment of SCD patients with advanced ONFH.
Medical advances in SCD management such as penicillin prophylaxis, hydroxyurea therapy, and chronic red blood cell transfusions have improved survival well into adulthood.21-24 Many health services and outcomes research in SCD have focused on disease prevalence, incidence, and mortality,25-29 but few have addressed chronic SCD complications in general, or ONFH in particular.16,30 In our present study, we estimated the cumulative incidence and clinical burden of ONFH in patients with SCD in California, highlighted differences between SCD patients with and without ONFH, and determined the association between ONFH and other common SCD complications. We also estimated readmission rates and diagnoses following hip arthroplasty in our SCD cohort.
Methods
Database
California’s Office of Statewide Health Planning and Development (OSHPD) maintains records of all patients hospitalized in non-Federal hospitals in the state on the Patient Discharge Database (PDD). Since July 1990, the State of California has required these hospitals to report up to 25 diagnoses, or 20 procedures, or both, associated with each hospitalization and coded using the International Classification of Disease (9th revision, Clinical Modification; ICD-9-CM). Since 1996, all PDD diagnoses have also included a present-on-admission indicator. Since 2005, an Emergency Department Utilization (EDU) database of all hospital-associated emergency rooms has also been mandated. Besides diagnostic and procedure information, both databases have collected demographic data including age, sex, race/ethnicity, insurance coverage, types of admission, and disposition.
An encrypted form of the social security number, which is the record linkage number, identifies unique individuals and allows for serial linking of multiple hospitalization records over time. We excluded approximately 5% of patients who did not have a record linkage number from our analysis. We then linked our data to the California master death registry, which provided death information through 2011. California’s Health and Human Services Agency Committee for the Protection of Human Subjects and the University of California, Davis, institutional review board approved our retrospective cohort study, and the study was conducted in accordance with the Declaration of Helsinki.
Patient identification
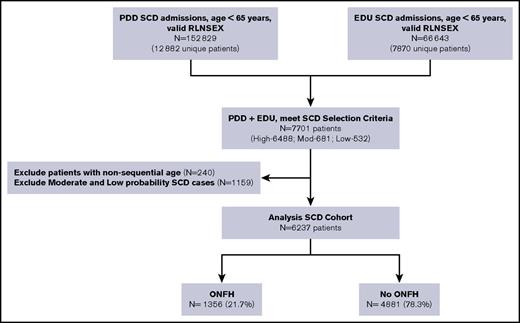
We identified all SCD patients who had PDD and EDU encounters from 1991 to 2013 using search algorithms informed by the Registry and Surveillance System in Hemoglobinopathies project, validated in studies from the Public Health Research, Surveillance, and Epidemiology in Hemoglobinopathies,27 and recently published by our group.31 We searched for specific ICD-9-CM codes for SCD found in any of the 25 diagnosis fields (supplemental Table 1). To be included in this analysis, subjects were required to have a high probability of SCD, which we defined as (1) ≥2 separate admissions with an SCD code in the principal (first position) diagnosis, or (2) ≥1 admission with an SCD code in the principal position and an SCD code in a secondary position on ≥2 additional admissions. We found a principal diagnosis of SCD in 63% of all inpatient hospitalizations and 37% of all emergency department visits in our SCD cohort.
Our senior author (T.W.) reviewed a random sample of discharge records, representing 10% of the entire derived cohort, for face validity. Patients ages ≥65 years at first encounter were deemed unlikely to have SCD and therefore excluded from the cohort. On the basis of concomitant diagnoses, age at entry onto the databases, and presence of sickle cell trait (ICD-9-CM code 282.5), we determined that most cases were likely false positives. Thus, the algorithm emphasized specificity over sensitivity (Figure 1). To further confirm the fidelity of serial records, we carefully reviewed all patient ages in chronological order. If age at preceding admission was more than 2 years greater than the immediate subsequent admission, then these linked cases were invalidated and excluded from the SCD cohort. We opted against using ICD-9-CM codes for hemoglobin genotype to improve upon SCD specificity because 67% of the SCD cases had multiple genotypes coded across different admissions. We excluded all SCD patients who underwent hip surgery prior to their first ONFH diagnosis (n = 22).
California SCD cohort diagram, 1991-2013. RLNSEX, Record Linkage Number merged with SEX.
California SCD cohort diagram, 1991-2013. RLNSEX, Record Linkage Number merged with SEX.
Covariates and outcomes
We obtained patient sex and race/ethnicity information from their first encounter in the PDD or EDU. We dichotomized SCD into more or less severe disease on the basis of frequency of admissions on either database. Each individual with SCD who averaged ≥3 admissions for any indication per year (PDD and EDU combined) was defined as having more severe SCD, and those with an average of <3 admissions per year per patient had less severe SCD. We based our SCD severity classification on previously published data showing higher mortality in SCD patients with ≥3 hospitalizations per year,32 a criterion that was later used as one of the indications for starting hydroxyurea treatment in the Multicenter Study of Hydroxyurea in Sickle Cell Anemia study.33
The diagnostic code for acute chest syndrome (ACS), a serious cardiopulmonary SCD complication associated with increased mortality in adult patients,34-36 became available in October 2003. We defined ACS cases using ICD-9-CM code 517.3 in any diagnosis position in either the PDD or EDU. We identified the primary outcome of interest (incidence of first ONFH) using specific ICD-9-CM codes in either the PDD or EDU (supplemental Table 1). Secondary outcomes included prevalence of hip arthroplasty in ONFH patients and postsurgical readmission rates for any indication, with specific attention paid to vaso-occlusive crises, venous thromboembolism, infection, and prosthesis malfunction.
Statistical methods
We used descriptive statistics and univariate comparisons with the χ2 test to determine differences between SCD patients with and without ONFH. We calculated person-time incidence rates by age decile to determine age range at peak ONFH onset. Because the US Food and Drug Administration (FDA) approved hydroxyurea for treatment of SCD with certain severe complications in 1998, we also calculated ONFH incident rates per decade in the prehydroxyurea (1991-2000) and posthydroxyurea (2001-2013) eras. Using the cumulative incidence function and age as a time scale, we estimated the cumulative incidence of ONFH and adjusted for the competing risk of death.37 We defined age as age at entry into database to age at first ONFH diagnosis, age at death, or age at the end of the study in 2013, whichever occurred first. We further stratified the cumulative incidence curve by SCD severity and antecedent ACS diagnoses, and assessed for differences using Gray’s test for equality. Using multivariable Cox proportional hazards regression, and stratifying by birth cohort to adjust for changes in diagnosis or treatment over time, we analyzed factors associated with ONFH diagnosis, including sex, race/ethnicity, and SCD severity.
In a subanalysis from October 1, 2003, to December 31, 2013, we assessed antecedent ACS as a time-dependent covariate to first ONFH diagnosis. We also determined rates of all readmissions to the PDD and EDU within 30 to 90 days after hip replacement surgery, specifically examining readmissions for painful vaso-occlusive crises (VOCs), venous thromboembolism (VTE), infections, and prosthesis malfunction. We used age as a time scale for the cumulative incidence and regression models and presented our results as hazard ratios (HRs) with 95% confidence intervals (CIs). We analyzed all our data using SAS 9.4 software.
Results
Characteristics of SCD patients with ONFH
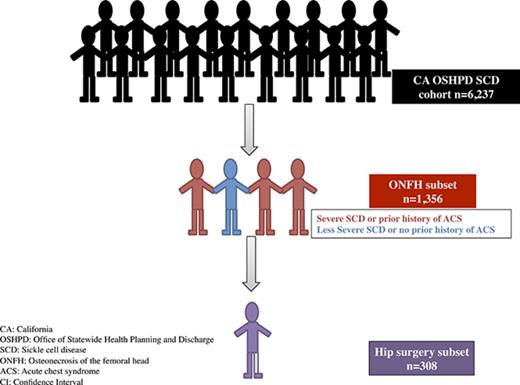
We identified 6237 unique SCD patients followed over a median duration of 15 years (0-23 years) and for a total of 75 529 person-years. Of these, 1356 (22%) were diagnosed with ONFH (Figure 1), with 75% of the ONFH subgroup (n = 1002) having specific ICD-9-CM codes 733.42 and 733.43. Median ages were 27 years at ONFH diagnosis and 36 years at hip replacement surgery. Baseline patient characteristics between ONFH cases and non-ONFH comparators were most notably different in disease severity and antecedent ACS categories (Table 1). Individuals in the ONFH group were followed for a median duration of 18 years (quartile 1 [Q1] = 11.6 years, Q3 = 22.1 years), in comparison with 14 years (Q1 = 7.5 years, Q3 = 19.9 years) median follow-up in the non-ONFH group.
After adjusting for race/ethnicity and stratifying by birth cohort, we found insignificant differences in ONFH diagnosis by sex (Table 2). The incidence rate for ONFH in those with more severe SCD was 3.00 per 100 person-years, in comparison with 1.06 per 100 person-years in those with less severe SCD (incidence rate ratio = 2.83). In a subanalysis of the SCD cohort from 2003 to 2013, the incidence rate for ONFH in those with antecedent ACS was 2.18 per 100 person-years, in comparison with 0.8 per 100 person-years in those without prior history of ACS (incidence rate ratio = 2.73). The incidence rate of ONFH in the prehydroxyurea era (1991-2000) was 2.37 per 100 person-years (95% CI, 2.18-2.56), in comparison with 1.52 per 100 person-years (95% CI, 1.41-1.63) in the posthydroxyurea era (2001-2013), for an incident rate ratio of 1.56. This difference persisted across all comparable age groups (Table 3).
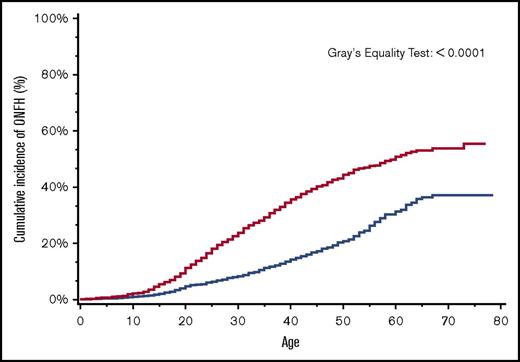
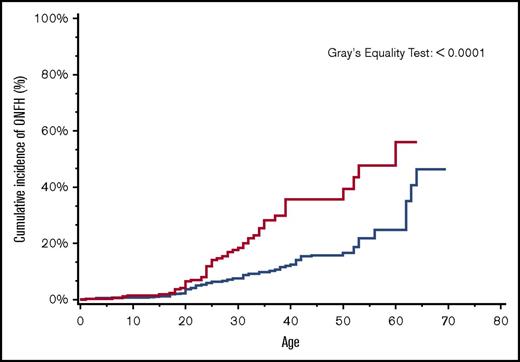
By age 30 years, the overall cumulative incidence of ONFH was 15% (95% CI, 14.4-16.5), with a significantly higher cumulative incidence of 24% among patients with more severe SCD (95% CI, 22.0-25.5) in comparison with 8% (95% CI, 7.4-9.5) in those with less severe SCD (Figure 2). Similarly, in a subanalysis of the SCD cohort after ACS codes became available in 2003, ONFH cumulative incidence in those with antecedent ACS was 18% (95% CI, 13.1-24.4) in comparison with 8% (95% CI, 5.8-9.6) in those without antecedent ACS (Figure 3).
Cumulative incidence of ONFH among SCD patients by SCD severity, California, 1991-2013 (n = 6237). SCD patients with more severe disease (red line); SCD patients with less severe disease (blue line).
Cumulative incidence of ONFH among SCD patients by SCD severity, California, 1991-2013 (n = 6237). SCD patients with more severe disease (red line); SCD patients with less severe disease (blue line).
Cumulative incidence of ONFH among SCD patients by antecedent ACS, California, 2003-2013 (n = 1538). SCD patients with antecedent ACS (red line); SCD patients without antecedent ACS (blue line).
Cumulative incidence of ONFH among SCD patients by antecedent ACS, California, 2003-2013 (n = 1538). SCD patients with antecedent ACS (red line); SCD patients without antecedent ACS (blue line).
Multivariate Cox regression analyses of the entire SCD cohort (1991-2013), stratified by birth cohort, confirmed that SCD severity (HR, 2.52, 95% CI, 2.23-2.84) was associated with ONFH. In a subanalysis of data from 2003 to 2013, both SCD severity (HR, 2.77, 95% CI 2.38-3.23) and antecedent ACS (HR, 1.61, 95% CI, 1.35-1.91) were independently associated with ONFH (Table 2).
Perioperative and postoperative outcomes of hip surgery in SCD patients with ONFH
Twenty-three percent (n = 308) of the 1356 patients with ONFH underwent hip replacement surgery at a median age of 36 years. Of these, 27% were readmitted (inpatient or emergency department) within 30 days of discharge after hip replacement surgery, and 48% were readmitted by 90 days. Vaso-occlusive crises accounted for 14% of readmissions by 30 days of discharge from the hip surgery admission, and 28% of readmissions by 90 days postdischarge. Venous thromboembolism occurred in <1% of patients readmitted over those same time periods. Nonspecific blood stream and surgical wound infections accounted for 8% of readmissions within 30 days of discharge and 12% within 90 days of discharge from hip replacement surgery admission (Table 4). Prosthetic hip joints malfunctioned in <4% of postsurgical patients and only within the 30-day postdischarge period. There was no mean difference in admissions rates in the 2 years prior to hip surgery in comparison with postoperative years 2 and 3 (we excluded data from the first year after hip surgery to avoid capturing immediate postoperative complications).
Discussion
The current study shows that ONFH commonly affects patients with SCD and often requires surgical intervention. SCD patients with more severe disease (as defined herein) or those with a history of ACS seem at heightened risk of ONFH. Following hip replacement surgery, vaso-occlusive crises and other complications commonly occur. ONFH morbidity has mostly been described in small, cross-sectional, single-institution studies,38-43 with findings that might not be generalizable to a larger population of SCD patients. In our novel approach, we built a large SCD cohort from the socioeconomically diverse state of California, using an iterative process based on methods described in other SCD epidemiologic studies.26-29,44-46 We then assessed the clinical burden of ONFH and hip postsurgical outcomes in the entire cohort, using regression techniques to identify potential predictors for ONFH diagnosis and need for hip arthroplasty.
Twenty-two percent of our SCD cohort developed ONFH, which is more than twice the percentage previously reported in 2 studies.6,16 In the smaller of the studies, Matos et al detected an 11% prevalence of ONFH in a cross-sectional analysis of 72 SCD study subjects younger than 21 years old.6 In a subanalysis of the much larger Cooperative Study of Sickle Cell Disease, Milner et al found that approximately 10% of the nearly 2600 SCD patients recruited from 23 clinical centers across the United States had plain film radiographic evidence of ONFH at study entry.16 Because ONFH prevalence increases with age and other comorbid conditions,7,47,48 both the younger patients in the Matos et al study and the shorter follow-up duration and sole use of plain film imaging in the study by Milner et al possibly account for the discrepancy in ONFH prevalence between these older studies and ours.
ONFH incidence rates
We found an overall ONFH incidence rate of 1.79 cases per 100 person-years in our SCD cohort. The Cooperative Study of Sickle Cell Disease investigators prospectively monitored more than 1700 SCD subjects without ONFH at study entry with bilateral hip joint plain films and reported a 9% overall incidence of ONFH over 3 years of follow-up.16 They included hemoglobin genotype in their analysis and found ONFH incidence rates, per 100 patient-years, of 4.47 in subjects with coinherited homozygous SCD (hemoglobin SS [HbSS]) and α-thalassemia, 2.35 in subjects with hemoglobin SS without α-thalassemia, and 1.91 in subjects with hemoglobin SC disease (compound heterozygote with hemoglobin S and hemoglobin C). The lower incident rate for ONFH in our observational study, in comparison with Milner et al’s prospective cohort study, possibly reflects the absence of routine imaging to screen patients for asymptomatic ONFH in clinical practice.
In our SCD cohort, the median age at ONFH diagnosis was 27 years, whereas Milner et al found differential ages of ONFH onset based on hemoglobin genotype. Within their HbSS cohort, subjects with coinherited α-thalassemia developed ONFH at a median age of 28 years, whereas those without coinherited α-thalassemia were diagnosed with ONFH at a median age of 36 years. Subjects with hemoglobin SC disease developed ONFH at a median age of 40 years. Our SCD cohort’s median age at ONFH onset was similar to the HbSS with coinherited α-thalassemia subgroup from Milner et al and the HbSS group from a more recent study from the Johns Hopkins University Sickle Cell Center for Adults.49
ONFH risk factors
In a subanalysis of our study from 2003 to 2013, patients with more severe SCD were more likely to develop ONFH than were those with less severe disease at an HR of 2.77 and a 95% CI of 2.38-3.23, although to a lesser degree, patients with SCD and antecedent ACS were also more likely to develop ONFH than were those without prior ACS (HR, 1.61; 95% CI, 1.35-1.91). Our findings corroborate prior studies that showed osteonecrosis aggregating with VOC and ACS, collectively termed the viscosity–vaso-occlusion subphenotype of SCD by Kato et al.50 Other disease-related complications such as pulmonary hypertension, hemolysis, priapism, and leg ulcers were grouped under the hemolysis–endothelial dysfunction subphenotype,50 which we did not investigate in our current study.
Interestingly, we observed that ONFH incidence rates were lower in the posthydroxyurea era across all age groups. We speculate that hydroxyurea use decreased frequency of VOCs and ACS, thus decreasing ONFH incidence if they are causally linked. However, FDA approval of hydroxyurea does not equate to universal prescription (at appropriate doses) by SCD providers or consistent medication adherence by patients with SCD. Because OSHPD lacks medication data, we were not able to directly associate reduced ONFH incidence to hydroxyurea therapy. SCD providers should have a low threshold to assess for ONFH in patients receiving hydroxyurea with mild hip joint symptoms, given the overlap between our identified risk factors and indications for starting this disease-modifying drug.
Hip surgery outcomes
Approximately 23% (n = 308) of the SCD patients with ONFH underwent hip arthroplasty at a median age of 36 years, which is similar to the mean age of 32 years previously reported in a single-institution study.42 In our SCD cohort, readmission rates within 30 days after hip surgery were similar to previously reported all-cause readmission rates51 and most often attributed to painful vaso-occlusive crises. Prior studies have shown suboptimal surgical outcomes in SCD patients undergoing hip surgery for advanced ONFH with complications such as bleeding and infections triggering vaso-occlusive pain crises in the perioperative period.9,14,42 The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study demonstrated that preemptive red blood cell transfusions (goal hemoglobin of 10 g/dL) in SCD patients undergoing moderate-risk surgeries reduced the risk of clinically significant complications within 30 days after surgery.52 We decided against including perioperative blood transfusions in our analysis because transfusions are considered minor procedures in the PDD and are likely inaccurately coded because they do not increase the diagnosis-related group severity for inpatient reimbursement. Furthermore, many participants in the preemptive transfusion group (intervention arm) of the TAPS study were transfused in the outpatient setting, and outpatient data were unavailable in our study.
Despite recently reported increased VTE incidence in our SCD cohort,31 there were no admissions for VTE within 30 days of discharge from hip replacement surgery. This is in contrast to 0.2% of readmissions for pulmonary embolism in older non-SCD adults undergoing hip replacement surgery in the United States and 0.3% rates of readmission for pulmonary embolism in older non-SCD patients in Canada.53 In our SCD cohort, VTE diagnoses accounted for 1.3% (n = 4) readmissions between 30 and 90 days from discharge for hip replacement surgery.
Strengths and limitations
There are limitations to this study. Our cohort comprised SCD patients discharged from hospitals and emergency rooms; thus, we possibly introduced detection bias for ONFH by excluding healthier patients with SCD who are not seen in these settings. Migration across state lines may also result in losing patients to follow-up. Coding for hemoglobin genotype and blood transfusions are unreliable in the OSHPD dataset. Laboratory and medication data are also excluded. However, there are also strengths. Most published studies of SCD-related ONFH are based on single-institution investigations, with inferences drawn from small sample sizes. The higher cumulative incidence of ONFH in our study could be related to longer duration of follow-up and wider use of magnetic resonance imaging in more recent years. The incidence of hip arthroplasty is most likely accurate as this inpatient surgical procedure would be correctly coded for billing purposes. Despite lack of source documentation verification, OSHPD PDD data have been extensively used for health services and epidemiologic research, and accurately coded diagnoses are considered reliable.54
Conclusion
In our large retrospective cohort study of patients with SCD in California, we found that ONFH incidence increases with age, especially in those with severe SCD and antecedent ACS. Hip replacement surgery for ONFH occurs at a young age, in comparison with the general population, and postoperative readmissions for painful vaso-occlusive crises are common. However, our findings of high postoperative readmissions rates for VOC is comparable to an older study of all-cause readmission rates for adults with SCD51 and should not detract from the potential benefit of hip arthroplasty to decrease pain and improve mobility in SCD patients with symptomatic ONFH. Our current study adds to prior work in its scope: use of a population-based cohort, long follow-up duration, and analysis stratified by birth cohort to account for changes in ONFH diagnosis and treatment options over time.
As the SCD population ages,55,56 epidemiologic research should pivot toward assessing risk factors, defining morbidity, and identifying effective therapies for chronic SCD complications such as ONFH. For example, preclinical studies on bone physiology of a mouse model of homozygous SCD (HbSS) show accelerated bone loss, when compared with hemoglobin AA mice (normal adult hemoglobin). Treating the HbSS mice with zoledronic acid, a potent intravenous bisphosphonate, reversed the bone loss and corrected their abnormal bone physiology.57 This preclinical observation, along with other studies showing positive outcomes with use of bisphosphonates in non-sickle patients with steroid-induced or traumatic ONFH,58-61 has sparked our interest in investigating the potential therapeutic benefit of bisphosphonates in SCD-related ONFH.
The full-text version of this article contains a data supplement.
Acknowledgments
O.A. wishes to acknowledge her former primary mentor Jason R. Gotlib of Stanford University School of Medicine and Clinical Research Training Institute faculty mentor Jane S. Hankins of St. Jude Children’s Research Hospital, Memphis, TN, for their mentorship throughout this project and ongoing support. O.A. also wishes to acknowledge the American Society of Hematology Clinical Research Training Institute, of which she is an alumna.
O.A. conducted this study as a postdoctoral research fellow in the Hematology Division of Stanford University School of Medicine, with support from a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (National Institutes of Health [NIH] grants KL2 TR 001083 and UL1 TR 001085). T.W. was supported by a grant from the NIH, National Center for Advancing Translational Sciences (UL1 TR0001860), University of California Davis Clinical and Translational Science Center.
Authorship
Contribution: O.A. developed the research concept; O.A., A.B., and T.W. designed and performed research; O.A. and A.B. performed statistical analyses; O.A., A.B., T.H.M.K., and T.W. analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for O.A. is University of Washington School of Medicine, Seattle, WA.
Correspondence: Oyebimpe Adesina, University of Washington, Seattle Cancer Care Alliance, 825 Eastlake Ave E, Box 358081, MS G3-200, Seattle, WA 98109; e-mail: adesina@uw.edu.