Key Points
We used a registry containing all 1 163 524 blood donor returns that took place in Finland between 2010 and 2015 to evaluate cHb recovery.
Average recovery times for cHb to return to the level of the preceding donation were longer than the minimum allowed donation intervals.
Abstract
Measuring the concentration of capillary hemoglobin (cHb) is a standard procedure before blood donation. To further assess the time period needed for cHb recovery after blood donation and to have a more in-depth understanding of features of recovery, we used data-mining tools in a large, retrospective data pool containing all 1 163 524 donor returns that took place in Finland in 2010 to 2015. The results show that the average recovery times for cHb to return back to the level preceding donation were substantially longer, over 200 days in all age groups, than were the minimum allowed donation intervals. cHb recovery was especially poor in women under the age of 30 who returned to donate soon after the minimum allowed donation interval. It was of interest that frequent donors recovered substantially faster, with the average recovery times of ∼100 days in men and ∼200 days in women, than did infrequent donors, suggesting that there is a subpopulation of donors who can donate frequently without fear of iron deficiency. Return interval in fact explained only 1% of the variation in cHb recovery, which points to unknown, individual features, such as genetic or lifestyle factors, warranting further studies and suggesting that simply extending the allowed donation intervals may not suffice to improve cHb recovery. The study demonstrates that data mining of blood bank records is a powerful tool for depicting features of blood donor population.
Introduction
Blood banks rely on committed, regular blood donors to maintain a sufficient inventory of blood products. Blood collection practices vary between blood establishments, with most allowing male donors to donate at 8-week-minimum intervals, with some recommending longer intervals for female donors. These intervals can be considered to be generally safe.1-3 A total of 200 to 250 mg of iron, however, is drawn with each whole-blood donation; the amount accounts for 25% of average tissue iron stores in men and up to 75% in women.4,5 Studies have suggested that a portion of blood donors may become iron depleted or deficient.6-11 Depending on populations and policies, 5% to 8% of presenting blood donors are deferred because of too low a level of hemoglobin, and for example, in the United States, 35% of frequent blood donors were found to be iron deficient.8 Adverse health effects have been linked to iron deficiency and anemia, though the significance of iron deficiency without anemia in otherwise healthy individuals is still unclear.1,12
Safe donation intervals were established in the 1940s using hemoglobin recovery as a marker for donor recovery.13,14 These studies have only limited applicability today owing to factors such as study settings and changes in donor demographics. A recent detailed analysis of 49 Dutch donors suggested that blood banks should consider increasing the donation interval to 180 days.11 To further assess the time period needed for hemoglobin recovery after blood donation and to provide an alternative perspective, we used data-mining tools in a large, retrospective data pool of the 1 163 524 attempted and registered whole-blood donations that took place in Finland in 2010 to 2015.
Materials and Methods
The study population
The Finnish Red Cross Blood Service is a national blood bank that collects, processes, and distributes all donated blood in Finland. All collection sites have access to a shared database (eProgesa, MAK-SYSTEM, Paris, France), wherein data on all donations are recorded. All the data were obtained as part of the routine evaluation of prospective blood donors; hence the need for consent was waived. All donations were voluntary and nonremunerated. The minimum whole-blood donation interval was 91 days for women and 61 days for men.
For the purpose of the present study, data (ie, donor identification, dates of all 1 163 524 registered whole-blood donations and their capillary hemoglobin (cHb) measurements, and the age, sex, and blood group of the donor) were collected in a pseudo-anonymized research database from all donors registered for whole-blood donation, including donors deferred for any reason, from the 1 January 2010 to 31 December 2015. Data on whole-blood donors and deferred donors with cHb measurements were included, whereas donors attending thrombapheresis or plasmapheresis were excluded. The number of individual donors was 219 149. Supplemental Table 1 shows their age and sex distribution. For the purpose of the study, the age of each donor was defined as the age on 1 January 2013; hence each individual donor would belong to a single age group only. Age limits for blood donation were 18 to 65 years (18-59 years for first-time donors), and from 10 February 2014 onward, active donors ages 66 to 70 were also allowed to donate. A donor return was defined either as two consecutive whole-blood donations or a whole-blood donation with succeeding cHb measurement without donation; that is, the donor had fulfilled other eligibility criteria but failed the cHb screen. A donation followed by a deferral without cHb measurement was not defined as a donor return.
Hemoglobin recovery
The change in cHb concentration (∆Hb) for each donor return was calculated by comparing the cHb concentration at the return time with the cHb concentration on the preceding whole-blood donation. The time between these 2 measurements is referred to as donor return interval, ∆T. Individual donors were grouped on the basis of age, sex, and donation activity. The donation activity groups were frequent (over 15 for men, over 10 for women), intermediate (6-15 for men, 6-10 for women) and infrequent (5 or below for both men and women) and were based on average donation frequency in Finland, 1.6 donations per year. For each postdonation day, days 61 to 500 for men and days 91 to 500 for women, a mean ∆Hb was calculated on the basis of the individual ∆Hb of each donor return on that specific day. A negative ∆Hb value indicates that the donor returning for donation had a cHb on that particular day lower than the cHb on her or his preceding donation day.
The data were analyzed using R (version 3.3.1) and plotted with the ggplot2 R-library.15,16 All smooth curves in the plots were fitted with generalized additive models of R-library mgcv with default settings.17 To estimate the amount of ∆Hb variation explained by ∆T, we fitted a single generalized additive model with a smooth curve for each unique combination of the variables sex, age group, and activity group and ∆T as log-transformed to the whole data set. On the basis of the Akaike information criterion, this model was better than were the linear models, models with fewer smooth curves, or models using nontransformed ∆T. The log-transformation also resulted in more randomly distributed residuals.
Blood collection
The targeted volume in whole-blood collection was 465 mL (acceptable range, 415-515 mL).
Measurement of cHb
The blood donation questionnaire protocol, cHb measurement protocol, and quality-control procedure have been recently described in detail.18 Briefly, a fingerstick capillary blood sample was analyzed from all donors before donation or attempted donation with the point-of-care device (HemoCue Hb201+, HemoCue AB). The acceptable cHb range was 125 to 175 g/L for women and 135 to 195 g/L for men. If the measured cHb was not in the acceptable range or if the cHb had decreased by more than 20 g/L from the previous visit, further testing was performed according to the blood service guidelines. Donors who did not fulfill the Hb criteria were deferred from donating for a minimum of 3 months; anemic donors were counseled to seek medical assessment.
For quality control, device-to-device variability of the HemoCue system was monitored monthly with a reference sample. The coefficient of variation was typically around 1%. The maximum deviation allowed from the mean for any single device was 5%. The results were compared monthly to external data in a national external quality assessment scheme organized by Labquality Oy, Helsinki, Finland.
To evaluate the correlation between the cHb and venous Hb measurements, we performed venous Hb measurement for 2382 samples from 1719 blood donors who participated in the FinDonor 10 000 research project, in addition to the standard cHb measurement taken at the same time point. The FinDonor 10 000 research project focuses on the measurement of iron stores of donors and the factors influencing it; the project started in 2016 and is open to all blood donors who give their consent to the study. The venous Hb measurements were performed at HUSLAB, Helsinki University Central Hospital (Helsinki, Finland) according to standard methods. These donations took place during 2016 and hence were not included in the data mining analyses. The cHb levels showed a good correlation with the venous Hb (P < 1 × 10−6, r = 0.85; data not shown).
Iron replacement
Iron replacement of 30 tablets containing 100 mg of ferrous sulfate (20 mg of elemental iron) was offered to all women under the age of 50 and to all donors regardless of age or sex donating at intervals of less than 4 months. In May 2015 the content changed: the first choice provided was 20 tablets containing 25 mg of ferrous fumarate and 350 mg of animal hemoglobin (9 mg of elemental iron), and the alternative choice was 30 tablets containing 100 mg of ferrous sulfate (20 mg of elemental iron). An information leaflet explaining the need for iron store repletion after blood donation was distributed with iron only when the donor requested additional information. According to our unpublished self-report data on 415 donors, 76% of those offered the iron replacement complied, and 95% of them reported to have ingested the offered dose fully or partly.
Results
This study is based on records collected from all 1 163 524 donor returns that took place in Finland in 2010 to 2015. Their age and sex distributions and average cHb concentrations in relation to the number of registered donations are summarized in supplemental Table 1 and supplemental Figure 1.
Figure 1 shows the cHb recovery curves, that is, the average time in days required for cHb recovery in men and women divided into different age groups. Figure 1 also shows the distribution of donor return intervals. There was a clear peak of donation frequency soon after minimum donation interval, 61 days in men and 91 days in women. In addition, there appears to be a second smaller peak about 30 days later in men. We did not see a peak around the 1-year interval (data not shown), as was shown in a US study.10 It is clear that on average the recovery took longer than the minimum donation interval: in men the ∆Hb reached 0 in ∼200 days, and in women the recovery time was even longer, particularly in women under the age of 30. As expected the average ∆Hb was clearly below 0 in all groups soon after the minimum donation interval had passed, regardless of age group or sex.
Hemoglobin recovery curves including all donor returns, subdivided into age groups below 30 years old, 31 to 50 years old, and over 50 years old and sex. The black line shows the population average. The upper panels show distributions of the donor returns. The lower panels show the average recovery of capillary Hb (∆Hb) as a function of return period (∆T in days). The 95% confidence intervals are shown as gray shading.
Hemoglobin recovery curves including all donor returns, subdivided into age groups below 30 years old, 31 to 50 years old, and over 50 years old and sex. The black line shows the population average. The upper panels show distributions of the donor returns. The lower panels show the average recovery of capillary Hb (∆Hb) as a function of return period (∆T in days). The 95% confidence intervals are shown as gray shading.
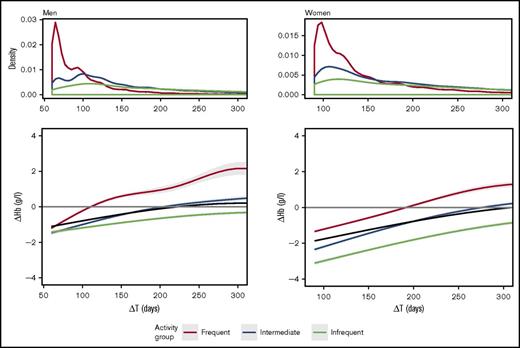
The data shown in Figure 1 include all donor returns in the study period. The recovery time as calculated here may be influenced by data of donors who donate very infrequently. For example, donors who donate only once a year cannot have an apparent recovery time of less than 1 year because their cHb will not be measured before that point. To understand the data better, we subdivided the data according to the donation frequency within the study period. Figure 2 shows the recovery times in frequent donors (over 10 for women and over 15 for men), intermediate donors (6-9 for women or 6-14 for men), and infrequent donors (5 or less) and the distribution of donation intervals in these groups. The distribution of donation intervals in frequent donors looked very similar to the distribution of all donor returns (Figure 1). However, it seems that the donors with intermediate frequency explained the secondary peak near 100 days. The recovery curves demonstrate that the average time for recovery in frequent donors, about 100 days in men and 200 days in women, was statistically significantly shorter than were the recovery times in the intermediate or infrequent donor return groups because the 95% confidence intervals did not overlap. The average ∆Hb of infrequent donors, interestingly, did not reach ∆Hb = 0 at all.
Hemoglobin recovery curves, including all donor returns, subdivided on the basis of donation frequency. Frequent donors (more than 15 for men, more than 10 for women) are shown in green, those of intermediate activity (6-15 for men, 6-10 for women) in red, and those of infrequent activity (5 or fewer for both sexes) in blue. The black line shows the population average. The upper panels show the distributions of the donor returns, and the lower panels show recovery (∆Hb) as a function of return interval (∆T in days). The 95% confidence intervals are shown as gray shading.
Hemoglobin recovery curves, including all donor returns, subdivided on the basis of donation frequency. Frequent donors (more than 15 for men, more than 10 for women) are shown in green, those of intermediate activity (6-15 for men, 6-10 for women) in red, and those of infrequent activity (5 or fewer for both sexes) in blue. The black line shows the population average. The upper panels show the distributions of the donor returns, and the lower panels show recovery (∆Hb) as a function of return interval (∆T in days). The 95% confidence intervals are shown as gray shading.
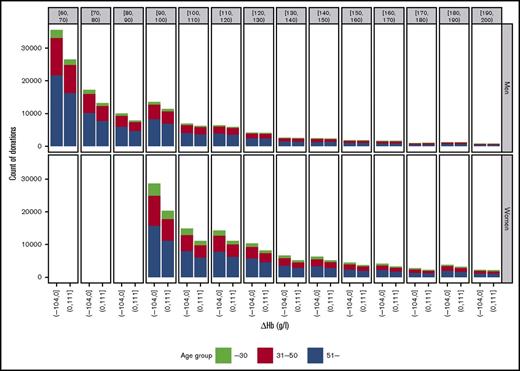
Figure 3 depicts the exact number of donor returns among the frequent donors divided into the groups with ∆Hb below 0 (left bars) and above 0 (right bars), at 10-day return intervals. The results clearly show that despite poor average recovery in those who returned soon after the minimum donation interval (Figure 1) had passed, almost half of the returns in those subgroups still had ∆Hb above 0, meaning that their cHb levels had recovered. In the analysis of data in 10-day intervals (Figure 3), half of the donor returns were recovered in 120 to 130 days in men and in 190 to 200 days in women.
Distribution of the number of donor returns with recovery below ∆Hb = 0 (left bars) and above ∆Hb = 0 (right bars) in 10-day return intervals. Each bar is divided into age groups with color codes.
Distribution of the number of donor returns with recovery below ∆Hb = 0 (left bars) and above ∆Hb = 0 (right bars) in 10-day return intervals. Each bar is divided into age groups with color codes.
It is important to note that in the present analysis the exact length of recovery period, ∆T required to reach ∆Hb = 0 in a given group, was dependent on how the curve was fitted to the data. We experimented with different fitting methods (ie, loess, generalized additive models with different parameters, and sliding windows of various sizes to estimate ∆T points, where on average the ∆Hb curves cross ∆cHb = 0) and found that the crossing points varied considerably. Hence the present results cannot be used to indicate exact donor recovery times but rather a rough description of major trends in the data.
The ∆Hb distributions at 10-day intervals were divided into groups of sex and age (supplemental Figure 2). Importantly, these distributions showed little variation. We found that the donor return interval, ∆T, explained only 1% of the variation in the ∆Hb. This apparent lack of correlation between ∆Hb and ∆T in our data are likely to explain why different curve-fitting methods give variable results.
Figure 4 shows the proportion of cHb-based deferrals as a function of time from previous donation in registered blood donors divided into age groups. In younger women (below 30 years old) the deferral rate due to low cHb was high. In this age group, but not so strongly in any others, there was a clear decrease in the deferral rate when the donation intervals were longer. A relatively consistent cHb-based deferral rate of 2% to 5% was seen in the older age groups in women. In men, there was no similar trend for higher deferral rates due to a low cHb in donations taking place soon after the minimum interval had passed. The deferral rates in men were below 2.5%.
Deferral rates due to low cHb as a function of time for different age groups. The black line shows the population average.
Deferral rates due to low cHb as a function of time for different age groups. The black line shows the population average.
Discussion
In the present study, we used a large national donor registry to assess whether the recommended donation intervals in Finland allow sufficient time for recovery of cHb concentrations in whole-blood donors. In total, more than 1 100 000 donor returns from the period of 2010 to 2015 were analyzed with data-mining tools. This kind of approach complements the more targeted but necessarily much smaller studies on individual donors. Although the retrospective analysis certainly has its drawbacks, the large amount of data can reveal trends not readily visible in smaller studies. We recently reported considerable seasonal and time-of-day variations in cHb levels based on a similar approach.18 We cannot see that those findings should affect the present results because we included all data points regardless of the season or time of day.
A major finding of the present study was that the recovery time from blood donation, defined as cHb recovery, was substantially longer than was the shortest allowed donation interval in place in Finland. We are hesitant to extrapolate exact recovery times or donation interval recommendations from the present results owing to limitations of the data. That requires more direct measurements of iron status in donors. It is of note that the estimations of recovery periods in the present study were based on modeled data that are open to interpretation. The present results merely indicate that recovery times were longer than the minimum donation interval, a conclusion supported by other recent studies.8,9,11 There were many indicators of poor recovery, particularly in women who were under the age of 30: their recovery period was long (over 300 days), their average ∆Hb values remained very low when they returned soon after the minimum donation interval had passed, and their deferral rate was high. The slow recovery in younger women may reflect effects of pregnancy and breastfeeding. The mean age of delivery in Finland was 30 years during 2010 to 2015.19 In addition, a pregnancy usually leads to deferral of a minimum of 15 months (∼450 days), which naturally prolongs the apparent recovery time. Future intervention or more targeted studies should focus on the young, <30-year-old, female donors and apply more refined subdivisions to reveal risk subpopulations; iron deficiency and anemia are common among fertile women globally.20
Another major finding of the present study was that frequent donors who could be assumed to have depleted their iron stores, in fact, recovered faster than did infrequent donors. There may be some homeostatic regulation in operation; alternatively, those who for any reason recover quickly and more readily from blood donation become regular donors. A similar conclusion can also be drawn on the basis of a recent report describing more detailed iron measurements in a small group of blood donors.11 It will be of interest to apply genetic or lifestyle studies to identification of donor subpopulations with the ability to recover quickly and safely from frequent donations. It is also important to identify those at risk for iron deficiency. The fact that the recovery curves of frequent donors returned to a level that was even higher than the level of preceding donation can be interpreted as an indicator of replenishment of depleted iron stores. The increase in the cHb level may indicate that when frequent donors donated at longer intervals, their cHb concentrations had sufficient time to return to their normal levels. A recent study addressed a similar question by identifying factors associated with declining and stable Hb levels in blood donors.21 Genome-wide association studies22-24 have identified a number of loci regulating iron levels at a population level, but the value of these loci in predicting iron levels in a donor population is not known. It will be of interest to compare the frequencies of, for example, TMPRSS6 and HFE polymorphisms, which regulate iron levels, in frequent donors to those in the general population or infrequent donors. It may be possible to predict more personalized donation intervals in the future by utilizing genome data. It also is of note that donors in Finland were offered iron replacement. Previous studies have shown that the Hb levels in blood donors with depleted iron stores recovered faster when the donors ingested iron replacement.25 Because of lack of information concerning the actual use of provided iron replacement by donors, our present retrospective study could not evaluate the impact of iron replacement on cHb recovery in an appropriate way. The study design enabled us to investigate only the impact of our iron replacement policy on the cHb recovery at a very general level. Our iron replacement policy is targeted to cover the most vulnerable donor groups at risk for iron deficiency, but on the basis of the findings of the present study, it seems not to be an effective way to protect individual donors against prolonged cHb recovery.
With the present, retrospective study approach, we were not able to verify whether the cHb levels genuinely indicated iron depletion or deficiency. We, however, demonstrated that the cHb values as measured here correlated well with the venous Hb levels measured at the same time, suggesting that the cHb levels worked as well as the venous Hb levels. Both cHb and venous Hb measurements are used in blood banks. The good correlation also speaks for the use of cHb measurement rather than the more expensive and invasive venous Hb measurement in the standard blood bank activity.
In conclusion, we have shown that large data pools collected in donor databases can be used to reveal cHb recovery patterns in different subgroups of blood donors. These data can be further used for operational purposes in identifying groups at risk for iron deficiency or when deciding safe donation intervals. Data from donor databases can also be used for identifying novel research questions and relevant blood donor subgroups for further research. Using a data-mining approach, we have shown that an average of 100 to 200 days was needed for hemoglobin recovery in frequent blood donors. For infrequent donors the apparent recovery was substantially longer, but we could not exclude the existence of confounding factors in this population. More research is needed not only on safe and sustainable donation intervals and iron replacement in blood donors but also on actual health effects of low iron levels on the quality of life.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Juha Soikkeli, for help in data management, Sari Bäckman for discussions, and Niina Woolley for revising the language.
Authorship
Contribution: P.N., A.L., J.C., and J.P. designed the study; M.A. and A.L. analyzed the data; P.N. M.A., A.L. P.M., J.I. M.S. J.C., and J.P. interpreted the results; P.N. and J.P. wrote the manuscript; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jukka Partanen, Finnish Red Cross Blood Service, Kivihaantie 7, 00310 Helsinki, Finland; e-mail: jukka.partanen@veripalvelu.fi.