Abstract
Background
T-cell prolymphocytic leukemia (T-PLL) is a rare disease of extremely poor outcome.
The current standard of care involves intravenous Alemtuzumab with high response rates. Virtually all patients experience relapse and the 5-years overall survival is not higher than 5%. Allogenic bone marrow transplantation is the only curative option.
Case presentation
A 35 years-old male was diagnosed with T-PLL in early 2014 with skin infiltration, fatigue, hepatomegaly, splenomegaly (28 cm), and lymphadenopathies. Immunophenotypic features of circulating lymphocytes showed a T-cell lymphoproliferation (CD2+ CD3+ CD5+ CD7+ CD8+). TCL1 hyperexpression was assessed on bone marrow and lymph node biopsy samples. There was a rearrangement of chromosome 14 involving TCL1 on circulating cells.
Pentostatin was initiated for 2 cycles without success. IV alemtuzumab (30mg/m2) was then introduced. After 8 weekly cycles, a CT scan showed a reduction of the splenomegaly (22 cm), a stable hepatomegaly, and no remaining lymphadenopathy. No abnormal circulating lymphocytes were detectable. After 14 cycles, the patient showed cervical painful lymph nodes. A needle aspiration revealed relapse with T-PLL cells. We then introduced idelalisib (150 mg, twice daily). Alemtuzumab was discontinued. One week after the introduction of idelalisib, physical examination revealed the disappearance of all peripheral lymph nodes. After 2 weeks, the spleen size was reduced to 16 cm and a body PET-CT was negative, proving metabolic remission. No circulating lymphocyte could be found. The patient was considered as being in complete remission (CR) and underwent haplo-identical transplantation. Idelalisib was discontinued after three weeks of exposure before transplantation and reintroduced from days 30 to 60. At day 100 after transplantation, the patient was in persistent CR: a peripheral immunophenotypic study was performed, no clonal T population was found, chimerism was 100% of donor origin. A bone marrow aspirate was quantitatively and qualitatively normal. Seven months after transplant, the patient remains in CR with a grade 1 skin chronic GVHD.
Biological rationale
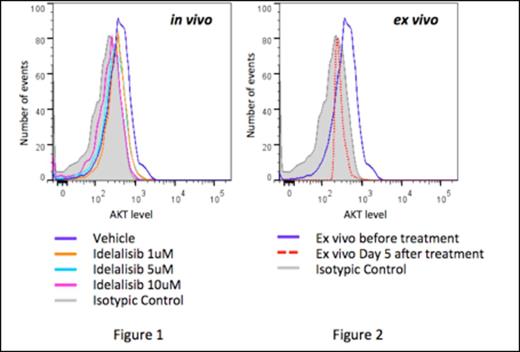
Idealisib is a delta isoform inhibitor of protein phosphatidylinositol 3 kinase (PI3K). TCL1 is an overexpressed oncoprotein described in the majority of T-PLL due to chromosomal 14 rearrangement. TCL1 modulates AKT activity, facilitating both AKT dimerization and phosphorylation upon PI3K stimulation. The PI3K produces PIP2 and PIP3, which are required to phosphorylate AKT. Even if TCL1 is not sufficient to transform T cells into T-PLL cells and requires secondary genetic events as suggested in murine models, AKT hyperactivation may participate in T-cell proliferation (through the GSK-3β and mTOR pathways), and survival (through the NFκB pathway). We hypothesized that the inhibition of the PI3K with idelalisib would inhibit growth and eventually cell survival of the T-PLL cell, depending on the secondary genetic events. In order to support this hypothesis, we investigated the biochemical and functional effects of idelalisib in vitro. Fresh primary T-PLL cells were obtained from the ascites of an another case of T-PLL (CD2+, CD3+, CD5+, CD7+, CD4/CD8+, complex caryotype involving chromosome 14). In vitro exposure of primary T-PLL cells to idelalisib led to a dramatic and dose-dependent decrease in AKT phosphorylation after 24 hours (figure 1) and ex vivo after 5 days (figure 2). However, no apoptotic response was observed, neither in vitro nor ex vivo, even though therapeutic levels of idelalisib were reached in the ascites.
Conclusion
We report the first case of idealisib successfully used as a salvage therapy in T-PLL, leading to complete remission. It was used as a bridge to transplantation for a patient who is still in complete remission 7 months after transplantation.
Our results provide a paradigm for the use of a PI3K inhibitor in T-PLL, which results in AKT dephosphorylation. The lack of functional response for the second patient suggests that the clinical response may depend on other oncogenic events, which could bypass AKT dependency. There is a need to identify a patient subgroup that may benefit from PI3K inhibition. In an attempt to identify molecular mechanisms underlying this resistance, comparative full exome sequencing is being performed for both patients and will be shown at the meeting.
Off Label Use: idelalisib, PI3-Kinase inhibitor. Tournilhac:Roche: Other: Travel support, Research Funding; Celgene: Other: Travel support; Mundiphrama: Honoraria, Other: Travel Support, Research Funding; GSK: Other: Travel Support, Research Funding; Janssen Cilag: Honoraria, Other: Travel support; Gilead: Other: Travel Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal