Abstract
CD33 (Siglec-3) is a marker of myeloid progenitor cells, mature myeloid cells, and most myeloid leukemias. Although its biologic role remains unknown, it has been demonstrated to function as a sialic acid–specific lectin and a cell adhesion molecule. Many of the Siglecs (including CD33) have been reported to be tyrosine phosphorylated in the cytosolic tails under specific stimulation conditions. Here we report that CD33 is also a serine/threonine phosphoprotein, containing at least 2 sites of serine phosphorylation in its cytoplasmic domain, catalyzed by protein kinase C (PKC). Phosphorylation could be augmented by exposure to the protein kinase–activating cytokines interleukin 3, erythropoietin, or granulocyte-macrophage colony-stimulating factor, in a cytokine-dependent cell line, TF-1. The CD33 cytoplasmic tail was phosphorylated by PKC in vitro, in a Ca++/lipid-dependent manner. CHOK1 cells stably expressing CD33 with cytoplasmic tails of various length also showed phorbol myristate acetate (PMA)-dependent phosphorylation of CD33. Inhibition of CD33 phosphorylation with pharmacologic agents resulted in an increase of sialic acid–dependent rosette formation. Furthermore, the occupancy of the lectin site affected its basal level of phosphorylation. Rosette formation by COS cells expressing a form of CD33 lacking its cytoplasmic domain was not affected by these same agents. These data indicate that CD33 is a phosphoprotein, that its phosphorylation may be controlled by PKC downstream of cytokine stimulation, and that its phosphorylation is cross-regulated with its lectin activity. Notably, although this is the first example of serine/threonine phosphorylation in the subfamily of CD33-like Siglecs, some of the other members also have putative target sites in their cytoplasmic tails.
Introduction
CD33 is a 67-kd glycoprotein found predominantly on myeloid cells, including early myeloid precursors in the bone marrow and certain subsets of mature circulating myeloid cells.1-3 It has also been reported on dendritic cells, cord blood–derived natural killer (NK) cells, in vitro–expanded T cells, and some biphenotypic leukemias.4-7 Although initially described as a marker for normal and leukemic myeloid progenitor cells, it has received renewed interest due to its demonstrated lectin activity for α2-6– and α2-3–enlinked sialylated oligosaccharides expressed on red blood cells (RBCs) and certain myeloid cells.8,9 These observations established CD33 as a member of a group of lectins termed Siglecs,10 a family that includes sialoadhesin, CD22, myelin-associated glycoprotein 1 (MAG-1)11,12 as well as the newly described Siglecs 5 to 10.13-27 The defining features of this family are their amino acid sequence homology in their first 2 N-terminal Ig-like domains, their membership in the immunoglobulin superfamily, and their lectin activity for sialylated glycoconjugates.8,13-18Siglecs show a sialic acid linkage specificity of the lectin domain to a varying degree, such that they are capable of discriminating between α2-3–, α2-6–, and α2-8–linked sialic acid residues. This feature makes specific Siglecs capable of distinguishing between some of the products of the 15 different sialyltransferases identified to date.19,28 29
A combination of in vitro and in vivo studies has indicated that some Siglecs have roles in cell activation and development. The Siglec-3–related Siglec family, CD22, and MAG are clustered together on chromosome 19q (in humans) or chromosome 7 (in mice), suggesting a common ancestral origin.30-32 However, it is possible that some of the gene duplications in the Siglec-3 family occurred after separation of the ancestors of primates and rodents.26CD22, expressed on B cells, becomes tyrosine phosphorylated in response to IgR signaling, which results in the recruitment of several protein tyrosine kinases and protein tyrosine phosphatase 1C.33-36Mice with null alleles of CD22 demonstrate B-cell hyperresponsiveness and some abnormalities in immunoglobulin production.37,38MAG has a 90–amino acid cytoplasmic domain, which is the target of both protein tyrosine kinases and serine/threonine kinases including protein kinase C (PKC).39-41 Cells expressing MAG (including Chinese hamster ovary [CHO] cells transfected to express MAG) down-regulate neurite outgrowth and, conversely, exhibit inhibited growth on neurites.42 MAG null mice exhibit microscopically detectable abnormalities in myelin sheath structure and neurologic abnormalities in adulthood.43,44 Sialoadhesin differs in chromosomal location and in lacking a cytoplasmic domain.17,45 However, sialoadhesin has been clearly demonstrated to function as an adhesion molecule in vivo.46
The cytosolic tail of CD33 has 2 tyrosine-based putative signaling motifs, the membrane-proximal one conforming to a canonical ITIM motif. Indeed, tyrosine phosphorylation of CD33 has been identified using phosphotyrosine antisera together with the pharmacologic manipulation of cellular phosphorylation with sodium pervanadate.47-49An association between CD33 phosphorylation, CD33 cross-linking, and calcium flux was also demonstrated, along with the recruitment of the protein tyrosine phosphatases SHP-1 and SHP-2, as well as an increased rosetting ability of a CD33 with a mutated Tyr340.47 Here we report serine/threonine phosphorylation of CD33 in several different cell lines, including a cytokine-dependent myelocyte cell line (TF-1), several cytokine-independent cell lines (HL-60, U937), and in COS cells transiently or CHO cells stably transfected to express CD33 with a CD33-encoding plasmid. These results indicate that CD33 is a serine/threonine phosphoprotein and a connection between this modification and its lectin activity, thereby possibly modifying the potential function on hematopoietic cells.
Materials and methods
Cell lines and reagents
COS-7 cells were the gift of Dr N. Varki (University of California San Diego [UCSD]); HL-60 and U937 cells were a gift of Dr R. Pilz (UCSD). TF-1 cells were provided by Dr R. Taetle (Arizona Cancer Center, Tucson, AZ) and were maintained as described.50 Where indicated, TF-1 cells were cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 U/mL; gift of Dr R. Taetle), interleukin (IL)-3 (10 ng/mL; Calbiochem, San Diego, CA), or erythropoietin (EPO; 10 U/mL; Amgen, Thousand Oaks, CA).
Cell labeling and immunopurification of CD33
One to 3 mL of cells (4 × 106/mL) was biotinylated with sulfo-NHS-biotin at 1 mg/mL in phosphate-buffered saline (PBS), pH 8.5, containing 1 mM MgCl2, 0.1 mM CaCl2, for 60 minutes on ice. Cells were washed, lysed in 20 mM Tris-Cl, pH 8.0, 0.15 M NaCl, 2 mM EDTA, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μM pepstatin, and 1% aprotinin. For [32P]-orthophosphate-labeled samples, lysis buffer containing 50 mM NaF, 0.2 mM sodium vanadate, and 0.01 M sodium phosphate according to Sefton51was used. Following centrifugation, lysates were absorbed onto My9 (anti-CD33; Immunogen, Cambridge, MA) linked to AffiGel-10 resin (Bio-Rad Laboratories, Hercules, CA) and processed as described.52 CD33 was eluted with 0.1 M acetic acid, pH 2.5, 0.1% NP-40, for 10 minutes at 37°C, and the eluates were neutralized with Tris pH 9, 1% sodium dodecyl sulfate (SDS), and precipitated with acetone overnight. For peptide N-glycosidase F treatment, precipitates were processed according to Freeze.53 For sialidase digestion, samples were diluted with 1 volume 0.1 M sodium acetate, pH 5.5, followed by 10 mUArthrobacter ureafaciens sialidase, for 1 hour at 37°C. The samples were analyzed by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, blotted, probed with streptavidin–horseradish peroxidase (HRPO), and detected by chemiluminescence (ECL, Pierce Chemical, Rockford, IL) using BioMax film (Eastman Kodak, Rochester, NY). Because none of the αCD33 antibodies was able to detect Western-blotted CD33, equivalence of loading could not be confirmed directly. Instead, detection of equal amounts of My9 antibody chains on the membranes confirmed no protein losses during sample preparation, and differences in CD33 phosphorylation were consistently found in repeated experiments without fail.
Cells were labeled with [32P]-orthophosphate by washing twice in warmed phosphate-free α modified Eagle medium (αMEM) followed by incubation in the same media with 100 μCi/mL (3.7 MBq) [32P]-orthophosphate. After 60 minutes, cells were either harvested directly or treated for an additional 12 minutes with either phorbol myristate acetate (PMA; 18 nM), dimethyl sulfoxide (DMSO; 1.3% final concentration), or dibutryl cyclic adenosine monophosphate (cAMP; 1 mM).54-57CD33 was immunopurified and analyzed as described above. For quantitation, the fluorograms were scanned and analyzed using ImageQuant 1.1 software (Molecular Dynamics, Sunnyvale, CA). Phosphoamino acid analysis was performed as described51 by high- voltage thin-layer chromatography on cellulose plates (EM Science, Gibbstown, NJ), using unlabeled phosphoamino acids (detected by ninhydrin spray reagent) as internal standards. For phosphopeptide studies, the radioactive band was excised from the polyvinylidene difluoride (PVDF) membrane, blocked with 50 μg ovalbumin in 0.1 M ammonium carbonate, pH 8, then digested sequentially with 5 μg trypsin (sequencing grade, Sigma Chemical, St Louis, MO) and 10 μg chymotrypsin (Calbiochem). More than 50% of the bound radioactivity was recovered in the soluble fraction, which was subsequently analyzed as described.51
The TF-1 cells were labeled with [32P]orthophosphate for 60 minutes as above after cytokine deprivation for 15 hours. Following labeling, cytokine was added, the cells incubated for an additional 10 minutes at 37°C, chilled, and CD33 immunopurified.52
DNA constructs and cell transfection
The complementary DNA (cDNA) coding for CD33 (gift of Dr B. Seed, Harvard University, Cambridge, MA) was in the eukaryotic expression vector πH3M.13 The truncated CD33 construct pCD33T299Δ was created by polymerase chain reaction (PCR; forward primer 5′ cccaagcttctagagatcc 3′; reverse primer 5′ cgggtctctgcagtcaggtgtcattgctgcc 3′), trimmed withHindIII and PstI, and ligated into πH3M. Transfections in COS cells were performed using Lipofectamine (Gibco BRL, La Jolla, CA).
CD33 cytoplasmic regions of various lengths were produced using the following primers on the CD33 parent cDNA (pcDNA3.1-CD33): sense: cccaaaatcctcatccctggc, antisense: ΔB) actgcagtcagtgggtcttcactatgaag; ΔC) actgcagtcaggggccatgtaacttgg; ΔD) actgcagtcacacagtaggggcggc; ΔE) actgcagtcaattcatcccatgaaag; ΔF) actgcagctcactgggtcctgacc. The products were cleaved using XbaI and PstI and ligated into pcDNA3.1-CD33. CHOK1 cells were stably transfected and CD33 expression on isolated clones for each construct was estimated by FACS analysis, using the CD33-specific antibody My9 as the primary antibody. Cells were stimulated with PMA or treated with DMSO as described above. Clones with similar CD33 expression levels were chosen, labeled with [32P]-orthophosphate and purified.52 Total counts per minute per milligram cell protein were calculated to normalize CD33 preparations. After SDS-PAGE separation, CD33 labeling was detected fluorometrically using BioMax film, and quantified using ImageQuant 1.1 software (Molecular Dynamics).
Glutathione-S-transferase tail fusion protein production
The cytoplasmic tail of CD33 (residues His285-Gln364) was produced by PCR (forward primer 5′gctggaattcaccggcggaaagcagcccggacagc3′; reverse primer 5′cgagagctcgacccaggactggagactcataagc3′) sequenced and ligated into pGEX (Pharmacia, Peapack, NJ) using EcoRI andXhoI. PGEXhCD33cyto/mock transfected Escherichia coli was grown and glutathione-S-transferase (GST) fusion protein prepared as described by the manufacturer.
PKC activity assay
In vitro PKC activity on the CD33 cytoplasmic tail was determined in 20 mM Hepes, pH 7.4, 1 mM dithiothreitol (DTT), 5 mM MgCl2, 0.1 mM adenosine triphosphate (ATP), 1 μCi (0.037 MBq) γ32P-ATP, 3 μg protein substrate in 55 μL Hepes. Then, 140 μM/3.8 μM phosphatidylserine/diacylglycerol membranes and 0.5 mM CaCl2 were added to produce PKC-activating conditions; equal volumes of Hepes and 0.5 mM EGTA were added for inactivating conditions. Human purified PKC was added in a 5-μL volume, and the reaction allowed to proceed for 10 minutes at 30°C. The reaction was stopped by acetone precipitation. The sample was analyzed by reducing SDS-PAGE. The gel was Coomassie stained, dried, and then photographed as well as autoradiographed.
Erythrocyte rosetting assay
COS cells, transfected to express CD33 as above, were lifted 24 hours after transfection, replated into 24-well cluster plates (precoated with poly-d-lysine), and allowed to expand for 48 hours. Cells were washed with serum-free αMEM, pH 6.9, 4 mM CaCl2, and then digested with 0.1 U/mL sialidase for 60 minutes at 37°C. Thereafter, the cells were washed gently in PBS, 4 mM EDTA, and twice in PBS, 5 mg/mL bovine serum albumin (PBA). Human erythrocytes were washed 4 times in 10 mM 3-[N-Morpholino]propanesulfonic acid (MOPS), pH 7.0, 0.14 M NaCl, 5 mM EDTA, and once in PBA. RBCs were desialylated by treatment of a 30% vol/vol suspension in 10 mM MOPS, pH 6.7, 0.15 M NaCl, 4 mM CaCl2 with 0.1 U/mL Vibrio cholerae sialidase for 3 hours at 37°C. RBCs were added to yield a final concentration of 0.2% vol/vol. The trays were incubated for 30 minutes at 4°C, washed 4 to 6 times using PBA, and then rosettes fixed for 10 minutes using PBS/4% formaldehyde, stained by incubation for 10 minutes using 2-amino-9-ethylcarbazole (AEC), and then returned to PBS/formaldehyde. Rosettes were defined as COS cells containing 4 or more bound RBCs. For antibody-blocking studies, My9 was added at 10 μg/mL during the sialidase treatment step. For treatment of [32P]-orthophosphate-labeled cells with kinase or phosphatase inhibitors, transfected cells were initially treated with sialidase, then labeled with [32P]-orthophosphate as above. Okadaic acid, bisindolylmaleimide (BIM), or staurosporine was included during the 60 minutes of [32P]-orthophosphate phosphate labeling, with PMA added during the final 12 minutes of labeling, where indicated. If the cells were to be used for rosetting, they were first treated with sialidase, then labeled with [32P]-orthophosphate, then rinsed with PBA and RBCs added. Rosettes were allowed to form at 37°C.8
Results
CD33 is a serine/threonine phosphoprotein
Examination of the amino acid sequence of the cytoplasmic domain of CD33 predicted that CD33 may be a phosphoprotein. Specifically, 11 serine, 11 threonine residues, and 2 tyrosine residues are present, along with potential consensus sequences for both serine/threonine and tyrosine (ITIMs) kinases.13,58 59 This prediction was tested directly by examining immunoprecipitated CD33 from U937 cells metabolically labeled with [32P]-orthophosphate. This approach identified a cell-surface glycoprotein with a total mass of about 75 kd, which, after desialylation, shifted to about 49 kd (data not shown). By this approach, CD33 was immunoprecipitated from 2 human myeloid cell lines (HL-60 and U937) that had been metabolically labeled for 60 minutes with [32P]-orthophosphate. A single peptide of about 75 kd was identified, indicating that CD33 is a phosphoprotein. In both cell types, a 12-minute treatment with PMA resulted in an approximately 3- to 8-fold increase in [32P]-orthophosphate incorporation. DMSO stimulated phosphorylation 2- to 4-fold in HL-60 cells (Figure 1).
CD33 phosphorylation in myeloid cell lines is induced by phorbol esters.
U937 cells or HL-60 cells were labeled with [32P]-orthophosphate for 1 hour and then treated for 12 minutes with either PMA (18 nM), DMSO (1.5%), or control (0.001% DMSO) for 12 minutes. CD33 immunoprecipitates were isolated using My9, blotted onto nitrocellulose, and detected by fluorography.
CD33 phosphorylation in myeloid cell lines is induced by phorbol esters.
U937 cells or HL-60 cells were labeled with [32P]-orthophosphate for 1 hour and then treated for 12 minutes with either PMA (18 nM), DMSO (1.5%), or control (0.001% DMSO) for 12 minutes. CD33 immunoprecipitates were isolated using My9, blotted onto nitrocellulose, and detected by fluorography.
Phosphoamino analyses were performed on [32P]-labeled CD33 immunopurified from resting, PMA-stimulated, or DMSO-stimulated cells.51 These results demonstrated exclusively phosphoserine in all cases, with trace amounts of phosphothreonine in PMA-stimulated HL-60 cells (Figure 2). In no cases could phosphotyrosine be detected in HL-60 or U937 cells, even after extended exposure. Thus, CD33 may be phosphorylated directly by PKC or by a serine/threonine kinase that is activated downstream following PKC activation.
CD33 phosphoamino acid analysis in U937 or HL60 cells detects only phosphoserine.
[32P]-orthophosphate-labeled CD33 from PMA-stimulated U937 (A) or HL-60 cells (B) was isolated by My9 adsorption and SDS-PAGE as described in “Materials and methods.” Following hydrolysis in 6 N HCl, the resulting phosphoamino acids were mixed with phosphoserine (pS), phosphothreonine (pT), and phosphotyrosine (pY) standards and analyzed by 2-dimensional high-voltage thin-layer cellulose chromatography. Samples were applied at (+). The radioactivity above the origin represents partially digested phosphopeptides, whereas that in the upper left corner represents free phosphate.
CD33 phosphoamino acid analysis in U937 or HL60 cells detects only phosphoserine.
[32P]-orthophosphate-labeled CD33 from PMA-stimulated U937 (A) or HL-60 cells (B) was isolated by My9 adsorption and SDS-PAGE as described in “Materials and methods.” Following hydrolysis in 6 N HCl, the resulting phosphoamino acids were mixed with phosphoserine (pS), phosphothreonine (pT), and phosphotyrosine (pY) standards and analyzed by 2-dimensional high-voltage thin-layer cellulose chromatography. Samples were applied at (+). The radioactivity above the origin represents partially digested phosphopeptides, whereas that in the upper left corner represents free phosphate.
Serine/threonine kinases catalyze CD33 phosphorylation
The effects of stimulators of protein kinase A, as well as different inhibitors of serine/threonine kinases, were further examined to more precisely define the roles of different kinases in CD33 phosphorylation. U937 cells were labeled with [32P]-orthophosphate, and stimulated with dibutryl cAMP, PMA, or PMA in the presence of either one of 2 inhibitors of serine/threonine-specific kinases, staurosporine and BIM. Dibutryl cAMP does not lead to enhanced phosphorylation, compared to untreated cells (Figure 3A). The enhanced phosphorylation by PMA can be totally blocked by either protein kinase inhibitor (Figure 3A). Moreover, staurosporine, but not BIM, is capable of reducing the resting level of CD33 phosphorylation by approximately 80%. Dose-response curves for inhibition demonstrated approximately 50% inhibition could be achieved with either straurosporine or BIM at 10 nM and 5 nM, respectively.60 The phosphorylation of CD33 in transiently transfected COS cells was also stimulated by PMA (4-fold increase) but not by dibutryl cAMP (Figure 3B). The same pattern of stimulation and inhibition seen in HL-60 and U937 cells was also observed in CD33-COS cells (data not shown). Phosphoamino acid analyses demonstrated the exclusive presence of phosphoserine (data not shown). Thus, in both myeloid cells that naturally express CD33, and in a transfected nonmyeloid cell line, CD33 is phosphorylated as a downstream event following PKC stimulation.
Effect of pharmacologic manipulation of protein kinase activity on CD33 phosphorylation.
(A) U937 cells were labeled with [32P]-orthophosphate for 1 hour in the absence or presence of 2 μM BIM or 2 μM staurosporine for 60 minutes. Subsequently, PMA was added to 18 nM, or dibutryl cAMP to 1 mM, as indicated. Following 12 minutes at 37°C, the cells were chilled and lysed, and CD33 isolated and analyzed. PMA treatment leads to an increase of CD33 phosphorylation, which is inhibited by pretreatment with BIM or even further reduced by pretreatment with staurosporine. Dibutryl cAMP does not influence the level of CD33 phosphorylation. (B) Phosphorylation of CD33 in transiently transfected COS cells is enhanced by PMA, but not by dibutryl cAMP. COS cells, transfected with pCD33 48 hours earlier, were labeled with [32P]-orthophosphate for 60 minutes followed by 12 minutes treatment with PMA (18 nM) or dibutryl cAMP (1 mM).
Effect of pharmacologic manipulation of protein kinase activity on CD33 phosphorylation.
(A) U937 cells were labeled with [32P]-orthophosphate for 1 hour in the absence or presence of 2 μM BIM or 2 μM staurosporine for 60 minutes. Subsequently, PMA was added to 18 nM, or dibutryl cAMP to 1 mM, as indicated. Following 12 minutes at 37°C, the cells were chilled and lysed, and CD33 isolated and analyzed. PMA treatment leads to an increase of CD33 phosphorylation, which is inhibited by pretreatment with BIM or even further reduced by pretreatment with staurosporine. Dibutryl cAMP does not influence the level of CD33 phosphorylation. (B) Phosphorylation of CD33 in transiently transfected COS cells is enhanced by PMA, but not by dibutryl cAMP. COS cells, transfected with pCD33 48 hours earlier, were labeled with [32P]-orthophosphate for 60 minutes followed by 12 minutes treatment with PMA (18 nM) or dibutryl cAMP (1 mM).
CD33 is phosphorylated on 2 serine residues
Phosphopeptide maps were analyzed to establish the number of potential phosphorylation sites. The examination of CD33 immunopurified from either CD33 transfected COS cells or U937 cells (both PMA stimulated) by 2-dimensional thin-layer chromatography identified 2 phosphopeptides in each sample with similar mobilities (Figure4A,B). These results indicate the same serine residues are likely phosphorylated in human myeloid (U937) and primate uroendothelial (COS) cells.
Phosphopeptide analysis demonstrates 2 phosphopeptides.
(A) Tryptic-chymotryptic peptides were analyzed from [32P]-orthophosphate-labeled CD33 isolated from PMA-stimulated CD33-transfected COS cells (i) or U937 cells (ii). Samples were initially purified by SDS-PAGE, blotted onto PVDF, and then digested sequentially with trypsin and chymotrypsin. Isolated phosphopeptides were examined by 2-dimensional thin-layer chromatography as described in “Materials and methods.” Two peptides of similar mobilities were identified in each sample. (B) Amino acid sequence of the cytoplasmic domain of CD33, starting with Lys283, with serine residues indicated by asterisks (*) and gaps indicating expected points of cleavage following combined trypsin-chymotrypsin digestion.
Phosphopeptide analysis demonstrates 2 phosphopeptides.
(A) Tryptic-chymotryptic peptides were analyzed from [32P]-orthophosphate-labeled CD33 isolated from PMA-stimulated CD33-transfected COS cells (i) or U937 cells (ii). Samples were initially purified by SDS-PAGE, blotted onto PVDF, and then digested sequentially with trypsin and chymotrypsin. Isolated phosphopeptides were examined by 2-dimensional thin-layer chromatography as described in “Materials and methods.” Two peptides of similar mobilities were identified in each sample. (B) Amino acid sequence of the cytoplasmic domain of CD33, starting with Lys283, with serine residues indicated by asterisks (*) and gaps indicating expected points of cleavage following combined trypsin-chymotrypsin digestion.
CD33 phosphorylation by PKC in vitro
Protein kinase C–dependent phosphorylation of CD33 could be demonstrated in vitro, using a purified, E coli–expressed GST-CD33 cytoplasmic tail fusion protein and purified human PKC, clearly showing that CD33 is a potential PKC target (Figure5). Addition of lipids and Ca++ as PKC activators led to an increased phosphorylation level, whereas reduction of phosphorylation by the calcium chelator EGTA confirms the PKC mediation of labeling. GST alone was not phosphorylated under these conditions.
Human CD33 cytoplasmic tail is phosphorylated by purified human PKC in vitro.
Coomassie-stained SDS-PAGE (A) and autoradiograph of the same gel (B). The cytoplasmic tail of CD33 was expressed as a GST fusion protein inE coli, purified, and [32P]-orthophosphate PKC labeled under activating conditions (+ indicates Ca++ and lipids; −, no Hepes and EGTA) and nonactivating conditions (+, Hepes and EGTA; −, no Ca++ and lipids). PKC-mediated labeling of the GST-CD33 tail region is reduced under nonactivating conditions, proving phosphorylation by the purified human PKC. GST alone is not phosphorylated by PKC under these conditions.
Human CD33 cytoplasmic tail is phosphorylated by purified human PKC in vitro.
Coomassie-stained SDS-PAGE (A) and autoradiograph of the same gel (B). The cytoplasmic tail of CD33 was expressed as a GST fusion protein inE coli, purified, and [32P]-orthophosphate PKC labeled under activating conditions (+ indicates Ca++ and lipids; −, no Hepes and EGTA) and nonactivating conditions (+, Hepes and EGTA; −, no Ca++ and lipids). PKC-mediated labeling of the GST-CD33 tail region is reduced under nonactivating conditions, proving phosphorylation by the purified human PKC. GST alone is not phosphorylated by PKC under these conditions.
S307 is the putative site for phosphorylation on CD33
CD33 constructs with various tail lengths were stably expressed in CHOK1 cells to determine peptides susceptible to PKC-mediated phosphorylation. The expression level of constructs of various lengths (CD33-ΔB, Δ at Val294 (no cytoplasmic tail); CD33-ΔC, Δ at Pro319; CD33-ΔD, Δ at Val332; CD33-ΔE, Δ at Pro350; and CD33-ΔF, full-length CD33) were confirmed by FACS analysis (Figure 6A). Clones were PMA activated or mock activated (Figure 6B), showing the presence of a PMA-dependent phosphorylation site in construct ΔC between Val294 and Pro319. The Ser307 protein kinase motif residing in construct ΔC was predicted as the only strong PKC phosphorylation site by PROSITE (http://www.expasy.ch/prosite). Longer constructs (ΔD-ΔF) showed a comparable increase in phosphorylation after PMA activation (average increase of 4 tests: ΔB, no increase; ΔC, × 1.35; ΔD, × 1.70; ΔE, × 1.45; ΔF, × 1.35).
Expression and phosphorylation of truncated CD33 constructs on CHOK1 cells.
(A) FACS analysis of CD33 expression on the surface of CHOK1 cells, as detected by My9 anti-CD33 antibody. All constructs were expressed at about the same level, except construct ΔE, which was expressed at an approximately 20 times reduced level. (B) Autoradiograph of CD33 with tails of various length (as defined in text) after PMA-dependent phosphorylation with [32P] in vivo. The applied amount of ΔE was 20 times that of the other constructs to compensate for reduced expression. Constructs ΔC through ΔF show phosphorylation in a PMA-dependent way, suggesting PKC activity on CD33 in vivo. The PMA-dependent phosphorylation of ΔC suggests Ser307 protein kinase as one PKC phosphorylated motif. The increase in phosphorylation after 15 minutes of PMA treatment compared to DMSO treatment alone is shown. (C) Amino acid sequence of the cytoplasmic tail of human CD33. Letters ΔB through ΔF indicate the length of the various constructs; the most C-terminal amino acid of each construct is shown. Putative phosphorylation sites Ser307 and Ser342 are indicated.
Expression and phosphorylation of truncated CD33 constructs on CHOK1 cells.
(A) FACS analysis of CD33 expression on the surface of CHOK1 cells, as detected by My9 anti-CD33 antibody. All constructs were expressed at about the same level, except construct ΔE, which was expressed at an approximately 20 times reduced level. (B) Autoradiograph of CD33 with tails of various length (as defined in text) after PMA-dependent phosphorylation with [32P] in vivo. The applied amount of ΔE was 20 times that of the other constructs to compensate for reduced expression. Constructs ΔC through ΔF show phosphorylation in a PMA-dependent way, suggesting PKC activity on CD33 in vivo. The PMA-dependent phosphorylation of ΔC suggests Ser307 protein kinase as one PKC phosphorylated motif. The increase in phosphorylation after 15 minutes of PMA treatment compared to DMSO treatment alone is shown. (C) Amino acid sequence of the cytoplasmic tail of human CD33. Letters ΔB through ΔF indicate the length of the various constructs; the most C-terminal amino acid of each construct is shown. Putative phosphorylation sites Ser307 and Ser342 are indicated.
CD33 phosphorylation also occurs in a myeloid cell line
The above experiments were all performed with nonhematopoietic cells or hematopoietic cell lines that may overexpress serine/threonine kinases.61 Therefore, we examined CD33 phosphorylation in TF-1 cells, a human myeloid cell line that requires either EPO, IL-3, or GM-CSF for growth in culture.50 62 CD33 was phosphorylated in these cells, and the level of phosphorylation could be increased by exposure to any of the 3 cytokines (data not shown). The effect of GM-CSF was only a modest increase of approximately 25%, whereas exposure to EPO increased phosphorylation by 2.6-fold and IL-3 by 2-fold. Thus, CD33 phosphorylation also occurs in a human myeloid cell line that has retained the requirement of cytokine stimulation for long-term growth in vitro.
Decreased phosphorylation results in increased erythrocyte rosetting
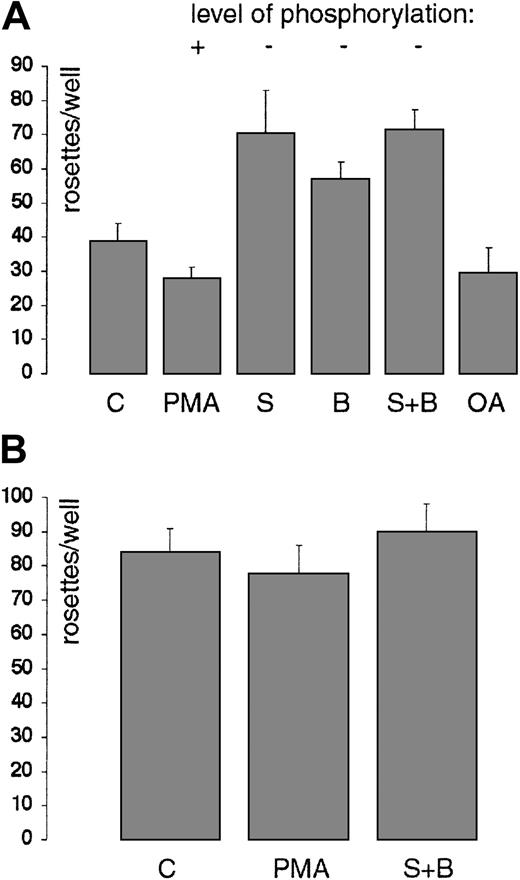
CD33 has been identified as a sialic acid–specific lectin, a member of the family of Siglecs.10,11,12 No rosetting activity of CD33-transfected COS cells was detected toward asialo RBCs or toward native (sialylated) RBCs if CD33 was initially blocked with My9 (data not shown), confirming that CD33 has a lectin activity for sialylated glycoconjugates.8 To determine if CD33 phosphorylation may affect lectin activity, the rosetting assay was performed with CD33+COS cells treated with specific activators or inhibitors of protein kinases as used above. CD33-transfected COS cells were treated with sialidase, returned to complete culture media for treatment with protein kinase inhibitors or activators as indicated in “Materials and methods,” and then the rosetting assay was performed. This demonstrated a reproducible effect of protein kinase inhibition on the number of RBC rosettes formed (Figure 7A). Staurosporine and BIM treatment increased CD33-dependent rosette formation (Figure 7A), whereas PMA treatment somewhat repressed rosetting. Okadaic acid, which inhibits the serine/threonine protein phosphatases 1 and 2A and thus may induce phosphorylation, had a similar effect.
CD33 lectin activity and serine phosphorylation interrelate.
(A) Effect of protein phosphorylation manipulation on RBC rosette formation. Working in a 24-well cluster tray, CD33-transfected COS cells were treated with either staurosporine (S, 2 μM), BIM (B, 2 μM), both staurosporine and BIM (S + B), or okadaic acid (OA, 0.4 μM) for 60 minutes, or with PMA (18 nM) for 12 minutes, all at 37° C. Rosettes were then allowed to form and were fixed, stained with AEC, and counted. Duplicate wells were counted, and the average ± SD shown (P < .01). Decrease of CD33 phosphorylation leads to an increase in lectin binding of RBCs; conversely, an increase in CD33 phosphorylation leads to a lowered rosetting ability. (B) Rosetting of RBCs with CD33T299Δ. COS cells transiently transfected with pCD33T299Δ rosette RBCs in the standard rosetting assay. Treatment with either PMA or staurosporine plus BIM (S + B) is without effect. Results of representative experiments are shown.
CD33 lectin activity and serine phosphorylation interrelate.
(A) Effect of protein phosphorylation manipulation on RBC rosette formation. Working in a 24-well cluster tray, CD33-transfected COS cells were treated with either staurosporine (S, 2 μM), BIM (B, 2 μM), both staurosporine and BIM (S + B), or okadaic acid (OA, 0.4 μM) for 60 minutes, or with PMA (18 nM) for 12 minutes, all at 37° C. Rosettes were then allowed to form and were fixed, stained with AEC, and counted. Duplicate wells were counted, and the average ± SD shown (P < .01). Decrease of CD33 phosphorylation leads to an increase in lectin binding of RBCs; conversely, an increase in CD33 phosphorylation leads to a lowered rosetting ability. (B) Rosetting of RBCs with CD33T299Δ. COS cells transiently transfected with pCD33T299Δ rosette RBCs in the standard rosetting assay. Treatment with either PMA or staurosporine plus BIM (S + B) is without effect. Results of representative experiments are shown.
CD33 without a cytoplasmic tail has an enhanced ability to form rosettes
As an alternative approach to these questions, a CD33 construct was designed containing a stop codon after Thr299to yield a truncated cytoplasmic domain. This construct could not be labeled with [32P]-orthophosphate (data not shown). COS cells transfected with this construct (pCD33T299Δ) rosetted RBCs, although consistently a higher number of rosettes were seen. Significantly, pretreatment of these cells with either inhibitors or activators of protein kinases did not change the level of rosettes seen (Figure 7B).
Ligand binding reduces the basal level of CD33 phosphorylation
We next examined if either sialidase treatment alone, or sialidase treatment followed by binding to RBCs, would affect CD33 phosphorylation. Sialidase treatment itself affected phosphorylation by only ± 10% (data not shown). Next, the effect of occupancy of the lectin site on CD33 by sialylated ligands on its phosphorylation was examined. For these experiments, CD33-transfected COS cells were labeled with [32P]-orthophosphate, treated with sialidase, and then incubated with either native or desialylated RBCs (to control for any effects secondary to exposure of cells to the suspension of RBCs). These studies demonstrated that binding RBCs reduced the steady-state level of CD33 phosphorylation observed in non–PMA-stimulated cells by about 70% (Figure8, lane 1 versus lane 3). The enhanced levels of phosphorylation seen after PMA stimulation were not affected by the presence of either native or asialo RBCs (Figure 8).
Ligand binding reduces basal level of CD33 phosphorylation.
CD33- transfected COS cells were labeled with [32P]-orthophosphate and treated with sialidase; the rosetting assay was performed with either native or asialo RBCs, as described in “Materials and methods.” Duplicate wells were treated with PMA or left untreated. Following rosetting, the cells were washed and the remaining cells were lysed. CD33 was immunopurified and analyzed as previously. Binding of native RBCs reduces the basal level of CD33 phosphorylation (lane 3) compared to incubation with asialo RBCs, which are not bound (lane 1). PMA stimulation of CD33 is not affected by ligand binding (lane 4 and lane 2).
Ligand binding reduces basal level of CD33 phosphorylation.
CD33- transfected COS cells were labeled with [32P]-orthophosphate and treated with sialidase; the rosetting assay was performed with either native or asialo RBCs, as described in “Materials and methods.” Duplicate wells were treated with PMA or left untreated. Following rosetting, the cells were washed and the remaining cells were lysed. CD33 was immunopurified and analyzed as previously. Binding of native RBCs reduces the basal level of CD33 phosphorylation (lane 3) compared to incubation with asialo RBCs, which are not bound (lane 1). PMA stimulation of CD33 is not affected by ligand binding (lane 4 and lane 2).
Discussion
Intracellular protein phosphorylation represents one of the more critical and dynamic mechanisms by which cellular functions are regulated, underscoring the interest in establishing the phosphorylation patterns of proteins. An analysis of the cytoplasmic domain of CD33 reveals several potential sites of serine/threonine phosphorylation. Of the 11 serine residues, 4 are found in close proximity to basic amino acid residues and agree approximately with established PKC acceptor sequences (Ser305, Ser307, Ser314, and Ser342)59; however, PROSITE analysis identified S307 as the strongest PKC acceptor site. In all cell lines examined, phosphorylated CD33 was identified by a radiochemical approach. Phosphoamino acid analysis identified predominantly phosphoserine, with lesser amounts of phosphothreonine and no phosphotyrosine.
The application of different pharmacologic agents known to specifically modify serine/threonine phosphorylation indicates PKC is involved either directly or indirectly in these events. The PKC activator PMA resulted in 3- to 8-fold increase in protein phosphorylation, whereas dibutryl cAMP, which stimulates guanosine-dependent kinases, was without any measurable effect. BIM, a highly specific inhibitor of PKC, totally abrogated the PMA-dependent increase in protein phosphorylation, as did staurosporine, which is more widely active against several serine/threonine kinases including the calmodulin-dependent kinases, myosin light-chain kinase, and protein kinases A and G. These observations demonstrate that PKC is involved in CD33 phosphorylation, either by directly acting on CD33 or as an upstream mediator of another kinase. In contrast, the basal level of phosphorylation seen in resting cells was inhibited by staurosporine but not BIM, indicating that other kinases may be involved as well. As one possibility, the kinase that acts directly on CD33 may be under the control of PKC, such that PKC activators increase phosphorylation yet basal levels of phosphorylation are not decreased by PKC inhibition. Alternatively, 2 or more kinases may act independently on CD33.
Several investigators have established the presence of thyosine phosphorylation in CD33 isolated from several myeloid cell lines by using treatment of cells with sodium pervanadate, followed by immunochemical phosphoamino acid analysis using antiphosphotyrosine antibodies. Thus, the tyrosine phosphorylation reported by others presumably occurs only under specific stimulation conditions. Using an alternative approach of radiochemical labeling with [32P]-orthophosphate followed by immunopurification and phosphoamino acid analysis, we demonstrated exclusively the presence of phosphoserine, with smaller amounts of phosphothreonine. These contrasting results indicate the possibility of parallel phosphorylation pathways involved with CD33 function. Because no phosphotyrosine could be identified radiochemically in our hands, the 2 different modifications may be mutually inhibitory or could occur on different populations of CD33, or in cells in different points of the cell cycle. Significantly, however, both techniques of protein phosphorylation analysis leave unanswered the important question of what percentage of the total CD33 population may be modified at any point in time.
The in vivo physiologic function of CD33 remains largely unknown. All members of the CD33-like subfamily (Siglec 5-10) carry ITIM motifs; however, neither Ser307 nor Ser342 is strictly conserved within this family. Siglec 5, 7, 8, 9, and 10 possess serine residues that align at or close to the position of CD33 Ser307 and possess a good PKC consensus. Although Ser342 is also found in Siglec 5, 8, 9 and 10, a good PKC motif is generally missing. Therefore, we predict Ser307 as one of the PKC phosphorylation sites. Other members of the Siglec family include CD22, MAG, and sialoadhesin. CD22 plays a role in B-cell responsiveness,63,64 MAG a role in neurite outgrowth,65-67 and sialoadhesin in macrophage adhesion.68-71 A relationship between CD33 cross-linking, CD33 tyrosine phosphorylation, and calcium flux was also demonstrated.48 Our data indicate a relationship between phosphorylation of CD33 and its ability to function as a lectin in these in vitro assays. Taylor et al47 demonstrated an increase in RBC rosette formation when Tyr340 was mutated. This inside-out signaling phenomenon is very similar to the effect of serine phosphorylation in CD33. A decrease in CD33 phosphorylation leads to a higher rosetting activity, implying an affinity modification of the lectin domain by phosphorylation of the cytoplasmic region due to an as yet unknown mechanism. If additional data indicate CD33 may function as an adhesion molecule in vivo, the phosphorylation state of the cell expressing CD33 may play a regulatory role in this regard. Vitale et al reported that CD33 inhibits the proliferation of normal or leukemic myeloid cells, suggesting a regulatory role in normal myelopoiesis, and that engagement of CD33 induces apoptosis of leukemic cells.72 73
The abundance of serine residues in the cytoplasmic tail of CD33 makes site-specific phosphorylation analysis challenging. The 2-dimensional peptide maps indicate more than one site of phosphorylation. PKC-dependent phosphorylation patterns of truncated CD33 constructs were analyzed because increases in phosphorylation were seen with increasing tail length. The first increase was seen between the ΔVal294 and ΔPro319 constructs, indicating phosphorylation at Ser305, Ser307, or Ser314. Ser307 is the most likely phosphorylation site due to its exact match to the PROSITE PKC motif. The second increase was observed for the 3 longest constructs, ΔD, ΔE, and ΔF. Constructs ΔE and ΔF include Ser342, which matches the pattern HisxxSerLeuAsn, which has been identified to be a good PKC acceptor sequence based on in vitro peptide phosphorylation studies.59
The physiologic significance of phosphorylation events observed in transformed tumor cells grown in culture with native function(s) is more difficult to address. Here we identified further that CD33 phosphorylation occurred under cytokine control in a cell line (TF-1) that required cytokines for long-term growth. Cytokine stimulation of these cells triggers multiple kinase cascades, including tyrosine and serine/threonine kinases. Further, PMA-dependent phosphorylation of CD33 was also seen with primary human CD34+CD33+ progenitor cells, harvested by leukophoresis following cytokine mobilization, indicating that this modification is not unique to transformed cell lines (data not shown). The paucity of material available here prevented more detailed biochemical analysis of the phosphorylation seen here.
The effect of manipulation of CD33 phosphorylation on RBC rosetting, though modest, was reproducible and consistent. Although increasing phosphorylation with PMA had only a marginal effect, decreasing its phosphorylation with kinase inhibitors reproducibly increased rosette formation. Conversely, occupancy of the lectin site with RBCs reduced CD33 phosphorylation. Thus, phosphorylation and lectin site occupancy appear to be antagonistic events. The failure of kinase inhibitors or activators to affect rosetting by pCD33T299Δ supports the interpretation that the effects of these agents on rosetting is not the consequence of other effects they may have on the cell. The lack of linear correspondence of RBC binding to [32P]-orthophosphate labeling may reflect that (1) labeling studies represent net radioactivity incorporated rather than basal levels of phosphorylation or (2) RBCs likely may not be the natural ligand for CD33, and the relationship of ligand binding to phosphorylation may be more pronounced with its natural ligand. At present the stoichiometry of CD33 phosphorylation in resting and PMA-stimulated cells is not known. No difference in SDS-PAGE mobility of CD33 either maximally phosphorylated or dephosphorylated was seen (even after de-N-glycosylation). Consequently, other techniques will need to be used to establish the basal levels of CD33 phosphorylation.
Of note, other Siglecs are also phosphoproteins. CD22 is rapidly tyrosine phosphorylated following B-cell antigen receptor triggering, and tyrosine phosphorylation is followed by the recruitment of specific cytosolic phosphatases to the membrane surface. MAG is both tyrosine and serine/threonine phosphorylated, possibly directly by PKC.39,74 For CD33, a relationship between the occupancy of the lectin site on the extracellular domain with intracellular phosphorylation events has been reported.47 We now show that another phosphorylation event independent of ITIMs also reduces the lectin activity of CD33, and occupancy of the CD33 lectin domain reduces basal levels of CD33 serine phosphorylation. Because CD33 interacts with sialylated structures on the same cell, the molecule could signal a change in sialylation to the cytoplasm. Another mechanism would be an inside-out signal regulated by the phosphorylation state of the cell (mediated by PKC and other kinases), possibly resulting in changes of structural features such as clustering, orientation, or dimerization.
At present, the natural biologic function of CD33 is not known. The RBC rosetting assay represents an in vitro assay of its lectin activity and RBCs are functioning as a surrogate ligand. The expression of CD33 on both immature myeloid cells as well as specific populations of mature, circulating immune effector cells (monocytes and dendritic cells) suggests a role beyond myeloblast development. The data presented here demonstrate its involvement in “kinase cascades” that follow cytokine stimulation and a relationship between its phosphorylation and lectin activity. Future experiments will seek to establish both the specific kinase(s) responsible for its phosphorylation and the biochemical mechanism(s) relating them.
The technical assistance of Jeannette Moyer for some of the work presented is gratefully acknowledged. Drs J. Hardwick and B. Sefton assisted with the phosphoamino acid and phosphopeptide studies, and Dr P. Law kindly provided the human peripheral blood progenitor cells. We thank Drs Jeff Esko, Ajit Varki, and Els Brinkman-Van der Linden for their helpful discussions and for carefully proofreading this manuscript.
Supported by a grant from the V Foundation, Cary, NC, Deutsche Forschungsgemeinschaft grant GR1748 (to K.G.), and Program Project Grant POIHL57345.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kay Grobe, Glycobiology Research and Training Center, 9500 Gilman Dr, La Jolla, CA 92093; e-mail: kgrobe@ucsd.edu.

![Fig. 1. CD33 phosphorylation in myeloid cell lines is induced by phorbol esters. / U937 cells or HL-60 cells were labeled with [32P]-orthophosphate for 1 hour and then treated for 12 minutes with either PMA (18 nM), DMSO (1.5%), or control (0.001% DMSO) for 12 minutes. CD33 immunoprecipitates were isolated using My9, blotted onto nitrocellulose, and detected by fluorography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499001.jpeg?Expires=1763603706&Signature=Y5HsAU1~K1JKPMp9u5n7ZVPFz5fhtyGA23JqcjrDqLIp5HVK-beMxJ8GVpUuY1EuKxeSoeFhBB4~TMFpR4AVrRACr56ae9lY2LgPxzr4R9mA1ugP4ZzKslbIOfk~7gp5zPboUpMJZFOQjJGrXEUn~N-gYmBQmNG4k7xAMCR1~zrxJlW38TRVBvIqHYynC142psESNjnUYGuPr~JxxqGx0F~MX5PIcBmWavNyJftmUY5YLCzv9dgNIhJfoK59oFJdLLSNnKSUWl4zZbeSatI6ulG6OTHDAwsCkAvtUXGsr9wfxSrV84m00x4ucPI~b3N3s8OsnVjYqQv5pu68aut~VQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. CD33 phosphoamino acid analysis in U937 or HL60 cells detects only phosphoserine. / [32P]-orthophosphate-labeled CD33 from PMA-stimulated U937 (A) or HL-60 cells (B) was isolated by My9 adsorption and SDS-PAGE as described in “Materials and methods.” Following hydrolysis in 6 N HCl, the resulting phosphoamino acids were mixed with phosphoserine (pS), phosphothreonine (pT), and phosphotyrosine (pY) standards and analyzed by 2-dimensional high-voltage thin-layer cellulose chromatography. Samples were applied at (+). The radioactivity above the origin represents partially digested phosphopeptides, whereas that in the upper left corner represents free phosphate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499002.jpeg?Expires=1763603706&Signature=GyUJLR7o4NwMfX2PlQbXI8VH4NBS3AKa0sWk4XdoJgLBJjREcb0fvjUxM1tqrMC59gUzgRK70G-O19RkwzpTYY936uTCsDeLLcJ122xt17oGRTPBrXRfEkPTLzYPFJf96qttns1jYDY2tzPoL5lJpBoZvGDL-eJ6nLiUjr2DBgvV82fRyTe7nWHEuF7SOBgWJ6GyCnpBA~vmeb7nC64KtO8qEL3qHLAJBKpyb1gO4VN6fmCsglIf37BmoDNzOZbL0RK4tMSFFp78vh4QMUS9U9G-YOjntb8zMa~3OEXy1UtwJ69~P3JhpMkM2piLRediDk9wvbNDXjotSIBa5gsUaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of pharmacologic manipulation of protein kinase activity on CD33 phosphorylation. / (A) U937 cells were labeled with [32P]-orthophosphate for 1 hour in the absence or presence of 2 μM BIM or 2 μM staurosporine for 60 minutes. Subsequently, PMA was added to 18 nM, or dibutryl cAMP to 1 mM, as indicated. Following 12 minutes at 37°C, the cells were chilled and lysed, and CD33 isolated and analyzed. PMA treatment leads to an increase of CD33 phosphorylation, which is inhibited by pretreatment with BIM or even further reduced by pretreatment with staurosporine. Dibutryl cAMP does not influence the level of CD33 phosphorylation. (B) Phosphorylation of CD33 in transiently transfected COS cells is enhanced by PMA, but not by dibutryl cAMP. COS cells, transfected with pCD33 48 hours earlier, were labeled with [32P]-orthophosphate for 60 minutes followed by 12 minutes treatment with PMA (18 nM) or dibutryl cAMP (1 mM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499003.jpeg?Expires=1763603706&Signature=rVY7hRaFwLxYwic0F~eX9qECXtbqKvCPVok49kI1HANkkJhFAdaUqMsXUlPHSoYgVegCnNEL5ofTn3XnbmsHzICojWh20DjxJqclQ81rtSHUrJJ2dNR5sDSR03SXWwZHfGU9i2nsR9JKVIM7XyYZwVbIZCLMq4uzMpjshW7E2NvFmh60Gu-gfcO7R20cEVIgUnUJGDtTkHmjg64fPb33BLLbia572kW3AoLBzWlkA9SgSH2QI5RtX79CLIl8j8SXE56uG3zwbCgEwxVb1GNqap1HtUUEJrOkSSWPIS4Qirsz~mLILRgPQrD2PyPp-WOxOV4pAg3aB~VDguBTNuwk8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Phosphopeptide analysis demonstrates 2 phosphopeptides. / (A) Tryptic-chymotryptic peptides were analyzed from [32P]-orthophosphate-labeled CD33 isolated from PMA-stimulated CD33-transfected COS cells (i) or U937 cells (ii). Samples were initially purified by SDS-PAGE, blotted onto PVDF, and then digested sequentially with trypsin and chymotrypsin. Isolated phosphopeptides were examined by 2-dimensional thin-layer chromatography as described in “Materials and methods.” Two peptides of similar mobilities were identified in each sample. (B) Amino acid sequence of the cytoplasmic domain of CD33, starting with Lys283, with serine residues indicated by asterisks (*) and gaps indicating expected points of cleavage following combined trypsin-chymotrypsin digestion.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499004.jpeg?Expires=1763603706&Signature=vQSaA190X5KzDiaKueUTA9iZ3W8Oe1v0O-ElK9xrLWdZ1oBLmA04eJjmPR2Y2k-YSR6nf~3LTxszfm38BP2xqaPYhLmC99fS8E-AyH7C7QkMJPAJJJPOb0kOEIuh4j5~KnwVpjyWChInfg4PR6T~e659LvCdz86SckkmmaExPJS6Jj6HrxJ-qtEkhwg9CPQsFAVAJxEKRoxdMU0No7SI5iR9EMXYCOflTDQTZhE5p~woWqEvP2zVq1fXTePaQnHyFA46a-336r5SivUEg51RFqNnfcAwfwElFeFiIdCK4o5igIeI5OE0yLoO0pczkKyIIFOMVl5cLTKX6iesfX~K1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Human CD33 cytoplasmic tail is phosphorylated by purified human PKC in vitro. / Coomassie-stained SDS-PAGE (A) and autoradiograph of the same gel (B). The cytoplasmic tail of CD33 was expressed as a GST fusion protein inE coli, purified, and [32P]-orthophosphate PKC labeled under activating conditions (+ indicates Ca++ and lipids; −, no Hepes and EGTA) and nonactivating conditions (+, Hepes and EGTA; −, no Ca++ and lipids). PKC-mediated labeling of the GST-CD33 tail region is reduced under nonactivating conditions, proving phosphorylation by the purified human PKC. GST alone is not phosphorylated by PKC under these conditions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499005.jpeg?Expires=1763603706&Signature=jJ1vpSIqzrvDvuhf69u86YGPe9f40CjYJzslpMoONzahneZ5Db3T2LYF4FXT1W1~2S7Qm~sPDapCtatXv3CTcJJ~M3WtsZpnttg3MZVflmjCAnZO-ljB82sFguxMCWb7h3DQ5Po-qsh2WKUkPUT4vayh-ydO-gNckv8B1ARwB8fi9woeDkYqlsKNl6lHh4c-CQw87MHv4VcVZKE-qz7bqD6sSaLp9fOj33p8D74P5QCZpGRTTnAhMU44fBgXSZ3YUV3YTwSWPMyahQ1idmlc-Pv1nOOXpf80VUHnLspbs2SP1m3a5ZJUoC24iHZFblMjYLBGCM0JMziZVJ9lZBFCEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Expression and phosphorylation of truncated CD33 constructs on CHOK1 cells. / (A) FACS analysis of CD33 expression on the surface of CHOK1 cells, as detected by My9 anti-CD33 antibody. All constructs were expressed at about the same level, except construct ΔE, which was expressed at an approximately 20 times reduced level. (B) Autoradiograph of CD33 with tails of various length (as defined in text) after PMA-dependent phosphorylation with [32P] in vivo. The applied amount of ΔE was 20 times that of the other constructs to compensate for reduced expression. Constructs ΔC through ΔF show phosphorylation in a PMA-dependent way, suggesting PKC activity on CD33 in vivo. The PMA-dependent phosphorylation of ΔC suggests Ser307 protein kinase as one PKC phosphorylated motif. The increase in phosphorylation after 15 minutes of PMA treatment compared to DMSO treatment alone is shown. (C) Amino acid sequence of the cytoplasmic tail of human CD33. Letters ΔB through ΔF indicate the length of the various constructs; the most C-terminal amino acid of each construct is shown. Putative phosphorylation sites Ser307 and Ser342 are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499006.jpeg?Expires=1763603706&Signature=heQZT70Nx2HmnBQcTsd23veYrCDrW7dCTNcFfv6ZKQvUODGQJIqUbyw9Ld0A088pV0Rs7BzqryJ9RgtsEfFxxgQtZWzpAJ5Zpl08etRD2Bc-8nGGZ6wN87cT~zcXbPklmDUMVyQhpXFXnf9abgZFQ5KxeBGPtTzanVq2VkGiA1-Rqsvj~eFoE~Qssz6zhh10SYEy~kQkW7K9hKVgelDUqIPRxW6kjZGx3W2PswrbmChUXwCEIgVRSiCfBTWGG~uIe7hYU95Be1uXQpkNmXh5EP4TsZ7UJdPSPrWXPkAEiqTmshQ~0V~~2~ca9v2ZeFIBZtrpMPvD7D95tV0ogpop~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Ligand binding reduces basal level of CD33 phosphorylation. / CD33- transfected COS cells were labeled with [32P]-orthophosphate and treated with sialidase; the rosetting assay was performed with either native or asialo RBCs, as described in “Materials and methods.” Duplicate wells were treated with PMA or left untreated. Following rosetting, the cells were washed and the remaining cells were lysed. CD33 was immunopurified and analyzed as previously. Binding of native RBCs reduces the basal level of CD33 phosphorylation (lane 3) compared to incubation with asialo RBCs, which are not bound (lane 1). PMA stimulation of CD33 is not affected by ligand binding (lane 4 and lane 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3188/6/m_h80922499008.jpeg?Expires=1763603706&Signature=ODwlSkTFaMeAiQkQDF3DxbD3D62AcB6BwUr0amwQEm7CGUp8om03RxwNRScxuVcHsQnV9phZC0oYcR~KnH1om5HzBPk59v9uNkWn1bo4WdAx~FbrhXdoojw0jCJuC1-LkNOpStpUg0ZJpKpQT5mqCry7ft7srTnDa~vOIXEaJmHYMzoc1JOEaqmRiEeOrrQgbNuLA4yDJMdtMA90mV9~NsNAk5cuSDVcbfjwY-hMAijGIBRbx6SbUO8loRFimNIcU9jzetwAI1vbLxn2fjPYqv0xdYb7E6-rxTh3n1jUG9H~IU~sAomf39wuwuOlLaCx1lGveHYAEU0T4JYr4owzyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal