Abstract
The role of maintenance therapy in multiple myeloma is controversial. Recent studies have shown an improvement in both progression-free and overall survival for patients receiving maintenance treatment with a combination of interferon and glucocorticoids, compared with interferon alone. The role of glucocorticoids alone as maintenance therapy has not been previously addressed. We compared alternate-day, oral prednisone at 2 different dose levels (10 mg versus 50 mg) for remission maintenance among previously untreated myeloma patients following a response to induction with standard-dose vincristine, doxorubicin, and dexamethasone with prednisone (VAD-P) or VAD-P plus quinine (VAD-P/Q). There were 250 eligible patients registered on Southwest Oncology Group study 9210 and randomized to receive VAD-P or VAD-P/Q. There were 125 patients achieving at least a 25% tumor reduction following induction therapy who were randomized to either physiologic (10 mg) or pharmacologic (50 mg) doses of alternate-day, oral prednisone until disease progression. At the time of study entry, patient characteristics were similar in VAD-P and VAD-P/Q patients and in the 2 arms randomized to maintenance therapy. After a median follow-up of 53 months, there was no difference in either progression-free or overall survival between the 2 induction regimens. However, from the time of maintenance randomization, both progression-free (14 versus 5 months; P = .003) and overall survival (37 versus 26 months; P = .05) were significantly improved in patients receiving 50 mg as compared with 10 mg alternate-day prednisone. There was no difference in treatment-related adverse events between the groups. Thus, 50 mg, oral, alternate-day prednisone is effective maintenance treatment for multiple myeloma patients who achieve a response to induction chemotherapy.
Introduction
Multiple myeloma is a bone marrow malignancy of clonal plasma cells and is1 characterized by osteolytic bone destruction, renal failure, anemia, and an increased risk of infections. Tumor responses are achieved in approximately half of patients who receive standard doses of cytotoxic drugs.1-3High-dose chemotherapy followed by autologous transplantation increases overall and complete response rates and improves overall survival compared with conventional treatment.4 However, all patients will relapse with incurable disease.
Since all patients ultimately recur following induction chemotherapy, attempts to prolong the remission duration with maintenance treatment have been made. However, the role of maintenance therapy in myeloma patients remains controversial. Studies comparing maintenance chemotherapy to unmaintained remission have failed to demonstrate any added benefit from maintenance for responsive patients, provided therapy was reinstituted promptly at relapse.5 Interferon maintenance may prolong remission by several months, but most studies have not shown an improvement in survival.6-11 Although 2 recently published meta-analyses suggest that myeloma patients receiving alpha 2 interferon as maintenance therapy may have a slight prolongation in overall survival,12,13 these studies included many trials that were published only in abstract form. It is well documented that glucocorticoids have antitumor activity in myeloma.14-18 For patients either responding or showing stable disease following conventional chemotherapy, a single-arm study demonstrated that maintenance therapy with oral prednisone (50 mg 3 times per week) and interferon resulted in a long duration of remission and overall survival.19 In a Southwest Oncology Group (SWOG) study, 89 patients responding to induction vincristine, doxorubicin, and dexamethasone (VAD) chemotherapy were randomized to receive maintenance therapy with either maintenance prednisone (50 mg 3 times per week with interferon or interferon alone).20 Progression-free survival was increased from 9 to 19 months for patients given the combination compared with patients given interferon alone. The improved outcome in these 2 studies may result from the glucocorticoid alone. Recently, the MD Anderson group (Houston, TX) randomized 84 patients responding to intermittent oral melphalan and high-dose oral dexamethasone (MD) to maintenance treatment with either α-interferon (3 mIU subcutaneously 3 times weekly) or pulse oral dexamethasone (20 mg/m2 for 4 days monthly) until relapse.21 Importantly, among patients randomized to maintenance treatment, the duration of induction therapy was limited to a median of only 2.5 months. Although there was no difference in duration of response or overall survival between the 2 maintenance treatments, the authors showed that more patients responded to resumption of MD treatment following relapses from interferon (82%) compared with dexamethasone (44%) maintenance treatment. However, the latter results were not surprising since the patients receiving steroid maintenance therapy were again treated with a steroid-containing regimen, MD, at the time of relapse. In the present study, we undertook to compare alternate-day, oral prednisone at pharmacologic doses (50 mg) versus physiologic doses (10 mg) for remission maintenance among myeloma patients responding to VAD with prednisone (VAD-P) or VAD-P with quinine (VAD-P/Q).
Patients and methods
Patients
A total of 262 previously untreated patients with myeloma were registered on this study (SWOG 9210) between April 1993 and December 1997. Patients with all stages of disease were eligible, provided that patients classified as stage I had evidence of progressive disease. A quantifiable serum M-component of immunoglobulin G (IgG), IgA, IgD, or IgE, and/or urinary Bence Jones protein was also necessary. Patients who had symptoms of congestive heart failure, who were using cardiac medications, or who had an allergy to quinine were excluded. Patients were stratified for stage of myeloma (I-II versus IIIA versus IIIB) and risk category (good versus poor). Staging was accomplished by means of the Durie and Salmon system.22 Poor-risk patients included those who had received prior radiotherapy to more than 20% of the bone marrow, who were older than 70 years of age, or whose serum creatinine was 176.8 μM or higher.
Study design
Written, informed consent was obtained from all patients prior to enrollment. Because previous small studies showed that quinine and verapamil may inhibit P-glycoprotein and overcome drug resistance in multiple myeloma,23-25 patients were randomized to receive induction chemotherapy with either VAD-P (arm I) or VAD-P/Q (arm II). Good-risk patients registered to arm I were treated with 0.4 mg vincristine per day and 9.0 mg/m2doxorubicin per day, both administered by continuous infusion on days 1 through 4; 40 mg dexamethasone per day orally on days 1 through 4; and 50 mg prednisone every other day orally on days 9, 11, 13, 15, 17, and 19. Treatment was repeated at 21-day intervals for at least 6 months or until patients achieved at least 25% regression. For good-risk patients randomized to arm II, 400 mg quinine was administered 3 times a day orally on days 1 through 6; VAD was administered on days 2 through 5; and prednisone on days 10, 12, 14, 16, 18, and 20. Poor-risk patients on both arms received a lower dose (6.75 mg/M2 per day) of doxorubicin initially, but were administered the standard dose after the first cycle, provided there was no undue toxicity. Poor-risk patients randomized to arm II received the same dose and schedule of quinine as good-risk patients.
Arm I patients who showed less than 25% regression after 9 months of treatment or who progressed or relapsed during induction on arm I received VAD-P/Q (arm V). Arm II patients with less than 25% regression after 9 months of treatment or who progressed or relapsed during induction were removed from the study.
To be eligible for the maintenance registration, patients showed at least 50% tumor regression after 6 months of induction therapy, or at least 25% regression after 9 to 12 months of therapy. Eligible patients were randomized to either 10 mg (arm III) or 50 mg (arm IV) doses of alternate-day, oral prednisone until disease progression. Stratification at maintenance registration was done by type of induction therapy (VAD-P versus VAD-P/Q) and induction response (less than 25% regression on VAD-P versus 25% to 49% versus 50% to 74% versus at least 75% regression).
Assessments
Patients underwent clinical and laboratory evaluation at the following times: prior to induction randomization; weekly for the first 7 weeks and thereafter at the end of each cycle of induction therapy; prior to maintenance randomization; and monthly (clinical) or quarterly (laboratory) until relapse or progression.
Complete blood count, differential white blood cell count, serum β2-microglobulin, and hepatic and renal functions were assessed. Prior to induction, all patients underwent bone marrow aspiration and skeletal radiography. Protein electrophoresis and immunofixation were performed on both serum and 24-hour urine collection to determine the type and quantity of M-component. Performance status was assessed, and standardized SWOG toxicity criteria were applied. A bone marrow aspirate, complete blood count, blood chemistries, skeletal radiography, and M-component quantification were performed at the end of induction therapy.
Response criteria
Remission (R) in accordance with SWOG criteria was defined as at least a 75% reduction in the calculated tumor mass on at least 2 measurements at an interval of 6 weeks or longer. In addition, the following were also required for remission: a decrease in 24-hour urine tumor mass to 10% or less of the prestudy value and to less than 0.2 g/d on at least 2 measurements at a intervals of 3 weeks or longer, and no increase in the size and number of lytic lesions or serum calcium. Patients who achieved 50% to 74% decreases in the serum and/or tumor mass were defined as having a partial remission (PR). Patients with unconfirmed remission showed an initial measurement that indicated remission but lacked serial follow-up data to verify this finding. Patients with decreases of less than 50% but with increases of not more than 25% in tumor mass without an increase in lytic lesions were considered to have stable disease. Patients with a greater than 25% increase in tumor mass above the nadir level or with an increase in the size or number of lytic lesions or soft-tissue plasmacytomas were considered to have progressive disease or to have relapsed.
Statistical analysis
This report is based on follow-up data collected as of July 10, 2000, which was the time of the planned final analysis. The actuarial durations of progression-free survival and overall survival were plotted according to the method of Kaplan and Meier.26Differences between the curves were appraised by the log-rank method.27 All statistical comparisons used 2-tailedP values. For analysis of induction therapy (VAD-P versus VAD-P/Q), survival was determined from the start of induction chemotherapy. Survival during the maintenance phase of the study was determined from the day of randomization to the maintenance phase. Following randomization, all patients were monitored according to their treatment group even if treatment was discontinued owing to toxicity or noncompliance. Patients who died without evidence of progression were included in the analysis of response.
Results
Patient characteristics for induction therapy
A total of 262 patients were registered to receive induction therapy; 12 of these patients (4 on arm I and 8 on arm II) were ineligible. Ineligibility was due largely to incomplete documentation (n = 6) or lack of serum or urine paraprotein criteria (n = 4). The 250 eligible patients consisted of 126 randomized to receive VAD-P (arm I) and 124 to receive VAD-P/Q (arm II). The median follow-up duration for living patients from the start of induction chemotherapy was 53 months (range, 21-81 months). There were no significant differences between the arms with respect to any of the pretreatment clinical or laboratory characteristics (Table1). The median age was 61, and the majority of patients were stage IIIA (57%), with fewer patients either at stage I or II (24%) or at stage IIIB (19%). Most patients were considered good risk (65%).
Patient characteristics at induction randomization
| Characteristic . | Induction regimen . | |
|---|---|---|
| VAD-P (n = 126) . | VAD-P/Q (n = 124) . | |
| Median age, y (range) | 60 (26-81) | 62 (34-87) |
| Sex, male/female | 79/47 (63%/37%) | 71/53 (57%/43%) |
| Race, white/black/ Hispanic/other | 94/23/6/3 (75%/18%/5%/2%) | 94/24/4/2 (76%/19%/3%/2%) |
| Stage | ||
| I-II | 30 (24%) | 30 (24%) |
| IIIA | 69 (55%) | 74 (60%) |
| IIIB | 27 (21%) | 20 (16%) |
| Risk | ||
| Good | 80 (63%) | 82 (66%) |
| Poor | 46 (37%) | 42 (34%) |
| M component, IgG/IgA/other | 70/28/28 (56%/22%/22%) | 79/27/18 (64%/22%/15%) |
| Performance status | ||
| 0-1 | 84 (68%) | 83 (67%) |
| 2+ | 39 (32%) | 41 (33%) |
| Serum albumin | ||
| Less than 3 g/dL | 26 (25%) | 30 (24%) |
| At least 3 g/dL | 98 (75%) | 93 (76%) |
| Serum calcium | ||
| Less than 11.5 mg/dL | 109 (87%) | 116 (95%) |
| At least 11.5 mg/dL | 13 (13%) | 6 (5%) |
| Serum creatinine | ||
| Less than 2 mg/dL | 94 (76%) | 102 (82%) |
| At least 2 mg/dL | 30 (24%) | 22 (18%) |
| Serum β2-M | ||
| Less than 6 μg/mL | 64 (57%) | 86 (73%) |
| At least 6 μg/mL | 49 (43%) | 32 (27%) |
| Characteristic . | Induction regimen . | |
|---|---|---|
| VAD-P (n = 126) . | VAD-P/Q (n = 124) . | |
| Median age, y (range) | 60 (26-81) | 62 (34-87) |
| Sex, male/female | 79/47 (63%/37%) | 71/53 (57%/43%) |
| Race, white/black/ Hispanic/other | 94/23/6/3 (75%/18%/5%/2%) | 94/24/4/2 (76%/19%/3%/2%) |
| Stage | ||
| I-II | 30 (24%) | 30 (24%) |
| IIIA | 69 (55%) | 74 (60%) |
| IIIB | 27 (21%) | 20 (16%) |
| Risk | ||
| Good | 80 (63%) | 82 (66%) |
| Poor | 46 (37%) | 42 (34%) |
| M component, IgG/IgA/other | 70/28/28 (56%/22%/22%) | 79/27/18 (64%/22%/15%) |
| Performance status | ||
| 0-1 | 84 (68%) | 83 (67%) |
| 2+ | 39 (32%) | 41 (33%) |
| Serum albumin | ||
| Less than 3 g/dL | 26 (25%) | 30 (24%) |
| At least 3 g/dL | 98 (75%) | 93 (76%) |
| Serum calcium | ||
| Less than 11.5 mg/dL | 109 (87%) | 116 (95%) |
| At least 11.5 mg/dL | 13 (13%) | 6 (5%) |
| Serum creatinine | ||
| Less than 2 mg/dL | 94 (76%) | 102 (82%) |
| At least 2 mg/dL | 30 (24%) | 22 (18%) |
| Serum β2-M | ||
| Less than 6 μg/mL | 64 (57%) | 86 (73%) |
| At least 6 μg/mL | 49 (43%) | 32 (27%) |
VAD-P indicates vincristine, doxorubicin, and dexamethasone with prednisone; VAD-P/Q, VAD-P plus quinine; Ig, immunoglobulin; β2-M, gb2-microglobulin.
One patient randomized to VAD-P elected to receive melphalan-prednisone instead; this patient is not evaluable for toxicity. Two patients on the VAD-P/Q arm did not receive quinine, but are still considered evaluable for toxicity and response. Analyses of progression-free and overall survival included all eligible patients.
There were 84 patients on the VAD-P arm who experienced a maximum grade toxicity of 3 (Table 2). Two patients died of sepsis and one individual of thromboemboli. Of the patients on the VAD-P/Q arm, 15 experienced a maximum grade toxicity of 3. One patient became septic and died from a cerebral hemorrhage, and 2 others died from infections.
Response to and toxicity of induction therapy
| Characteristic . | Induction regimen . | |
|---|---|---|
| VAD-P (n = 126)* . | VAD-P/Q (n = 124)* . | |
| Response to induction therapy | ||
| R (75%-100% reduction) | 47 (38%) | 49 (40%) |
| PR (50%-74% reduction) | 23 (18%) | 21 (17%) |
| UR | 9 (7%) | 10 (8%) |
| SD | 27 (22%) | 15 (12%) |
| PD | 6 (5%) | 7 (6%) |
| Early death | 1 (1%) | 1 (1%) |
| Inadequate assessment | 12 (10%) | 20 (16%) |
| Total | 125 (100%) | 123 (100%) |
| Maximum grade any toxicity | ||
| At most Grade 2 | 40 | 28 |
| Grades 3-5 | 84 | 95 |
| Characteristic . | Induction regimen . | |
|---|---|---|
| VAD-P (n = 126)* . | VAD-P/Q (n = 124)* . | |
| Response to induction therapy | ||
| R (75%-100% reduction) | 47 (38%) | 49 (40%) |
| PR (50%-74% reduction) | 23 (18%) | 21 (17%) |
| UR | 9 (7%) | 10 (8%) |
| SD | 27 (22%) | 15 (12%) |
| PD | 6 (5%) | 7 (6%) |
| Early death | 1 (1%) | 1 (1%) |
| Inadequate assessment | 12 (10%) | 20 (16%) |
| Total | 125 (100%) | 123 (100%) |
| Maximum grade any toxicity | ||
| At most Grade 2 | 40 | 28 |
| Grades 3-5 | 84 | 95 |
R indicates remission; PR, partial remission; UR, unconfirmed remission; SD, stable disease; PD, progressive disease. See Table 1 for other abbreviations.
One patient in each arm had incomplete response data available.
Response rates and survival by induction therapy
Response (R + PR) rates were similar in the arms: 56% for VAD-P and 57% for VAD-P/Q (P = .85). Only 5% of patients on the VAD-P arm and 6% of patients on the VAD-P/Q arm had progressive disease while on induction therapy. There have been a total of 179 deaths among the 250 patients eligible for the study. There was no difference in progression-free or overall survival between the 2 arms (Figure 1). The median duration of progression-free survival was the same at 15 months for both arms (P = .22). The median overall survival was 27 months from the start of chemotherapy among patients who received VAD-P, in comparison with 33 months for patients who received VAD-P/Q (P = .38).
Kaplan-Meier estimates of survival among the study patients randomized to induction therapy.
Estimates of progression-free (A) and overall survival (B). Median progression-free survival is 15 months on both induction arms;P = .22. Median survival is 33 months for VAD-P/Q and 27 months for VAD-P; P = .38.
Kaplan-Meier estimates of survival among the study patients randomized to induction therapy.
Estimates of progression-free (A) and overall survival (B). Median progression-free survival is 15 months on both induction arms;P = .22. Median survival is 33 months for VAD-P/Q and 27 months for VAD-P; P = .38.
Patient characteristics for maintenance therapy
A total of 132 patients were registered to receive maintenance therapy; 6 of these patients (2 on arm III and 4 on arm IV) were ineligible. The 126 eligible patients consisted of 65 randomized to receive 10 mg prednisone and 61 randomized to receive 50 mg prednisone. The median time to maintenance randomization for the 10-mg and 50-mg groups was 202 days and 199 days, respectively. The premaintenance treatment patient characteristics, type of induction therapy, and response to induction therapy were similar in the 2 arms (Table3). In addition, the proportion of patients who had received intravenous pamidronate was similar in the high-dose (52%) as compared with the low-dose (50%) prednisone arms.
Patient characteristics at maintenance randomization
| Characteristic . | Maintenance regimen . | |
|---|---|---|
| Prednisone, 10 mg (n = 65) . | Prednisone, 50 mg (n = 61) . | |
| Median age, y (range) | 61 (35-81) | 63 (41-79) |
| Sex, male/female | 37/28 (57%/43%) | 38/23 (62%/38%) |
| Race, white/black/ Hispanic/other | 49/12/3/1 (75%/18%/5%/2%) | 47/9/3/2 (77%/15%/5%/3%) |
| Induction regimen | ||
| VAD-P | 31 (48%) | 33 (54%) |
| VAD-P/Q | 34 (52%) | 28 (46%) |
| Response to induction | ||
| 25%-49% reduction | 4 (6%) | 3 (5%) |
| 50%-74% reduction | 16 (25%) | 12 (20%) |
| 75%-100% reduction | 45 (69%) | 46 (75%) |
| Serum albumin, at least 3 g/dL | 98% | 94% |
| Serum calcium, less than 12 mg/dL | 100% | 100% |
| Serum creatinine, less than 2 mg/dL | 90% | 94% |
| Serum β2-M, less than 6 μg/mL | 91% | 95% |
| Maximum grade any toxicity on maintenance therapy | ||
| At most Grade 2 | 79% | 75% |
| Grade 3-5 | 21% | 25% |
| Characteristic . | Maintenance regimen . | |
|---|---|---|
| Prednisone, 10 mg (n = 65) . | Prednisone, 50 mg (n = 61) . | |
| Median age, y (range) | 61 (35-81) | 63 (41-79) |
| Sex, male/female | 37/28 (57%/43%) | 38/23 (62%/38%) |
| Race, white/black/ Hispanic/other | 49/12/3/1 (75%/18%/5%/2%) | 47/9/3/2 (77%/15%/5%/3%) |
| Induction regimen | ||
| VAD-P | 31 (48%) | 33 (54%) |
| VAD-P/Q | 34 (52%) | 28 (46%) |
| Response to induction | ||
| 25%-49% reduction | 4 (6%) | 3 (5%) |
| 50%-74% reduction | 16 (25%) | 12 (20%) |
| 75%-100% reduction | 45 (69%) | 46 (75%) |
| Serum albumin, at least 3 g/dL | 98% | 94% |
| Serum calcium, less than 12 mg/dL | 100% | 100% |
| Serum creatinine, less than 2 mg/dL | 90% | 94% |
| Serum β2-M, less than 6 μg/mL | 91% | 95% |
| Maximum grade any toxicity on maintenance therapy | ||
| At most Grade 2 | 79% | 75% |
| Grade 3-5 | 21% | 25% |
See Table 1 for abbreviations.
Of the patients randomized to the high-dose prednisone, one had this therapy discontinued early, one had treatment continued too long, and there was a delay in initiation of prednisone in one patient. One patient on the low-dose arm received high-dose prednisone, and one patient randomized to high-dose received no treatment; neither is evaluable for toxicity.
Thirteen patients on each arm experienced toxicity of grade 3 or higher (some specific toxicities are listed in Table4). One patient on high-dose prednisone died of cardiomyopathy and another individual of a respiratory infection.
Comparison of adverse events in high-dose and low-dose prednisone maintenance
| Toxicity . | Number of events grade 3 or higher4-150 . | |
|---|---|---|
| Prednisone, 10 mg (n = 61) . | Prednisone, 50 mg (n = 51) . | |
| Infections | 1 (0) | 2 (1) |
| Edema | 2 (0) | 8 (2) |
| Weight gain | 1 (0) | 0 |
| Personality changes | 0 | 4 (0) |
| Muscle weakness | 7 (1) | 4 (0) |
| Myalgias | 3 (0) | 2 (1) |
| Cushingoid appearance | 0 | 6 (0) |
| Toxicity . | Number of events grade 3 or higher4-150 . | |
|---|---|---|
| Prednisone, 10 mg (n = 61) . | Prednisone, 50 mg (n = 51) . | |
| Infections | 1 (0) | 2 (1) |
| Edema | 2 (0) | 8 (2) |
| Weight gain | 1 (0) | 0 |
| Personality changes | 0 | 4 (0) |
| Muscle weakness | 7 (1) | 4 (0) |
| Myalgias | 3 (0) | 2 (1) |
| Cushingoid appearance | 0 | 6 (0) |
Note that 13 patients on each arm experienced toxicities of grade 3 or higher as their worst degree of toxicity. Some patients had multiple toxicities of grade 3 or higher.
Survival by maintenance therapy
There have been 67 deaths among the 125 patients randomized to receive maintenance treatment. There was no difference in survival following maintenance randomization on the basis of the degree of response from induction therapy (36 and 33 months for the R and PR groups, respectively; P = .90).
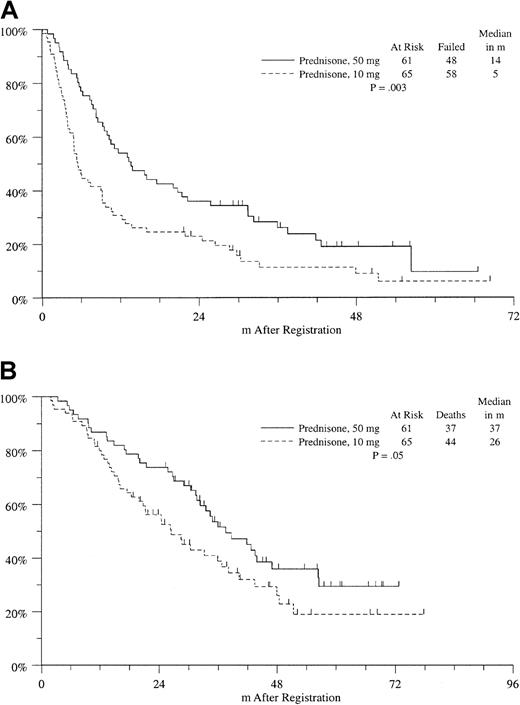
On the other hand, the median progression-free survival was significantly improved in the high-dose prednisone arm compared with the low-dose prednisone arm (14 versus 5 months;P = .003), with a median follow-up for living patients of 44 months from randomization to maintenance therapy (Figure2A). Median overall survival from maintenance randomization was also significantly prolonged in the high-dose group compared with the low-dose group (37 versus 26 months;P = .05) (Figure 2B). There was no difference in specific or overall treatment-related adverse events between these arms (Table4).
Kaplan-Meier estimates of survival among the study patients randomized to maintenance therapy.
Progression-free survival (A) and overall survival (B). Survival was measured from the time of randomization to maintenance therapy. Median progression-free survival is 14 months on the high-dose arm and 5 months on the low-dose arm; P = .003. Median survival is 37 months on the high-dose arm and 26 months on the low-dose arm;P = .05.
Kaplan-Meier estimates of survival among the study patients randomized to maintenance therapy.
Progression-free survival (A) and overall survival (B). Survival was measured from the time of randomization to maintenance therapy. Median progression-free survival is 14 months on the high-dose arm and 5 months on the low-dose arm; P = .003. Median survival is 37 months on the high-dose arm and 26 months on the low-dose arm;P = .05.
Discussion
This study addressed the role of glucocorticoids as maintenance therapy and the question of whether the addition of multidrug resistance inhibitors can improve the efficacy of induction chemotherapy. The results clearly demonstrated that maintenance therapy with 50 mg alternate-day prednisone significantly improved overall survival among patients responding to induction treatment. Although previous small studies had suggested the potential usefulness of blocking P-glycoprotein with quinine in myeloma,25 the addition of this drug to VAD-P did not improve outcome in this study.
Studies have demonstrated a number of newer chemotherapeutic protocols,1,28,29 and high-dose chemotherapy followed by autologous bone marrow transplantation4,30 can substantially reduce the tumor burden in myeloma patients. However, none of these treatments are curative. Thus, maintenance therapy might be useful in prolonging survival by inhibiting proliferation and inducing apoptosis of cells that are unable to be eliminated by chemotherapy. Subcutaneous interferon has been evaluated as maintenance therapy for myeloma patients during the past decade.6-13Initial results of an Italian study were encouraging and showed that the use of this agent improved the duration of remission.6However, most randomized studies and meta-analyses evaluating maintenance interferon showed at best a modest increase in progression-free survival without any, or with minimal, overall survival benefit.7-13 This may be explained by recent in vitro studies showing that interferon decreases the amount of monoclonal immunoglobulin produced by malignant plasma cells without inhibiting their growth.31
Two recent studies suggested that the combination of interferon and glucocorticoids were effective as maintenance therapy for myeloma patients.19,20 Since maintenance interferon does not improve outcome, this benefit may be observed with the use of glucocorticoids alone. Although pulse dexamethasone produced a similar outcome to 3-times-a-week interferon maintenance therapy among patients responding to MD induction therapy,21 the type and length of induction therapy (melphalan-containing compared with VAD-like regimens) and the type, dose, and schedule of steroids may be critical to their efficacy during maintenance therapy. In fact, patients received only a median of 2.5 months (maximum, 4.9 months) of induction MD therapy,21 and this short induction period is unlikely to be long enough to produce a maximal antimyeloma effect with the use of a melphalan-based regimen. Thus, we compared alternate-day, oral prednisone at a physiologic dose (10 mg) with a pharmacologic dose (50 mg) as maintenance therapy for patients showing a more than 25% reduction in tumor burden to induction therapy with VAD-P or VAD-P/Q. Patients who received the higher dose of prednisone showed improved progression-free and overall survival. These data provide clear evidence of the benefit and safety of 50 mg alternate-day prednisone as maintenance treatment for myeloma patients who respond to conventional chemotherapy. Whether the efficacy of this maintenance regimen is limited to myeloma patients responding to a VAD-like induction therapy, as was used in this study, is unknown, however. The fact that these patients were already responding to a regimen containing a glucocorticoid, dexamethasone, before receiving the prednisone treatment may explain the efficacy of this maintenance regimen in this study.
The efficacy of glucocorticoids as initial or relapse therapy for myeloma is well established. These drugs are known to suppress the production of cytokines important in myeloma growth and bone disease, such as interleukin-6 (IL-6) and IL-1β, in vitro as well as induce apoptosis of myeloma cells.32-35 In addition, glucocorticoids reduce NF-κB activity,36 and this effect enhances apoptosis of malignant plasma cells.37 NF-κB also stimulates IL-6 production from myeloma stromal cells38 and enhances bone resorption.39 Thus, the clinical benefits of glucocorticoids may result from the induction of tumor cell apoptosis as well as from the reduction of the availability of growth-promoting and bone-resorbing cytokines. It is also important to recognize that chronic glucocorticoid use is associated with significant potential toxicities, including hyperglycemia, osteoporosis, aseptic necrosis of bone, infectious complications, weight gain, myopathy, and mood changes. However, when administered as alternate-day prednisone at 50 mg in this trial, the drug was well tolerated without significant toxicity although specific assessments of bone mineral density, bone-resorption markers, and aseptic necrosis of bone were not done as part of this study.
Maintenance treatment with alternate-day oral prednisone, at 50 mg, of multiple myeloma patients who have responded to conventional chemotherapy improves both progression-free and overall survival. This effective form of maintenance therapy is safe, well tolerated, and inexpensive. Similar studies should be initiated in myeloma patients undergoing high-dose chemotherapy with autologous stem cell support. This is the first demonstration of the efficacy of maintenance therapy with glucocorticoids in any chronic B-cell malignancy. Since many other types of these tumors are responsive to glucocorticoid treatment, it should also be determined whether these agents are effective as maintenance therapy in patients with other B-cell tumors, including non-Hodgkin lymphoma and chronic lymphocytic leukemia.
Supported in part by the following Public Health Service Cooperative agreement grants awarded by the National Cancer Institute, US Department of Health and Human Services: CA38926, CA32102, CA58348, CA13612, CA35261, CA37981, CA04920, CA52654, CA45807, CA22433, CA35281, CA35128, CA46441, CA12644, CA12213, CA58861, CA42777, CA04919, CA35176, CA16385, CA46113, CA20319, CA35431, CA35119, CA96429, CA35090, CA58416, CA27057, CA76447, CA45377, CA35178, CA35262, CA67663, CA46136, CA63845, CA46282, CA52386, CA45560, CA76462, CA35192, and CA14028.
S.E.S. is deceased.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John J. Crowley, Southwest Oncology Group (SWOG-9210), 14980 Omicron DR, San Antonio, TX 78245-3217; e-mail:johnc@swog.fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal