Abstract
We conducted a retrospective study to determine whether the presence of moyamoya collaterals influenced the risk of recurrence of cerebrovascular events (CVEs: stroke or transient ischemic attack) in patients with sickle cell disease placed on chronic transfusions after a stroke. Forty-three patients with homozygous sickle cell anemia (HbSS) and 1 with HbSOArab (16 females, 28 males) who had suffered strokes while under the age of 18 were studied. All patients had been on transfusions aimed at maintaining the sickle hemoglobin (HbS) level below 30%. They were followed for a mean of 6.6 years (2.2 to 20.4 years). The presence of collaterals was diagnosed based on either magnetic resonance angiography or conventional angiography. Eighteen (41%) of the 44 patients suffered recurrent CVEs. Nineteen (43%) (6 females, 13 males) patients had moyamoya collaterals. Eleven (58%) of these 19 experienced 21 total recurrent CVEs, including 4 strokes in 4 patients (21%). In comparison, 7 (28%) of 25 patients without moyamoya collaterals experienced 9 recurrent CVEs (P < .05) with only 1 recurrent stroke (4%). Moyamoya patients were also more likely to have 2 recurrent CVEs (42% vs 8%,P < .05) as well as poorer neuropsychological testing results. A proportional hazards regression analysis indicated that patients with moyamoya were more than twice as likely to incur a subsequent CVE (hazard ratio, 2.40; 95% confidence interval, 0.85, 6.75). We conclude that up to 41% of patients with sickle cell disease experience recurrent CVEs after an initial stroke despite chronic transfusions and that the risk of recurrence is significantly higher for those who have moyamoya collaterals.
Introduction
Ischemic stroke develops in 7% to 11% of children with homozygous sickle cell anemia (HbSS).1-5 In most cases strokes are the result of large-vessel occlusive disease and watershed ischemia confirmed by cerebral angiography.6-8Moyamoya (Japanese for “hazy puff of smoke”) disease describes an angiographic pattern consisting of large-vessel occlusion and a telangiectatic network of collateral vessels of unknown etiology usually seen in children who present with acute hemiplegia.9,10 This is the result of progressive development of collaterals after occlusion of large cerebral vessels. Moyamoya syndrome or pattern refers to the same angiographic changes when they are the result of diseases such as neurofibromatosis, tuberous sclerosis, sickle cell, periarteritis nodosa, postradiation vasculopathy, or infections.11 Conventional angiograms performed after a stroke in children with sickle cell anemia were first reported in 1972 to have a moyamoya pattern.8
After a stroke, an estimated 44% to 67% of HbSS patients experience recurrent infarctions.2,4,12 Chronic transfusion therapy programs that have the goal of maintaining sickle hemoglobin (HbS) levels below 30% can reduce the risk of recurrent stroke to 13% but seem to have little effect on the recurrence of other transient neurologic events or hemorrhagic strokes.13 Transfusions seem to be needed long-term because up to 50% of patients on transfusion have a recurrent stroke after transfusions are discontinued.14 Nonetheless, strokes can recur despite chronic transfusion,15 although it has been reported that maintaining HbS levels below 10% can prevent most recurrences.3 Moyamoya syndrome has been recently shown to be a predictor of recurrent stroke after cerebral arterial infarction in non–sickle cell patients.16 It is unclear if moyamoya pattern in sickle cell anemia is associated with an increased risk of recurrent stroke or transient ischemic attack (TIA). To investigate this issue, we retrospectively collected data to evaluate the recurrence risk of stroke or TIA in children with HbSS who were on a transfusion therapy program after having an initial acute clinical stroke.
Patients and methods
We reviewed the records of all patients with sickle cell anemia in our institution who had an acute stroke when they were less than 18 years of age between January 1980 and August 1999. All were enrolled in the chronic transfusion program. Patients received transfusions every 3 to 5 weeks aimed at maintaining the level of HbS below 30% and were followed for a minimum of 1 year after occurrence of initial stroke. This study was deemed to be exempt from the need for informed consent by the Institutional Review Board at the Medical University of South Carolina.
For the purpose of this study, we defined a cerebrovascular event (CVE) to include a stroke or TIA, with each considered a different-type event. Infarctive and hemorrhagic strokes were defined as neurologic events lasting more than 24 hours associated with or without radiographic evidence of new areas of infarction or hemorrhage, respectively. TIA was defined as focal neurologic symptoms such as weakness or hypoesthesia of fewer than 24 hours' duration and without radiographic changes.5 13
Data collected from charts and radiologic reviews on all patients included baseline complete blood counts, including levels of HbS and fetal hemoglobin, age at initial acute stroke, and associated clinical and radiographic manifestations. Similar data were recorded for any subsequent CVE during their follow-up evaluations. Most patients diagnosed before 1991 had computed tomography scans after their stroke, and magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) was performed as part of their follow-up. After 1994 we followed a uniform protocol for evaluating strokes and TIA. All patients had MRI/MRA as soon as possible after the initial event and each subsequent event. They also had documented neurology consultations after each event. Neurology consultation and imaging studies were repeated yearly. Before MRA was available, cerebral angiograms were performed on patients who experienced recurrent events. All imaging studies were reviewed by one neuroradiologist (J.K.C.).
MRI examinations were performed on a 1.5 T superconducting magnet (Sigma, General Electric Medical Systems, Milwaukee, WI). MRA examinations consisted of axially acquired 3-dimensional Fourier transformation (3DFT) time of flight studies of the cerebral vessels, supplemented in some cases by 2DFT and 3DFT phase contrast MRAs. Several early patients underwent conventional angiography consisting of cut-film anteroposterior and lateral views supplemented by digital subtraction angiography for analysis of subtle collateral flow patterns. Nonionic contrast (Omnipaque 300, Winthrop Pharmaceuticals, New York, NY) was used in all cases. Patients' HbS levels were maintained at 25% or less, and patients were well hydrated before and during the procedure. Normal oxygen saturation was maintained throughout the procedure and was monitored by pulse oximetry.
MRI studies were reviewed to assess the presence and locations of cerebral infarcts. MRAs and conventional angiograms were reviewed to identify the moyamoya pattern. Moyamoya findings were defined by vascular stenosis and occlusion of the supraclinoid portions of the internal carotid arteries or the proximal parts of the anterior and middle cerebral arteries, accompanied by patterns of collateral blood flow through the basal ganglia and/or meningeal anastomoses.17 18
Patients with moyamoya collaterals were compared with patients without moyamoya on the following measures: gender, current age, age at first stroke, length of follow-up, proportion having a subsequent CVE, proportion having 2 subsequent CVEs, proportion having a subsequent stroke, proportion having subsequent TIA, and baseline laboratory values. Preliminary results from neuropsychological functioning tests have been previously reported on patients from these 2 groups.19
Using nonparametric log-rank statistics, the time to subsequent CVE was compared between moyamoya patients and nonmoyamoya patients. Kaplan-Meier techniques were used to calculate the median time to subsequent CVE and the median time to subsequent stroke. To estimate hazard ratios comparing event rates in moyamoya patients with nonmoyamoya patients, proportional hazards regression models were created. The primary analysis included all eligible patients and all CVEs, while secondary analyses excluded data from 3 CVEs incurred by 3 patients (2 moyamoya and 1 nonmoyamoya) who were not being transfused secondary to noncompliance at the time of the event.
Results
Forty-four patients (16 females and 28 males) were studied who had initial acute strokes between January 1, 1980, and August 1, 1999; all but one (HbSOArab) had HbSS. Characteristics of patients with and without moyamoya are listed in Table 1.
Characteristics of study patients
| Variable . | Patients with moyamoya (n = 19) . | Patients without moyamoya (n = 25) . | Total study population (N = 44) . |
|---|---|---|---|
| Sex: male/total (% male) | 13/19 (68.4) | 15/25 (60.0)* | 28/44 (63.6) |
| Age, y, as of 7/1/2000, mean ± SD | 17.1 ± 7.0 | 15.5 ± 7.1* | 16.2 ± 7.0 |
| Age, y, at first stroke, mean ± SD | 7.8 ± 4.3 | 7.4 ± 3.9* | 7.6 ± 4.0 |
| Follow-up time, y, after first stroke median (interquartile range) | 8.1 (5.0 to 11.5) | 4.7 (3.6 to 13.4)* | 6.6 (4.2 to 12.5) |
| Recurrent CVE (%) | 11/19 (57.9) | 7/25 (28.0)† (P = .0457) | 18/44 (40.9) |
| Two recurrent CVEs (%) | 8/19 (42.1) | 2/25 (8.0)† (P = .0075) | 10/44 (22.7) |
| Recurrent TIA (%) | 10/19 (52.6) | 6/25 (24.0)* (P = .0505) | 16/44 (36.4) |
| Recurrent stroke (%) | 4/19 (21.1) | 1/25 (4.0)* (P = .0775) | 5/44 (11.4) |
| Median time, y, to subsequent CVE | 3.08 | ‡ | 13.26 |
| Laboratory test values at time of initial stroke | |||
| HbS (%) | 83.0 ± 15.1 | 86.5 ± 7.2* | 84.6 ± 12.2 |
| Fetal hemoglobin (%) | 10.3 ± 6.6 | 10.2 ± 7.6* | 10.3 ± 6.8 |
| White blood cell count (× 109/L) | 17.9 ± 7.7 | 18.1 ± 4.6* | 17.9 ± 6.8 |
| Hemoglobin (g × dL) | 7.6 ± 1.1 | 7.5 ± 1.2* | 7.5 ± 1.1 |
| Reticulocytes (%) | 12.5 ± 4.8 | 10.0 ± 5.0* | 11.6 ± 4.9 |
| Platelets, (× 106/μL) | 403.3 ± 95.3 | 440.1 ± 120.6* | 414.9 ± 102.0 |
| Variable . | Patients with moyamoya (n = 19) . | Patients without moyamoya (n = 25) . | Total study population (N = 44) . |
|---|---|---|---|
| Sex: male/total (% male) | 13/19 (68.4) | 15/25 (60.0)* | 28/44 (63.6) |
| Age, y, as of 7/1/2000, mean ± SD | 17.1 ± 7.0 | 15.5 ± 7.1* | 16.2 ± 7.0 |
| Age, y, at first stroke, mean ± SD | 7.8 ± 4.3 | 7.4 ± 3.9* | 7.6 ± 4.0 |
| Follow-up time, y, after first stroke median (interquartile range) | 8.1 (5.0 to 11.5) | 4.7 (3.6 to 13.4)* | 6.6 (4.2 to 12.5) |
| Recurrent CVE (%) | 11/19 (57.9) | 7/25 (28.0)† (P = .0457) | 18/44 (40.9) |
| Two recurrent CVEs (%) | 8/19 (42.1) | 2/25 (8.0)† (P = .0075) | 10/44 (22.7) |
| Recurrent TIA (%) | 10/19 (52.6) | 6/25 (24.0)* (P = .0505) | 16/44 (36.4) |
| Recurrent stroke (%) | 4/19 (21.1) | 1/25 (4.0)* (P = .0775) | 5/44 (11.4) |
| Median time, y, to subsequent CVE | 3.08 | ‡ | 13.26 |
| Laboratory test values at time of initial stroke | |||
| HbS (%) | 83.0 ± 15.1 | 86.5 ± 7.2* | 84.6 ± 12.2 |
| Fetal hemoglobin (%) | 10.3 ± 6.6 | 10.2 ± 7.6* | 10.3 ± 6.8 |
| White blood cell count (× 109/L) | 17.9 ± 7.7 | 18.1 ± 4.6* | 17.9 ± 6.8 |
| Hemoglobin (g × dL) | 7.6 ± 1.1 | 7.5 ± 1.2* | 7.5 ± 1.1 |
| Reticulocytes (%) | 12.5 ± 4.8 | 10.0 ± 5.0* | 11.6 ± 4.9 |
| Platelets, (× 106/μL) | 403.3 ± 95.3 | 440.1 ± 120.6* | 414.9 ± 102.0 |
Not significantly different from patients with moyamoya (P > .05).
P < .05.
Median time to subsequent CVE after initial stroke is not estimable for patients without moyamoya because most nonmoyamoya patients did not have a subsequent stroke. However, it can be determined that a lower bound for this measure is 5.01 years and that the median time to subsequent CVE after initial stroke is significantly (P = .0429) greater among patients without moyamoya.
Presence of moyamoya
Of the 44 patients studied, 19 (43%; 6 females and 13 males) were found to exhibit a moyamoya pattern. The imaging studies performed on these patients are described in Table 2and summarized below. Moyamoya was diagnosed by MRA in 16 patients an average of 2.4 years (range, −0.3 to 11.4 years) after the initial stroke. In 1 patient, who had abnormal transcranial Doppler findings and was randomized to the observation arm of the Stroke Prevention Trial in Sickle Cell Disease (STOP), moyamoya collaterals were observed 3 months before he had a stroke.20 Eleven of these 16 patients were diagnosed with moyamoya within 1 year of initial stroke. Moyamoya was diagnosed by conventional angiography in 3 patients an average of 3.1 years (range, 0.1 to 9.0 years) after initial stroke. Moyamoya vessels were present most frequently in the lenticulostriate distributions (9 of 19; 47%). Twenty-five patients (57%; 10 females and 15 males) were found not to have moyamoya collaterals, but 21 of 25 had developed some large vessel abnormality by the end of this study. The imaging studies performed on these patients are described in Table 3.
Summary of imaging studies completed for moyamoya patients
| Patient no. . | Initial stroke; age, y . | Trfx . | CVE recurrences . | Initial scans, y . | Scan diagnosis 1 . | Initial MRA/CA . | Scan diagnosis 2 . | F/U MRA . | F/U MRA scan diagnosis . | Prog . | Latest MRA scan diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | '92; 4.4 | '92 | '93 | MRA, '92 | S, NMV | '92 | MM | '92, '93, '94, '95, '96, '97, '99 | '93, MM | + | ACA occlusion |
| 2 | '96; 3.9 | '96 | '99, '99 | CT, MRA, '96 | S, NMV | '96 | NMV | '96, '97, '99 | '96, MM | + | L MCA occlusion |
| 3 | '93; 11.4 | '93 | '93, '93 | MRA, '93 | S, MM | '93 | MM | '93, '94, '95, '96, '97, '99 | '93, MM | + | B ICA occlusions |
| 4 | '91; 12.0 | '91 | '92 | CT, MR, CA, '91 | S, MM | CA, '91 | MM | '92, '93, '94, '96 | '92, MM | − | B ICA, L MCA occlusions |
| 5 | '96; 16.9 | '96* | '98, '99 | CT, MRA, '96 | S, MM | '96 | MM | '97 | '96, MM | − | L MCA occlusion |
| 6 | '81; 15.7 | '82* | '82, '82, '90, '93 | CA, '90 | S, MM | CA, '90 | MM | '93, '94 | '94, MM | + | B ICA occlusion, B ACA, MCA stenosis |
| 7 | '91; 6.9 | '91 | '95, '95 | CT, CA, '91 | S, MM | CA, '91 | MM | '92, '94, '95, '96, '97, '99 | '94, MM | + | B MCA occlusion, PCA stenosis |
| 8 | '86; 6.4 | '87 | '87, '91 | CT, '86 | S | '93 | MM | '94 | '93, MM | − | B ACA, MCA occlusions |
| 9 | '92; 7.9 | '92 | '92, '95 | CT, MRA, '92 | S, MM | '92 | MM | '92, '93, '94, '95, '96, '98 | '92, MM | − | R ACA occlusion, L MCA, ACA stenosis |
| 10 | '95; 11.4 | '95 | '95, '96 | CT, MRA, '95 | S, MM | '95† | MM | '95, '96, '98, '99 | '95, MM | + | B MCA severe stenosis |
| 11 | '89; 2.4 | '89 | '91 | MR, '90 | S | '92 | MM | '93, '94, '95, '96, '98 | '93, MM | + | B ICA, L ACA, R MCA occlusion |
| 12 | '85; 6.2 | '85 | MR, '91 | S | '92 | MM | '93, '94, '96, '97 | '93, MM | + | L MCA occlusion, L PCA stenosis | |
| 13 | '81; 8.9 | '81 | MRA, '92 | S, MM | '92 | MM | '95 | '95, MM | + | B MCA occlusion | |
| 14 | '93; 2.7 | '93 | MRA, '93 | S, NMV | '93 | NMV | '94, '95, '96, '98 | '94, MM | + | R MCA occlusion, B ACA stenosis | |
| 15 | '91; 9.5 | '91 | CT, MR, '91 | S | '92 | MM | '94, '95, '98 | '92, MM | − | L ICA occlusion | |
| 16 | '86; 7.6 | '86 | MRA, '92 | S, MM | '92 | MM | '93, '94, '96, '98 | '98, MM | + | L MCA occlusion, B PCA stenosis | |
| 17 | '96; 1.1 | '96 | MRA, '96 | S, MM | '96 | MM | '96, MM | B ACA, MCA severe stenosis | |||
| 18 | '93; 6.9 | '93 | CT, MR, '93 | S | '94 | MM | '95, '96, '98 | '95, MM | + | B ACA, R MCA stenosis | |
| 19 | '97; 6.0 | '97 | MRA, '97 | S, MM | '97 | MM | '98 | '98, MM | + | R MCA occlusion |
| Patient no. . | Initial stroke; age, y . | Trfx . | CVE recurrences . | Initial scans, y . | Scan diagnosis 1 . | Initial MRA/CA . | Scan diagnosis 2 . | F/U MRA . | F/U MRA scan diagnosis . | Prog . | Latest MRA scan diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | '92; 4.4 | '92 | '93 | MRA, '92 | S, NMV | '92 | MM | '92, '93, '94, '95, '96, '97, '99 | '93, MM | + | ACA occlusion |
| 2 | '96; 3.9 | '96 | '99, '99 | CT, MRA, '96 | S, NMV | '96 | NMV | '96, '97, '99 | '96, MM | + | L MCA occlusion |
| 3 | '93; 11.4 | '93 | '93, '93 | MRA, '93 | S, MM | '93 | MM | '93, '94, '95, '96, '97, '99 | '93, MM | + | B ICA occlusions |
| 4 | '91; 12.0 | '91 | '92 | CT, MR, CA, '91 | S, MM | CA, '91 | MM | '92, '93, '94, '96 | '92, MM | − | B ICA, L MCA occlusions |
| 5 | '96; 16.9 | '96* | '98, '99 | CT, MRA, '96 | S, MM | '96 | MM | '97 | '96, MM | − | L MCA occlusion |
| 6 | '81; 15.7 | '82* | '82, '82, '90, '93 | CA, '90 | S, MM | CA, '90 | MM | '93, '94 | '94, MM | + | B ICA occlusion, B ACA, MCA stenosis |
| 7 | '91; 6.9 | '91 | '95, '95 | CT, CA, '91 | S, MM | CA, '91 | MM | '92, '94, '95, '96, '97, '99 | '94, MM | + | B MCA occlusion, PCA stenosis |
| 8 | '86; 6.4 | '87 | '87, '91 | CT, '86 | S | '93 | MM | '94 | '93, MM | − | B ACA, MCA occlusions |
| 9 | '92; 7.9 | '92 | '92, '95 | CT, MRA, '92 | S, MM | '92 | MM | '92, '93, '94, '95, '96, '98 | '92, MM | − | R ACA occlusion, L MCA, ACA stenosis |
| 10 | '95; 11.4 | '95 | '95, '96 | CT, MRA, '95 | S, MM | '95† | MM | '95, '96, '98, '99 | '95, MM | + | B MCA severe stenosis |
| 11 | '89; 2.4 | '89 | '91 | MR, '90 | S | '92 | MM | '93, '94, '95, '96, '98 | '93, MM | + | B ICA, L ACA, R MCA occlusion |
| 12 | '85; 6.2 | '85 | MR, '91 | S | '92 | MM | '93, '94, '96, '97 | '93, MM | + | L MCA occlusion, L PCA stenosis | |
| 13 | '81; 8.9 | '81 | MRA, '92 | S, MM | '92 | MM | '95 | '95, MM | + | B MCA occlusion | |
| 14 | '93; 2.7 | '93 | MRA, '93 | S, NMV | '93 | NMV | '94, '95, '96, '98 | '94, MM | + | R MCA occlusion, B ACA stenosis | |
| 15 | '91; 9.5 | '91 | CT, MR, '91 | S | '92 | MM | '94, '95, '98 | '92, MM | − | L ICA occlusion | |
| 16 | '86; 7.6 | '86 | MRA, '92 | S, MM | '92 | MM | '93, '94, '96, '98 | '98, MM | + | L MCA occlusion, B PCA stenosis | |
| 17 | '96; 1.1 | '96 | MRA, '96 | S, MM | '96 | MM | '96, MM | B ACA, MCA severe stenosis | |||
| 18 | '93; 6.9 | '93 | CT, MR, '93 | S | '94 | MM | '95, '96, '98 | '95, MM | + | B ACA, R MCA stenosis | |
| 19 | '97; 6.0 | '97 | MRA, '97 | S, MM | '97 | MM | '98 | '98, MM | + | R MCA occlusion |
Trfx indicates year chronic transfusions were begun; MRA, MRA and MRI done; CT, computed tomography; MR, only MRI done; CA, cerebral angiogram; S, infarct; NMV, no moyamoya with vessel changes; MM, moyamoya; NMNL, no moyamoya, no vessel changes; F/U MRA, MRAs done after initial MRA/CA; F/U MRA scan diagnosis, the year of earliest progressive large vessel changes. If the vessels were static, this column lists the initial MRA diagnosis; Prog, progression of large vessel changes; +, progression; −, no progression; latest MRA scan diagnosis, all large vessel changes noted on the most recent MRA; ACA, anterior cerebral artery; L, left; MCA, middle cerebral artery; B, bilateral; ICA, internal carotid artery; PCA, posterior cerebral artery; R, right.
Noncompliant with chronic transfusions.
Had MRA studies prior to initial stroke as part of enrollment in the STOP study.
Summary of imaging studies completed for nonmoyamoya patients
| Patient no. . | Initial stroke; age, y . | Trfx . | CVE recurrences . | Initial scans, y . | Scan diagnosis 1 . | Initial MRA/CA . | Scan diagnosis 2 . | F/U MRA . | F/U MRA scan diagnosis . | Prog . | Latest MRA scan diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | '95; 4.7 | '95 | '98 | CT, MRA, '95 | S, NMV | '95 | NMV | '97, '98 | '95, NMV | − | L ICA stenosis |
| 21 | '80; 4.8 | '803-150 | '81 | CT, '80 | S | '92 | NMV | '93, '94, '96, '97 | '96, NMV | + | B PCA occlusions |
| 22 | '85; 13.5 | '85 | '85, '85 | CT, '85 | S | '93 | NMV | '94, '95 | '93, NMV | − | R ICA occlusion |
| 23 | '80; 10.2 | '80 | '93 | MR, '90 | S | '93 | NMV | '94 | '93, NMV | − | R PCA stenosis |
| 24 | '84; 13.8 | '84 | '89 | CT, '84 | S | '92 | NMV | '93, '94, '96 | '92, NMV | − | L ICA occlusion |
| 25 | '91; 6.4 | '91 | '92 | CT, MR, '91 | S | '92 | NMV | '93, '94, '96, '98 | '92, NMV | − | L MCA occlusion, L ICA stenosis |
| 26 | '98; 11.8 | '98 | '98, '00 | CT, MRA, '98 | NMNL3-151 | '98 | NMNL | '98, '99 | '98, NMNL | − | No vessel changes |
| 27 | '97; 5.5 | '97 | MRA, '97 | S, NMV | '97 | NMV | '98 | '97, NMV | − | L PCA stenosis | |
| 28 | '96; 12.9 | '96 | MRA, '96 | S, NMV | '96 | NMV | '96, NMV | R ICA occlusion, L ICA, ACA stenosis | |||
| 29 | '96; 8.9 | '96 | MRA, '95 | NMV3-152 | '95 | NMV | '96, '97, '99 | '96, NMV | + | B ACA, L MCA stenosis | |
| 30 | '93; 11.2 | '93 | MRA, '93 | S, NMNL | '93 | NMV | '94, '95, '96, '98 | '94, NMV | + | R MCA, PCA stenosis | |
| 31 | '81; 4.5 | '81 | CT, '87 | S | '92 | NMV | '93, '94 | '92, NMV | − | L MCA stenosis | |
| 32 | '87; 1.7 | '87 | CT, '87 | S | '92 | NMNL | '93, '94, '96, '98 | '92, NMNL | − | No vessel changes | |
| 33 | '93; 3.9 | '93 | CT, MRA, '93 | S, NMV | '933-153 | NMNL | '93, NMNL | No vessel changes | |||
| 34 | '96; 11.6 | '96 | MRA, '96 | S, NMV | '96 | NMV | '96, NMV | B ICA stenosis | |||
| 35 | '96; 8.3 | '96 | MRA, '95 | NMV3-152 | '95 | NMV | '96, '97, '99 | '96, NMV | + | R MCA, ICA stenosis | |
| 36 | '97; 7.1 | '97 | MRA, '97 | S, NMV | '97 | NMV | '98, '99 | '98, NMV | + | L MCA, R ACA stenosis | |
| 37 | '84; 2.6 | '84 | CT, '84 | S | '92 | NMNL | '93, '94, '96, '98 | '98, NMV | + | Decreased L MCA branches | |
| 38 | '94; 4.0 | '94 | CT, MRA, '94 | S, NMNL | '94 | NMNL | '95, '97, '99 | '94, NMNL | − | No vessel changes | |
| 39 | '98; 5.6 | '98 | MRA, '98 | S, NMV | '98 | NMV | '99 | '99, NMV | + | L ACA stenosis | |
| 40 | '96; 9.7 | '96 | CT, MRA, '96 | S, NMNL | '96 | NMNL | '98 | '98, NMV | + | R MCA occlusion, R ICA, L PCA stenosis | |
| 41 | '93; 1.7 | '93 | CT, MRA, '94 | S, NMV | '94 | NMV | '96, '98 | '98, NMV | + | L MCA, ICA stenosis | |
| 42 | '96; 10.6 | '96 | CT, MRA, '96 | S, NMV | '96 | NMV | '97, '98 | '97, NMV | + | R MCA, ICA, ACA, PCA stenosis | |
| 43 | '96; 8.1 | '96 | MRA, '96 | S, NMV | '96 | NMV | '98, '99 | '98, NMV | + | B ACA, ICA stenosis | |
| 44 | '96; 2.0 | '96 | MRA, '97 | S, NMV | '97 | NMV | '97, NMV | B ACA stenosis |
| Patient no. . | Initial stroke; age, y . | Trfx . | CVE recurrences . | Initial scans, y . | Scan diagnosis 1 . | Initial MRA/CA . | Scan diagnosis 2 . | F/U MRA . | F/U MRA scan diagnosis . | Prog . | Latest MRA scan diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | '95; 4.7 | '95 | '98 | CT, MRA, '95 | S, NMV | '95 | NMV | '97, '98 | '95, NMV | − | L ICA stenosis |
| 21 | '80; 4.8 | '803-150 | '81 | CT, '80 | S | '92 | NMV | '93, '94, '96, '97 | '96, NMV | + | B PCA occlusions |
| 22 | '85; 13.5 | '85 | '85, '85 | CT, '85 | S | '93 | NMV | '94, '95 | '93, NMV | − | R ICA occlusion |
| 23 | '80; 10.2 | '80 | '93 | MR, '90 | S | '93 | NMV | '94 | '93, NMV | − | R PCA stenosis |
| 24 | '84; 13.8 | '84 | '89 | CT, '84 | S | '92 | NMV | '93, '94, '96 | '92, NMV | − | L ICA occlusion |
| 25 | '91; 6.4 | '91 | '92 | CT, MR, '91 | S | '92 | NMV | '93, '94, '96, '98 | '92, NMV | − | L MCA occlusion, L ICA stenosis |
| 26 | '98; 11.8 | '98 | '98, '00 | CT, MRA, '98 | NMNL3-151 | '98 | NMNL | '98, '99 | '98, NMNL | − | No vessel changes |
| 27 | '97; 5.5 | '97 | MRA, '97 | S, NMV | '97 | NMV | '98 | '97, NMV | − | L PCA stenosis | |
| 28 | '96; 12.9 | '96 | MRA, '96 | S, NMV | '96 | NMV | '96, NMV | R ICA occlusion, L ICA, ACA stenosis | |||
| 29 | '96; 8.9 | '96 | MRA, '95 | NMV3-152 | '95 | NMV | '96, '97, '99 | '96, NMV | + | B ACA, L MCA stenosis | |
| 30 | '93; 11.2 | '93 | MRA, '93 | S, NMNL | '93 | NMV | '94, '95, '96, '98 | '94, NMV | + | R MCA, PCA stenosis | |
| 31 | '81; 4.5 | '81 | CT, '87 | S | '92 | NMV | '93, '94 | '92, NMV | − | L MCA stenosis | |
| 32 | '87; 1.7 | '87 | CT, '87 | S | '92 | NMNL | '93, '94, '96, '98 | '92, NMNL | − | No vessel changes | |
| 33 | '93; 3.9 | '93 | CT, MRA, '93 | S, NMV | '933-153 | NMNL | '93, NMNL | No vessel changes | |||
| 34 | '96; 11.6 | '96 | MRA, '96 | S, NMV | '96 | NMV | '96, NMV | B ICA stenosis | |||
| 35 | '96; 8.3 | '96 | MRA, '95 | NMV3-152 | '95 | NMV | '96, '97, '99 | '96, NMV | + | R MCA, ICA stenosis | |
| 36 | '97; 7.1 | '97 | MRA, '97 | S, NMV | '97 | NMV | '98, '99 | '98, NMV | + | L MCA, R ACA stenosis | |
| 37 | '84; 2.6 | '84 | CT, '84 | S | '92 | NMNL | '93, '94, '96, '98 | '98, NMV | + | Decreased L MCA branches | |
| 38 | '94; 4.0 | '94 | CT, MRA, '94 | S, NMNL | '94 | NMNL | '95, '97, '99 | '94, NMNL | − | No vessel changes | |
| 39 | '98; 5.6 | '98 | MRA, '98 | S, NMV | '98 | NMV | '99 | '99, NMV | + | L ACA stenosis | |
| 40 | '96; 9.7 | '96 | CT, MRA, '96 | S, NMNL | '96 | NMNL | '98 | '98, NMV | + | R MCA occlusion, R ICA, L PCA stenosis | |
| 41 | '93; 1.7 | '93 | CT, MRA, '94 | S, NMV | '94 | NMV | '96, '98 | '98, NMV | + | L MCA, ICA stenosis | |
| 42 | '96; 10.6 | '96 | CT, MRA, '96 | S, NMV | '96 | NMV | '97, '98 | '97, NMV | + | R MCA, ICA, ACA, PCA stenosis | |
| 43 | '96; 8.1 | '96 | MRA, '96 | S, NMV | '96 | NMV | '98, '99 | '98, NMV | + | B ACA, ICA stenosis | |
| 44 | '96; 2.0 | '96 | MRA, '97 | S, NMV | '97 | NMV | '97, NMV | B ACA stenosis |
See Table 2 for explanation of abbreviations.
Noncompliant with chronic transfusions.
Normal imaging but stroke based on clinical symptoms of hemiparesis lasting 4 weeks.
Had MRA studies prior to initial stroke as part of enrollment in the STOP study.
Transferred to our institution in 1999. Review of records from parent institution showed no recurrent CVE. Has not had follow-up scans.
Timing of imaging studies
Because of the nature of this study, imaging studies were performed at varying times after the initial event as described above. Nonetheless, 12 (63%) of 19 patients with moyamoya and 16 (64%) of 25 patients without moyamoya had MRAs or cerebral angiograms performed within 2 months of the initial event. Six (13%) of the 44 patients did not have any imaging studies performed within 6 months of the initial event and were diagnosed clinically. Infarcts were documented in subsequent studies in all 6 patients. Details of the timing of studies are presented in Tables 2 and 3.
Initial stroke
The mean age at the time of the first acute clinical stroke was 7.8 years (range, 1.1 to 16.9 years) for moyamoya patients and 7.4 years (range, 1.7 to 13.9 years) for those without moyamoya. The initial stroke for all 44 patients was infarctive in nature. One nonmoyamoya patient had a hemorrhagic parietal infarct and has not had a recurrent event in 13 years of follow-up. Most strokes were in the areas of distribution of the middle cerebral arteries, anterior cerebral arteries, or watershed areas (frontal and/or parietal lobes). Eight (18%) of 44 patients—2 with moyamoya—had evidence of old infarcts on scan at the time of their initial acute clinical stroke.
Follow-up time after initial stroke
At the time of this report, patients with moyamoya have been followed a median of 8.1 years (interquartile range, 5.0 to 11.5 years) after the initial stroke, while nonmoyamoya patients have been followed a median of 4.7 years (interquartile range, 3.6 to 13.4 years). One patient died after 13.5 years of follow-up due to complications of iron overload, and 2 patients moved out of the area after 4 and 11.5 years of follow-up, respectively.
Recurrent CVE
Eighteen (41%) of 44 of the patients studied experienced recurrent CVEs, including 5 patients with strokes. Of the 19 patients exhibiting moyamoya pattern, 11 (57.9%) experienced 21 total CVE (stroke or TIA) recurrences. Four patients had recurrent strokes, 10 had TIAs, and 3 patients experienced both. Four of the 11 patients experienced 7 recurrent events (2 strokes and 5 TIAs) with contralateral symptoms when compared with the initial event. Seven (28.0%) of 25 patients without moyamoya vessels experienced 9 total recurrent CVEs. One patient had a recurrent stroke (which occurred while off transfusions), and 6 had 1 or 2 TIAs. Two of 7 patients had recurrent events (1 stroke and 1 TIA) with contralateral symptoms compared with the initial event. Thus, patients with moyamoya were significantly more likely to have a recurrent CVE (stroke or TIA) during the period of this study (57.9% vs 28.0%;P < .05). They were also more likely to have 2 subsequent CVEs (42.1% vs 8.0%; P < .05). When recurrent strokes are considered by themselves, the data suggest that moyamoya patients had more subsequent strokes than nonmoyamoya patients (21.1% vs 4.0%, P = .078); however, this parameter did not reach statistical significance. Of the 8 patients with evidence of old infarcts on scan at the time of their initial acute clinical stroke, 1 moyamoya patient had a recurrent stroke, 1 moyamoya patient had a recurrent TIA, and 1 nonmoyamoya patient had a recurrent TIA.
Time to subsequent CVE
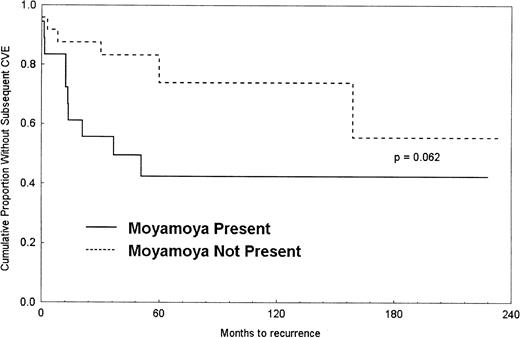
A Kaplan-Meier survival curve was generated to show the difference in time to second CVE between the 2 groups (Figure1). The median time to recurrent CVE after initial stroke for moyamoya patients was 3.1 years but was not estimable for nonmoyamoya patients because most of those patients did not have a recurrent CVE. The proportional hazards regression analysis indicated that patients with moyamoya were more than 2 times as likely, on average, to incur a subsequent CVE (hazard ratio [HR], 2.40; 95% confidence interval [CI], 0.85-6.75). When CVEs incurred while off transfusion were excluded, the results remained relatively unchanged (HR, 2.41; 95% CI, 0.93-6.22).
Large vessel abnormalities on imaging
All moyamoya patients had evidence of severe large vessel disease on every imaging study done, with 16 (84%) of 19 eventually developing complete occlusion and the remaining 3 of 19 having severe stenosis of at least 1 major vessel (Table 2). Two of the 19 patients (nos. 2 and 14) did not have collateral vessels visualized initially, but these were observed in studies performed 1 year or less after the initial event. Thirteen patients with moyamoya were shown to have progressive vessel narrowing on follow-up MRAs. Vessel disease was present but clearly less severe among patients without moyamoya (Table 3), because only 6 (24%) of 25 patients developed complete occlusion of a major vessel. In fact, another 6 (24%) of 25 nonmoyamoya patients had normal MRA findings at initial stroke presentation. One of these 6 (no. 26) experienced 2 recurrent CVEs (TIAs at 3 months and 23 months after initial stroke). This patient's MRAs remained normal through follow-up. Two of the 6 patients (nos. 37 and 40) went on to develop large vessel changes on MRA, with no signs of collateral vessels. Average time of follow-up for these 6 patients is 7.9 years (range, 2.2 to 16.3 years). Progressive large vessel disease was observed more often in moyamoya patients (13 of 19; 68%) when compared with nonmoyamoya patients (11 of 25; 44%). Some of the progressive abnormalities detected may have been due to improved imaging techniques.
Hematologic parameters
Mean baseline levels of HbS were similar between moyamoya and nonmoyamoya patients (83.0% vs 86.5%) and were not predictive of recurrent events. Other baseline hematologic values, including white count and proportion of fetal hemoglobin, were also not significantly predictive of recurrent neurologic events (Table 1).
CVEs off transfusion
We treated all patients who had an initial stroke with a program of chronic transfusion every 3 to 5 weeks aimed at keeping HbS below 30%. In the group of patients studied, 3 had recurrent events during a period of noncompliance with transfusion therapy. Two patients with moyamoya suffered recurrent CVEs (1 stroke and 1 TIA) while off transfusion. One of these patients was on hydroxyurea therapy at the time of recurrent TIA with questionable compliance. The only nonmoyamoya patient who had a recurrent stroke was not receiving transfusions at the time. The mean HbS level was 89.8% (range, 86.5% to 91.9%) in these 3 patients at the time of the recurrent CVE. In comparison, the mean HbS was 39.0% (range, 12.1% to 73.0%) at the time of recurrent events in patients on transfusion. When these 3 noncompliant patients were excluded from the analysis and only recurrent CVEs that occurred while patients were on transfusion were considered, the results remained unchanged. All 3 patients have been compliant with chronic transfusion since their recurrent events.
Silent stroke
In addition to the above patients who presented with an initial acute clinical stroke, the records of 7 HbSS patients who did not present with acute stroke but had MRI/MRA performed for other neurologic symptoms (falloff in school performance, headaches, seizures, syncope, mild memory defect) were reviewed. These 7 patients had no acute MRI changes but did have evidence of old silent infarcts that could have explained some of their presenting symptoms. Three of the patients had moyamoya. These patients have been followed an average of 4.0 years (range, 2.5 to 6.5 years) with no recurrent events. Based on the absence of an initial acute motor stroke presentation, these patients were excluded from statistical analyses in this study. Thus, on initial presentation, 8 (18.2%) of 44 study patients, plus 7 with other neurologic abnormalities in the absence of acute motor presentation—a total of 15 (29.4%) of 51—had evidence of old infarcts.
Discussion
Chronic transfusions are effective in preventing the recurrence of infarctive strokes in patients with sickle cell disease (SCD).6,13,21 The reasons why some patients develop recurrences15 while complying with transfusions are unknown. In this regard there are no reports correlating angiographic or MRA findings with the clinical course. In our series of patients with SCD who had suffered an infarctive stroke, a large proportion (41%) had recurrent strokes and/or TIAs despite chronic transfusions. This recurrence rate is higher than previously reported13 and may reflect the careful documentation of neurologic events. In this group of patients the prevalence of moyamoya collaterals was similar to what has been described by others,8 but the male preponderance was different.8 23 Patients with moyamoya collaterals were found to be at higher risk for recurrent strokes and TIAs compared with patients without these collaterals. Cerebral hemorrhages were not observed, perhaps due to the young age of our patients and the relatively short follow-up.
The significance of recurrent TIAs has not been well studied in SCD. In other settings it has been argued that a TIA is the mildest end of a spectrum of ischemic cerebrovascular disease that should not be considered as a separate illness from stroke.22 In one large series of patients with SCD, TIAs were found to be highly predictive of a first ischemic stroke.5 In contrast, TIAs were not found to be associated with or predictive of a second (recurrent) stroke in a different series of patients.13This was also the case among our patients, where only 3 of 16 who experienced recurrent TIAs developed strokes.
Patients with moyamoya vessels did worse on neuropsychological evaluations. Neuropsychological tests (Weschler Intelligence Scale, Woodcock-Johnson Psychoeducational Battery, Wide Range Assessment of Memory and Learning, Visual Motor Integration, and others) were performed on a group of 16 patients that included 14 patients reported above. The results revealed significant differences between patients with moyamoya and patients without moyamoya collaterals on verbal IQ, performance IQ, full-scale IQ, math achievement, visual memory, and aphasic symptomatology.19 Subjects with moyamoya collaterals consistently showed greater impairments in both verbal and visual-spatial intellectual abilities and were found to be achieving less well in all areas of psychoeducational abilities. These findings and the increase in recurrent CVEs suggest that moyamoya collaterals are a marker for severe global cerebrovascular disease. Further neuropsychological studies are, however, needed to verify these results.
The effects of transfusions on the cerebral vessel abnormalities in patients with SCD are unknown. One report suggested that certain vessel abnormalities could be reversed.21 In our series, 24 of 44 patients had MRA evidence of progressive large vessel disease after several years on a tightly regulated transfusion protocol. Progressive vessel changes were more common among patients with moyamoya. Thus, it seems unlikely that chronic transfusions will reverse the severe vascular damage seen in these patients. Two of our patients developed new moyamoya collaterals while on transfusions. In contrast, another patient had a normal MRA after initial stroke and has continued to show normal MRAs and unchanged MRIs despite 2 recurrent TIAs. It is therefore unclear whether transfusions will prevent the development of collaterals in patients who do not initially have them.
It has been reported that bone marrow transplantation can improve the patency of stenotic vessels and reverse the progression of vasculopathy in SCD.24 We have previously reported on a 9-year-old boy who had mild cognitive and memory defects but no stroke. He was found to have extensive moyamoya collaterals and a silent infarct before transplantation for recurrent acute chest syndrome.25 These findings remain unchanged 7 years after successful bone marrow transplantation, suggesting stabilization of vessel disease.
In our series, MRA defined the patterns of vascular abnormality and correlated well with conventional angiography when this was performed, as has been shown by others.7,18,26-28 MRI and MRA are relatively easy to perform and carry none of the risks associated with administration of contrast. These tests may provide significant information in SCD patients who have suffered a stroke or have “soft” signs of central nervous system dysfunction as demonstrated in our patients with silent infarcts. Future studies, with techniques such as diffusion and perfusion imaging, transcranial Doppler, and quantitative MRI may help clarify the pathophysiology of strokes and moyamoya formation.29
Our findings suggest that patients with SCD have a high rate of recurrent CVE after initial stroke. Furthermore, transfusions were more effective in preventing TIA and stroke recurrences in patients without severe vessel disease and moyamoya vessels. In a recent report, hydroxyurea and phlebotomy were used for stroke prevention in SCD with promising results.30 However, no radiologic or MRA data about these patients were provided. It may well be that hydroxyurea is more effective in preventing stroke among patients with less severe vessel disease. Noninvasive techniques such as MRA could be used prospectively to help identify patients at risk for recurrent CVE and to study the responses to intervention therapies. Thus, the knowledge of the presence or absence of moyamoya collaterals or other morphologic abnormalities may be important in the design of studies looking at the effects of other agents, such as hydroxyurea, in primary or secondary stroke prevention.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miguel R. Abboud, Dept of Pediatrics, Medical University of South Carolina, 165 Ashley Ave, PO Box 250911, Charleston, SC 29425; e-mail: abboudm@musc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal