Abstract
A serious complication of aplastic anemia (AA) is its evolution to clonal hematologic diseases such as myelodysplasia (MDS) and leukemia, which is usually associated with the appearance of a cytogenetic abnormality in bone marrow cells. We present here an analysis of a cohort of 30 patients with otherwise typical AA in whom clonal karyotypic evolution was observed during frequent periodic marrow examinations. The actuarial risk for this complication has been estimated in other studies at around 15% at 5 years. Conversion from normal to abnormal karyotype occurred at a constant rate after initial diagnosis, with about 50% of cases developing within the first 30 months. Transient chromosomal abnormalities were infrequent. Clinically, AA patients with clonal cytogenetic patterns were heterogenous; a variety of karyotypic defects with numerical and structural abnormalities of chromosome 7 accounted for 40% of all cases followed by trisomy 8, structural and numerical abnormalities of chromosome 13, deletion of Y chromosome, and complex cytogenetic abnormalities. Unlike in primary MDS, aberrancies of chromosome 5 and 20 were infrequent. The clinical course depended on the specific abnormal cytogenetic pattern. Most deaths related to leukemic transformation occurred in patients with abnormalities of chromosome 7 or complex cytogenetic alterations or both. Evolution of chromosome 7 abnormalities was seen most often in refractory patients who had failed to respond to therapy. In contrast, trisomy 8 developed in patients with good hematologic responses who often required chronic immunosuppression with cyclosporine A (CsA), and survival was excellent. Although AA patients with monosomy 7 showed a similar prognosis to those with primary MDS, trisomy 8 in AA appears to have a more favorable prognosis than in MDS.
Introduction
With the introduction of immunosuppressive therapy (IST) for aplastic anemia (AA), the survival of patients who are not treated with bone marrow (BM) transplantation has improved significantly.1 However, with long-term systematic observation, evolution of AA to other hematologic diseases has been frequently recognized as a serious late sequelae of this disease. Historically, paroxysmal nocturnal hemoglobinuria (PNH) was considered the most common clonal complication, but its incidence is now appreciated to be high at initial presentation of AA.2 3The second most common clonal disease occurring in the context of AA is myelodysplasia (MDS). Aberrant maturation, increased numbers of myeloblasts, and marrow hypercellularity are all characteristic of MDS, but persistent marrow hypocellularity in AA may preclude reliable morphologic analysis. The finding of a cytogenetic abnormality therefore is the most objective evidence of clonal evolution.
The acquisition of an abnormal karyotype in the setting of diagnosed AA is not infrequent, but estimates have been variable among published studies, due to differences in diagnostic criteria, patient populations, treatment protocols, and the frequency of follow-up BM examinations.4-15 In an early study from Seattle, an abnormal karyotype acquired in the course of the disease was reported in 3 of 183 AA patients.9 In a recent European analysis, abnormalities occurred in 23 of 170 patients, but in 4 cases, chromosomal changes were present at first diagnosis.6 In another report of 69 Italian AA patients, an abnormal karyotype was found in 18, but the findings were transient in 8 patients and in only 7 cases was the karyotypic abnormality known not to be present initially.8 Similarly, in a recent series of 13 patients with AA and abnormal cytogenetics, only 2 developed the abnormality later in the course of the disease.7 Of 40 Japanese children treated with granulocyte colony-stimulating factor (G-CSF) and cyclosporine A (CsA), cytogenetic evolution was found in 11.11 At our institution, abnormal cytogenetics excludes a diagnosis of AA, regardless of marrow morphology; in our National Institutes of Health (NIH) series of 122 patients treated with intensive immunosuppression, 14 have developed karyotypic abnormalities, with a risk of about 21% at 10 years.6 In a preliminary combined analysis of 189 patients treated with various immunosuppressive regimens on consecutive protocols, the actuarial risk of karyotypic conversion was estimated at 14% at 5 years and 20% at 10 years (J.M., unpublished observation).
Based on our stringent initial diagnostic criteria, the new appearance of a karyotypically abnormal clone has warranted a change of diagnosis from AA to MDS. To avoid observer bias, we purposely selected karyotype rather then morphologic criteria in our case definition. Using this approach, we could determine the clinical features and prognosis of patients who developed abnormal cytogenetics in the course of their AA.
Patients and methods
Patients
Patients with either AA evaluated or treated (or both) in the Hematology Branch of the National Heart, Lung and Blood Institute (NHLBI, Bethesda, MD) were studied. Informed consent for the laboratory tests and clinical examination was obtained according to protocols approved by the Institutional Review Board of the NHLBI.
Case definitions and diagnostic criteria
We designed an observational study to determine the clinical features and outcomes associated with the development of new cytogenetic abnormalities in patients with a history of AA treated with immunosuppressive drugs. Our case definition included: (1) an initial diagnosis of AA (see below) and normal karyotypic analysis at the time of diagnosis (patients for whom such an analysis was not obtained at the time of presentation or in which the study was not informative were excluded, and (2) new appearance of a karyotypically abnormal clone defined as 2 or more metaphase cells with the same extra chromosome(s) or structural rearrangement(s) or 3 or more cells with the same missing chromosome. The minimum number of cells to be analyzed in each BM was 20, provided there were that many present. Complete medical histories were available in all cases. For patients participating in treatment protocols at NIH karyotypic analysis was routinely performed at presentation, at 6 months, and at 1 year after IST and then annually. In addition, cytogenetic studies were performed for clinical indications, such as the appearance of circulating blasts or worsening blood counts. Patients who did not participate in NIH treatment protocols also fulfilled the above described diagnostic criteria, and their abnormal karyotype was confirmed at the NIH.
The diagnosis of AA was established by BM biopsy and peripheral blood counts according to the International Study of Aplastic Anemia and Agranulocytosis criteria; severity was classified by the criteria by Camitta et al.16 For the diagnosis of severe AA (sAA), in addition to a hypocellular BM without evidence of karyotypic abnormalities or morphologic dysplasia, patients fulfilled 2 of 3 blood criteria as previously described.16,19 Patients with AA, whose pancytopenia did not qualify as severe but with 2 of 3 blood parameters (absolute neutrophil count [ANC] < 1200, platelet count < 60 000/μL, and hemoglobin of < 8 g/dL), were classified as having moderate AA (mAA). When present, MDS was subclassified according to French-American-British (FAB) nomenclature and International Prognostic Scoring System (IPSS)17 18; hypocellular MDS was diagnosed if the overall biopsy cellularity was less than 25%. In general, the diagnosis of AA required exclusion of other pathologic conditions that can mimic its presentation. All bone marrow biopsies and aspirates were evaluated for morphology by an independent hematopathologist not associated with this study.
For the period between January 1989 and August 2001, we identified a total of 30 patients fulfilling the case definition criteria (Tables1 and2).
Clinical characteristics of patients
| . | Initial diagnosis . | Sex . | Age . | Counts at presentation . | Treatments before cytogenetic conversion . | Response to IST . | |||
|---|---|---|---|---|---|---|---|---|---|
| ANC × 103/μL . | Hemoglobin g/dL . | Reticulocytes × 103/μL . | Platelets × 103/μL . | ||||||
| 1 | sAA | M | 54 | 544 | 6.4 | 28 | 19 | ATG/CsA, androgens | NR |
| 2 | sAA | F | 77 | 48 | 10 | 3.8 | 11 | ATG/CsA, GM-CSF | R |
| 3 | sAA | M | 30 | 660 | 9 | 28 | 9 | ATG/CsA, GM-CSF | R |
| 4 | sAA | M | 51 | 350 | 2 | 9.4 | 21 | ATG/CsA, G-CSF | NR |
| 5 | sAA | F | 42 | 288 | 7.6 | 12 | 7 | ATG/CsA, G-CSF | NR |
| 6 | sAA | M | 8 | 470 | 4 | 5 | 14 | ATG/CsA, GM-CSF, G-CSF, SCF | NR |
| 7 | sAA | M | 63 | 115 | 7 | 7.2 | 6 | ATG/CsA, SCF/G-CSF | NR |
| 8 | sAA | M | 73 | 870 | 9.6 | 28 | 13 | ATG/CsA | NR |
| 9 | sAA | F | 55 | 600 | 9.8 | 30 | 20 | ATG/CsA, androgens | R |
| 10 | sAA | M | 11 | 450 | 10 | 65 | 9 | ATG/CsA | NR |
| 11 | sAA | F | 34 | 660 | 5 | 3 | 8 | ATG/CsA | R |
| 12 | sAA | M | 20 | 230 | 7 | 6 | 3 | ATG/CsA, SCF/G-CSF | NR |
| 13 | sAA | M | 48 | 330 | 9 | 6.8 | 39 | ATG/CsA | R |
| 14 | sAA | F | 45 | 1400 | 10 | 10 | 5 | ATG/CsA | R |
| 15 | sAA | F | 49 | 666 | 9.8 | 47 | 2 | ATG/CsA | R |
| 16 | sAA | M | 66 | 1000 | 5.4 | 32 | 7 | ATG/CsA | NR |
| 17 | sAA | F | 23 | 870 | 5.1 | 29 | 30 | G-CSF/EPO | NT |
| 18 | sAA | M | 67 | 1000 | 3 | 25 | 9 | ATG/CsA | R |
| 19 | sAA | M | 43 | 360 | 9.5 | 7 | 23 | ATG/CsA | R |
| 20 | mAA | F | 54 | 1080 | 6.9 | 40 | 14 | ATG/CsA | R |
| 21 | sAA | M | 61 | 360 | 9.1 | 41 | 20 | ATG/CsA | R |
| 22 | sAA | F | 61 | 120 | 3.5 | 14 | 15 | ATG/CsA, SCF/G-CSF, EPO | NR |
| 23 | sAA | F | 54 | 1068 | 8.2 | 24 | 5 | ATG/CsA, SCF/G-CSF, androgens | NR |
| 24 | sAA | M | 10 | 230 | 9 | 10 | 22 | ATG/CsA, SCF/G-CSF, androgens | NR |
| 25 | sAA | F | 16 | 774 | 6 | 19 | 18 | ATG/CsA | R |
| 26 | sAA | F | 8 | 885 | 9.5 | 50 | 9 | ATG/CsA | R |
| 27 | sAA | M | 48 | 820 | 8 | 21 | 18 | CTX/CsA | R |
| 28 | sAA | M | 27 | 580 | 7.5 | 14 | 10 | ATG/CsA | R |
| 29 | mAA | F | 53 | 1290 | 9 | 53 | 17 | ATG/CsA | R |
| 30 | sAA | M | 34 | 494 | 7.5 | 32 | 16 | ATG/CsA/MMF, ATG/CsA | NR |
| . | Initial diagnosis . | Sex . | Age . | Counts at presentation . | Treatments before cytogenetic conversion . | Response to IST . | |||
|---|---|---|---|---|---|---|---|---|---|
| ANC × 103/μL . | Hemoglobin g/dL . | Reticulocytes × 103/μL . | Platelets × 103/μL . | ||||||
| 1 | sAA | M | 54 | 544 | 6.4 | 28 | 19 | ATG/CsA, androgens | NR |
| 2 | sAA | F | 77 | 48 | 10 | 3.8 | 11 | ATG/CsA, GM-CSF | R |
| 3 | sAA | M | 30 | 660 | 9 | 28 | 9 | ATG/CsA, GM-CSF | R |
| 4 | sAA | M | 51 | 350 | 2 | 9.4 | 21 | ATG/CsA, G-CSF | NR |
| 5 | sAA | F | 42 | 288 | 7.6 | 12 | 7 | ATG/CsA, G-CSF | NR |
| 6 | sAA | M | 8 | 470 | 4 | 5 | 14 | ATG/CsA, GM-CSF, G-CSF, SCF | NR |
| 7 | sAA | M | 63 | 115 | 7 | 7.2 | 6 | ATG/CsA, SCF/G-CSF | NR |
| 8 | sAA | M | 73 | 870 | 9.6 | 28 | 13 | ATG/CsA | NR |
| 9 | sAA | F | 55 | 600 | 9.8 | 30 | 20 | ATG/CsA, androgens | R |
| 10 | sAA | M | 11 | 450 | 10 | 65 | 9 | ATG/CsA | NR |
| 11 | sAA | F | 34 | 660 | 5 | 3 | 8 | ATG/CsA | R |
| 12 | sAA | M | 20 | 230 | 7 | 6 | 3 | ATG/CsA, SCF/G-CSF | NR |
| 13 | sAA | M | 48 | 330 | 9 | 6.8 | 39 | ATG/CsA | R |
| 14 | sAA | F | 45 | 1400 | 10 | 10 | 5 | ATG/CsA | R |
| 15 | sAA | F | 49 | 666 | 9.8 | 47 | 2 | ATG/CsA | R |
| 16 | sAA | M | 66 | 1000 | 5.4 | 32 | 7 | ATG/CsA | NR |
| 17 | sAA | F | 23 | 870 | 5.1 | 29 | 30 | G-CSF/EPO | NT |
| 18 | sAA | M | 67 | 1000 | 3 | 25 | 9 | ATG/CsA | R |
| 19 | sAA | M | 43 | 360 | 9.5 | 7 | 23 | ATG/CsA | R |
| 20 | mAA | F | 54 | 1080 | 6.9 | 40 | 14 | ATG/CsA | R |
| 21 | sAA | M | 61 | 360 | 9.1 | 41 | 20 | ATG/CsA | R |
| 22 | sAA | F | 61 | 120 | 3.5 | 14 | 15 | ATG/CsA, SCF/G-CSF, EPO | NR |
| 23 | sAA | F | 54 | 1068 | 8.2 | 24 | 5 | ATG/CsA, SCF/G-CSF, androgens | NR |
| 24 | sAA | M | 10 | 230 | 9 | 10 | 22 | ATG/CsA, SCF/G-CSF, androgens | NR |
| 25 | sAA | F | 16 | 774 | 6 | 19 | 18 | ATG/CsA | R |
| 26 | sAA | F | 8 | 885 | 9.5 | 50 | 9 | ATG/CsA | R |
| 27 | sAA | M | 48 | 820 | 8 | 21 | 18 | CTX/CsA | R |
| 28 | sAA | M | 27 | 580 | 7.5 | 14 | 10 | ATG/CsA | R |
| 29 | mAA | F | 53 | 1290 | 9 | 53 | 17 | ATG/CsA | R |
| 30 | sAA | M | 34 | 494 | 7.5 | 32 | 16 | ATG/CsA/MMF, ATG/CsA | NR |
NR indicates nonresponsive; R, responsive; MMF, mycophenolate mofetil.
Clinical feature of cytogenetic evolution in AA
| . | Time to evolution (mo) . | First abnormal karyotype . | BM cellularity . | BM morphology . | Counts at conversion . | FAB . | Outcome . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ANC × 103/μL . | Hemoglobin g/dL . | Reticulocytes × 103/μL . | Platelets × 103/μL . | |||||||
| 1 | 58 | 45,XY,−7[20] | Hypo | L-shift, dyserythopoiesis | 320 | 9.1 | 19.7 | 9 | RAEB | Transfusion-dependent for red cells and platelets |
| 2 | 20 | 45,XY,−7[20] | Hypo | Megakaryocytes increased | 500 | 8 | 20.3 | 80 | RAEBt | Temporary remission after chemotherapy |
| 3 | 72 | 45,XY,−7[20] | Mixed | L-shift, dysplastic megakaryocytes | 250 | 13 | 51 | 129 | RAEB | MUD |
| 4 | 14 | 45,XY,−7[19]/46,XY[1] | Hypo | Megakaryocytes absent | 850 | 7.4 | 53 | 8 | RA | Expired |
| 5 | 30 | 46,XX,t(2;3)(p23;q29)[2]/45XX, idem,−7[20] | Normo | L-shift, dysplastic megakaryocytes | 340 | 8.5 | 60 | 135 | AML | Expired |
| 6 | 97 | 45,XY,−7[14]/46,XY,−7, +mar[7] | Normo | Myeloid dysplasia | 390 | 8.5 | 37 | 5 | AML | Expired |
| 7 | 53 | 45,XY,−7[13]/45,idem, del(12)(p12p13)[8] | Mixed | L-shift megakaryocytes decreased | 160 | 9.4 | 12.6 | 15 | AML | Expired |
| 8 | 28 | 45,XY,−7[20] | Mixed | Dysplastic megakaryocytes | 730 | 11.4 | 30.9 | 28 | RAEB | Expired |
| 9 | 7 | 47,XX,+8[3]/46,XX[14] | Hyper | Dysplastic megakaryocytes | 900 | 8 | 45 | 20 | RA | Partial hematologic remission |
| 10 | 13 | 45,XY,−7[20] | Hyper | No dysplasia | 550 | 7.5 | 18 | 22 | RAEBt | MUD, expired |
| 11 | 48 | 46,XX,+1,der(1;7) (q10p10)[14]/46,XX[6] | Normo | L-shift, dysplasia | 600 | 7 | 10 | 7 | RAEBt | MUD |
| 12 | 34 | 91,XXYY,−16[8]/91,XXYY, del(6)(q15q25),−16[3]/ 92,XXYY,del(6)(q15q25)[2]/ 92,XXYY,del(5) | Hyper | L-shift | 340 | 9.3 | 41 | 21 | AML | MUD |
| 13 | 5 | 47,XY,+8[18]/46,XY[2] | Mixed | Normal | 1760 | 12 | 37 | 110 | RA | CsA-dependent counts |
| 14 | 38 | 47,XY,+8[20] | Normo | Normal | 3300 | 13 | 90 | 130 | RA | CsA-dependent counts |
| 15 | 51 | 46,XX,del(13)(q12q14)[21]/ 46,XX[4] | Hyper | Megaloblastic changes | 2650 | 11.6 | 138 | 53 | RA | Stable counts |
| 16 | 6 | 45,X,−Y[5]/46,XY[15] | Hypo | L-shift | 2450 | 8.8 | 63 | 35 | RA | Expired |
| 17 | 11 | 47,XX,+8[8]/45,XX,−5[3]/46, XX[39] | Hypo | L-shift, megaloblastic changes | 1800 | 9.9 | 78 | 41 | RA | GF therapy |
| 18 | 39 | 45,X,−Y[14] | Normo | L-shift | 2000 | 8.6 | 107 | 37 | RA | CsA-dependent counts |
| 19 | 25 | 46,XY,del(13)(q12q14)[3]/46, XY[17] | Normo | Normal | 1900 | 10 | 63 | 118 | RA | Stable counts |
| 20 | 84 | 48,XXXc,+8[5]/47,XXXc[15] | Hypo | Megablastoid changes | 1850 | 11 | 34 | 90 | RA | CsA-dependent counts |
| 21 | 17 | 46,XY,del20(q11.2q13.2)[9]/46, XY[41] | Hypo | No dysplasia | 1600 | 12 | 67 | 80 | RA | Stable counts |
| 22 | 116 | 47,XX,add(X)(p22.3),add(3) (q26.2),+21 | Mixed | L-shift | 520 | 12 | 123 | 89 | AML | Expired |
| 23 | 34 | 46,XX,del(13)(q12q14)[4]/ 46,XX[16] | Hyper | L-shift | 1206 | 8 | NA | 8 | RAEB | Transfusion-dependent for red cells and platelets |
| 24 | 126 | 45,XY,−7[7]/46,XY[13] | Mixed | Myeloid dysplasia | 697 | 7 | 7 | 9 | RAEBt | MUD |
| 25 | 84 | 46,XX,del(17)(p11.2p13.1)[5]/ 46,XX[47] | Mixed | L-shift, dysplastic megakaryocytes | 1500 | 12 | 86 | 69 | RA | Stable counts |
| 26 | 20 | 46,XX,del(13)(q12q14)[4]/46, XX[16] | Mixed | Normal | 1052 | 7.9 | 20 | 20 | RA | CsA |
| 27 | 25 | 47,XY,+8[4]/46,XY[16] | Hypo | Normal | 2700 | 11 | 69 | 56 | RA | CsA-dependent counts |
| 28 | 28 | 47,XY,+8[20] | Hyper | Dysplastic megakaryocytes | 2290 | 15 | NA | 155 | RA | Stable remission |
| 29 | 8 | 47,XY,del(7)(p13p22)[3]/46, XY[17] | Hypo | Normal | 1350 | 15 | 47 | 48 | RA | CsA-dependent counts |
| 30 | 8 | 47,XY,+6[3]/46,46,XY[18] | Hypo | Normal, decreased megakaryocytes | 1350 | 8 | 20 | 10 | RA | Transfusion-dependent for red cells and platelets |
| . | Time to evolution (mo) . | First abnormal karyotype . | BM cellularity . | BM morphology . | Counts at conversion . | FAB . | Outcome . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ANC × 103/μL . | Hemoglobin g/dL . | Reticulocytes × 103/μL . | Platelets × 103/μL . | |||||||
| 1 | 58 | 45,XY,−7[20] | Hypo | L-shift, dyserythopoiesis | 320 | 9.1 | 19.7 | 9 | RAEB | Transfusion-dependent for red cells and platelets |
| 2 | 20 | 45,XY,−7[20] | Hypo | Megakaryocytes increased | 500 | 8 | 20.3 | 80 | RAEBt | Temporary remission after chemotherapy |
| 3 | 72 | 45,XY,−7[20] | Mixed | L-shift, dysplastic megakaryocytes | 250 | 13 | 51 | 129 | RAEB | MUD |
| 4 | 14 | 45,XY,−7[19]/46,XY[1] | Hypo | Megakaryocytes absent | 850 | 7.4 | 53 | 8 | RA | Expired |
| 5 | 30 | 46,XX,t(2;3)(p23;q29)[2]/45XX, idem,−7[20] | Normo | L-shift, dysplastic megakaryocytes | 340 | 8.5 | 60 | 135 | AML | Expired |
| 6 | 97 | 45,XY,−7[14]/46,XY,−7, +mar[7] | Normo | Myeloid dysplasia | 390 | 8.5 | 37 | 5 | AML | Expired |
| 7 | 53 | 45,XY,−7[13]/45,idem, del(12)(p12p13)[8] | Mixed | L-shift megakaryocytes decreased | 160 | 9.4 | 12.6 | 15 | AML | Expired |
| 8 | 28 | 45,XY,−7[20] | Mixed | Dysplastic megakaryocytes | 730 | 11.4 | 30.9 | 28 | RAEB | Expired |
| 9 | 7 | 47,XX,+8[3]/46,XX[14] | Hyper | Dysplastic megakaryocytes | 900 | 8 | 45 | 20 | RA | Partial hematologic remission |
| 10 | 13 | 45,XY,−7[20] | Hyper | No dysplasia | 550 | 7.5 | 18 | 22 | RAEBt | MUD, expired |
| 11 | 48 | 46,XX,+1,der(1;7) (q10p10)[14]/46,XX[6] | Normo | L-shift, dysplasia | 600 | 7 | 10 | 7 | RAEBt | MUD |
| 12 | 34 | 91,XXYY,−16[8]/91,XXYY, del(6)(q15q25),−16[3]/ 92,XXYY,del(6)(q15q25)[2]/ 92,XXYY,del(5) | Hyper | L-shift | 340 | 9.3 | 41 | 21 | AML | MUD |
| 13 | 5 | 47,XY,+8[18]/46,XY[2] | Mixed | Normal | 1760 | 12 | 37 | 110 | RA | CsA-dependent counts |
| 14 | 38 | 47,XY,+8[20] | Normo | Normal | 3300 | 13 | 90 | 130 | RA | CsA-dependent counts |
| 15 | 51 | 46,XX,del(13)(q12q14)[21]/ 46,XX[4] | Hyper | Megaloblastic changes | 2650 | 11.6 | 138 | 53 | RA | Stable counts |
| 16 | 6 | 45,X,−Y[5]/46,XY[15] | Hypo | L-shift | 2450 | 8.8 | 63 | 35 | RA | Expired |
| 17 | 11 | 47,XX,+8[8]/45,XX,−5[3]/46, XX[39] | Hypo | L-shift, megaloblastic changes | 1800 | 9.9 | 78 | 41 | RA | GF therapy |
| 18 | 39 | 45,X,−Y[14] | Normo | L-shift | 2000 | 8.6 | 107 | 37 | RA | CsA-dependent counts |
| 19 | 25 | 46,XY,del(13)(q12q14)[3]/46, XY[17] | Normo | Normal | 1900 | 10 | 63 | 118 | RA | Stable counts |
| 20 | 84 | 48,XXXc,+8[5]/47,XXXc[15] | Hypo | Megablastoid changes | 1850 | 11 | 34 | 90 | RA | CsA-dependent counts |
| 21 | 17 | 46,XY,del20(q11.2q13.2)[9]/46, XY[41] | Hypo | No dysplasia | 1600 | 12 | 67 | 80 | RA | Stable counts |
| 22 | 116 | 47,XX,add(X)(p22.3),add(3) (q26.2),+21 | Mixed | L-shift | 520 | 12 | 123 | 89 | AML | Expired |
| 23 | 34 | 46,XX,del(13)(q12q14)[4]/ 46,XX[16] | Hyper | L-shift | 1206 | 8 | NA | 8 | RAEB | Transfusion-dependent for red cells and platelets |
| 24 | 126 | 45,XY,−7[7]/46,XY[13] | Mixed | Myeloid dysplasia | 697 | 7 | 7 | 9 | RAEBt | MUD |
| 25 | 84 | 46,XX,del(17)(p11.2p13.1)[5]/ 46,XX[47] | Mixed | L-shift, dysplastic megakaryocytes | 1500 | 12 | 86 | 69 | RA | Stable counts |
| 26 | 20 | 46,XX,del(13)(q12q14)[4]/46, XX[16] | Mixed | Normal | 1052 | 7.9 | 20 | 20 | RA | CsA |
| 27 | 25 | 47,XY,+8[4]/46,XY[16] | Hypo | Normal | 2700 | 11 | 69 | 56 | RA | CsA-dependent counts |
| 28 | 28 | 47,XY,+8[20] | Hyper | Dysplastic megakaryocytes | 2290 | 15 | NA | 155 | RA | Stable remission |
| 29 | 8 | 47,XY,del(7)(p13p22)[3]/46, XY[17] | Hypo | Normal | 1350 | 15 | 47 | 48 | RA | CsA-dependent counts |
| 30 | 8 | 47,XY,+6[3]/46,46,XY[18] | Hypo | Normal, decreased megakaryocytes | 1350 | 8 | 20 | 10 | RA | Transfusion-dependent for red cells and platelets |
RA indicates refractory anemia; MUD, matched unrelated donor transplant.
Other studies performed
The presence of a PNH clone was determined historically by a Ham test or more recently using flow cytometry; the latter test was considered positive when more than 1% of glycosyl-phosphatidylinositol-anchored protein (GPI-AP)–deficient cells in blood were found as defined by negativity for surface staining for CD66b and CD16 in a distinctive population of cells.3Patients with the presence of PNH clone and otherwise fulfilling criteria of sAA or mAA were classified as having AA/PNH.
Therapy and response criteria
All but 2 patients received therapy with horse antithymocyte globulin (ATG; 40 mg/kg/d for 4 days) followed by 6 months of CsA adjusted to blood levels between 200 and 300 μg/L. One patient received 50 mg/kg cyclophosphamide for 4 days followed by CsA as initial therapy and one patient was treated only with a combination of growth factors (erythropoietin [EPO] and G-CSF). Six patients received 2 cycles of ATG. Several patients were also treated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and stem cell factor (SCF; Table 1). The response to immunosuppression was determined 6 months after treatment and defined as transfusion independence for red cells and platelets and improvement in blood counts such that they no longer fulfilled severity criteria.19
Statistical analysis
Means or medians (for nonnormal sample distribution) ± SD were calculated when appropriate. The unpaired Student ttest was applied to compare the means. For not normally distributed samples, an unpaired Wilcoxon test was used. Proportions were compared using χ2 test.
Results
Demographic and clinical features of AA patients who developed abnormal karyotype
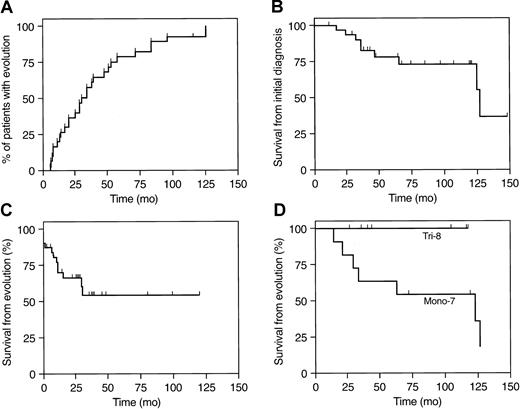
Patients who develop cytogenetic abnormalities in the course of AA have appeared to have heterogenous clinical characteristics and outcomes. To accurately address possible differences, we identified a large cohort of AA patients who had developed cytogenetic abnormalities. Loss of Y chromosome was observed in 2 patients and was included in this analysis but may be associated with aging, and its pathologic significance is not clear.29 Abnormalities involving chromosomes 7, 8, and 13 were most frequent among the group of 30 patients fulfilling case definition criteria (Tables 2 and3). All but 2 patients had presented with blood counts warranting a diagnosis of sAA; the remaining had mAA at presentation, which progressed to sAA. The average time from the initial diagnosis to cytogenic evolution was 39 ± 33 months (range, 5-126 months; Figure 1).
Frequency of cytogenetic abnormalities in AA
| . | Karyotypic abnormality . | All other3-150 . | |||
|---|---|---|---|---|---|
| +8 . | −73-150 . | −13/−13q . | −Y . | ||
| No. of patients | 7 | 12 | 4 | 2 | 5 |
| % of total | 23% | 40% | 13% | 7% | 16% |
| . | Karyotypic abnormality . | All other3-150 . | |||
|---|---|---|---|---|---|
| +8 . | −73-150 . | −13/−13q . | −Y . | ||
| No. of patients | 7 | 12 | 4 | 2 | 5 |
| % of total | 23% | 40% | 13% | 7% | 16% |
Structural and numerical abnormalities of chromosome 7.
Differential prognosis in AA patients who developed cytogenetic abnormalities.
(A) Cytogenetic evolution over the time within the group (30 patients = 100%). (B) Survival from the time of diagnosis (n = 30). (C) Survival from the time of evolution (n = 30). (D) Survival of patients with trisomy 8 (Tri-8; n = 7) and abnormalities of chromosome 7 (Mono-7; n = 11; P = .075).
Differential prognosis in AA patients who developed cytogenetic abnormalities.
(A) Cytogenetic evolution over the time within the group (30 patients = 100%). (B) Survival from the time of diagnosis (n = 30). (C) Survival from the time of evolution (n = 30). (D) Survival of patients with trisomy 8 (Tri-8; n = 7) and abnormalities of chromosome 7 (Mono-7; n = 11; P = .075).
The mean age of AA patients who developed karyotypic abnormalities was 42 years (range, 8-77 years) with a male-to-female ratio of 16:14. At presentation of AA, the mean platelet count was 13 900/μL (range, 2000-39 000/μL); ANC was 488/μL (range, 48-1400/μL); and the mean reticulocyte count (ARC) and hemoglobin concentration were 23 000/μL (range, 3000-65 000/μL) and 7.3 g/dL (range, 6-10 g/dL), respectively (Table 1). Clinically, of 29 patients treated with IST, 28 had received ATG/CsA and 1 had received cyclophosphamide and CsA in combination. Thirteen patients did not respond to immunosuppression and 16 did respond (54%). In addition, prior to cytogenetic evolution, 6 patients received therapy with SCF combined with G-CSF, 1 patient received EPO and G-CSF as long-term primary therapy, 2 patients received GM-CSF, and another 3 received G-CSF as a supportive therapy prior to and after IST (Table 1). After transformation to MDS, therapies included androgens, G-CSF, and some patients (see below) continued CsA.
All patients were tested for the presence of a PNH clone. In 21 cases assessed by flow cytometry, 12 had detectable GPI-AP–deficient granulocytes (57%), and in another 2 patients in whom flow cytometry was not performed, the presence of PNH was established by the Ham test. The prevalence of PNH in this group of patients was at least 50% and not statistically different from an historical cohort of AA (38% at presentation, 35% 1-4 years, and 50% in patients with > 4 years disease duration). There were no additional conversions to PNH within this group observed after cytogenetic evolution.
Clinical features and outcomes of clonal evolution in AA
In total, in comparison to initial presentation, there was a trend to higher blood counts at the time of diagnosis of clonal evolution (mean ANC of 1263/μL; range, 160-3300/μL), mean platelet count of 54 000/μL (range, 5000-155 000/μL), and mean hemoglobin concentration and ARC of 9.9 g/dL (range, 7-15 g/dL) and 52 000/μL (range, 7 000-13 800/μL), respectively; however, the individual blood counts depended on the response status to initial immunosuppression (see below). Marrow morphology was characterized by predominance of hypercellularity (41%) and patchy biopsy cellularity (27%), although continued hypocellularity was found in 1 of 3 of the patients. Frank dysplasia, including changes in megakaryocyte morphology, were found in 15 of 30 patients, and a left shift in myeloid differentiation was observed in 12. However, in 9 of 30 patients, there were no morphologic changes suggestive of MDS.
AA patients who developed secondary chromosomal abnormalities showed a mortality rate of about 27% with mean follow-up after evolution of 29 months (from the initial diagnosis, the total observation interval was 70 months; Figure 1). All but 2 deaths were related to complications of leukemia. In total, 13 patients (45%) developed either refractory anemia with excess blasts in transformation (RAEBt) or acute myeloid leukemia (AML; Table 2). Of these patients, 5 underwent unrelated matched BM transplantation.
Of the 30 patients, totally clonal hematopoiesis (100% of affected metaphases) was present at initial cytogenetic diagnosis in 13 patients. In all but 2 patients who initially presented with a mosaic of normal and abnormal karyotype, there was a steady increase in percentages of karyotypically abnormal metaphases during the observation interval. Disappearance of an abnormal clone was observed only in 2 patients, in both of whom, at evolution, only 20% to 25% cytogenetically abnormal metaphases were counted.
Abnormal karyotypes—clinical comparisons
Among patients who developed karyotypic abnormalities, trisomy 8 (n = 7; 23% of total) and partial or total loss of chromosome 7 (n = 12; 40% of total; Table 3) were most common. We have compared the clinical features of these 2 groups of patients (Table4). There were no significant differences between their ages at presentation of AA, time to evolution of cytogenetic abnormality, or blood counts at presentation (AA patients who later developed monosomy 7 showed a trend to a lower initial ANC). There was an obvious difference in initial responsiveness to IST. Among 6 patients with trisomy 8 treated with immunosuppression, all had responded to ATG and CsA and their blood counts continued to be dependent on CsA therapy. For patients with other cytogenetic abnormalities, similar CsA dependence of blood counts was observed only in 2 cases (1 patient with a transient 7p− and another with −Y). When compared with patients with numerical or structural defects of chromosome 7 at diagnosis of abnormal karyotype, those with trisomy 8 showed significantly higher mean ANC, hemoglobin concentration, and mean reticulocyte and platelet counts at the time of chromosomal evolution (Table 4).
Comparison of clinical features of trisomy 8 and monosomy 7
| . | Abnormality . | P . | |
|---|---|---|---|
| −74-154 . | +8 . | ||
| N | 114-150 | 7 | |
| Age (y) | 41 ± 24 | 42 ± 12 | .87 |
| Time to evolution (mo) | 47 ± 36 | 28 ± 25 | .21 |
| Mortality (%) | 50% | 0% | .0894-151 |
| Initial blood counts | |||
| ANC × 103/μL | 425 ± 250 | 811 ± 354 | .0152 |
| Hemoglobin (g/dL) | 7.2 ± 2.6 | 8 ± 1.7 | .48 |
| Reticulocytes × 103/μL | 18 ± 18 | 21 ± 12 | .67 |
| Platelets × 103/μL | 12 ± 5 | 18 ± 11 | .11 |
| Blood counts at evolution | |||
| ANC × 103/μL | 489 ± 217 | 2085 ± 768 | .0001 |
| Hemoglobin (g/dL) | 8.8 ± 1.8 | 11.4 ± 2.2 | .0169 |
| Reticulocytes × 103/μL | 29 ± 18 | 58 ± 23 | .0114 |
| Platelets × 103/μL | 40 ± 50 | 86 ± 49 | .07 |
| Response of AA to IST (%) | 25% | 100% | .0134-151 |
| . | Abnormality . | P . | |
|---|---|---|---|
| −74-154 . | +8 . | ||
| N | 114-150 | 7 | |
| Age (y) | 41 ± 24 | 42 ± 12 | .87 |
| Time to evolution (mo) | 47 ± 36 | 28 ± 25 | .21 |
| Mortality (%) | 50% | 0% | .0894-151 |
| Initial blood counts | |||
| ANC × 103/μL | 425 ± 250 | 811 ± 354 | .0152 |
| Hemoglobin (g/dL) | 7.2 ± 2.6 | 8 ± 1.7 | .48 |
| Reticulocytes × 103/μL | 18 ± 18 | 21 ± 12 | .67 |
| Platelets × 103/μL | 12 ± 5 | 18 ± 11 | .11 |
| Blood counts at evolution | |||
| ANC × 103/μL | 489 ± 217 | 2085 ± 768 | .0001 |
| Hemoglobin (g/dL) | 8.8 ± 1.8 | 11.4 ± 2.2 | .0169 |
| Reticulocytes × 103/μL | 29 ± 18 | 58 ± 23 | .0114 |
| Platelets × 103/μL | 40 ± 50 | 86 ± 49 | .07 |
| Response of AA to IST (%) | 25% | 100% | .0134-151 |
Statistical analysis: t test (normal distribution) or Wilcoxon test when appropriate.
Patient with a transient abnormality not included.
χ2 test.
Numerical and structural abnormalities.
The prognosis of patients with abnormalities of chromosome 7 appear much worse in comparison to those with trisomy 8 (Figure 1); although all patients with trisomy 8 remain alive and had not progressed to leukemia over a mean observation interval of 44 months (range, 5-84 months), those patients who developed numerical or structural chromosome 7 aberrancy showed high rates of mortality (50%) and transformation (RAEBt and AML in 6 of 11). Two patients who developed complex karyotypic abnormality also progressed to RAEBt or AML.
Discussion
Our report differs from previously published summaries in the large numbers of patients examined and the exclusion of cases in whom cytogenetics were abnormal at presentation of marrow failure. AA patients with true evolution of karyotypic changes were clinically diverse, and their prognosis was strongly linked to the type of cytogenetic defect. In agreement with other published data,5,8,14,15 the most common cytogenetic abnormalities were aberrations of chromosome 7 and trisomy 8; in a study from Seattle, monosomy 7 was found in 3 and trisomy 8 in 2 of 7 patients with karyotypic abnormalities,5 in a Dutch series, in 3 of 5 patients who evolved to MDS from AA,14 whereas among Italian patients,8 trisomy 8 was most frequent, present in 8 of 18 cases, followed by monosomy 7 in 2 of 18 patients (only a minority of these patients had initially normal cytogenetics at the time of presentation). In contrast to these results and to our own data, a recent meta-analysis showed trisomy 6 as most common in AA,20 but in all of the patients included in this study, the abnormal karyotype was present at initial diagnosis.
The appearance of a cytogenetic abnormality in a patient with AA provides strong evidence of clonal evolution to MDS. However, a high proportion of transient chromosomal changes, as reported in some studies, would diminish the diagnostic and prognostic implications of new cytogenetic defects. The low incidence of this finding in our study may be due to the stringent exclusion of patients who showed abnormal cytogenetics at presentation.
Myelodysplasia evolving from AA and primary MDS differ in the distribution of specific cytogenetic abnormalities. In primary MDS, abnormalities of chromosome 5 are most frequent (10%-37% of patients),21-28 but this chromosome is only occasionally affected in AA. 20q− is also found more often in primary MDS in comparison to AA.21,22 Conversely, monosomy 7, most prominent in late evolution of MDS from AA, occurs in a minority of primary MDS, 6.5% to 11%.20-27 Trisomy 8 appears to have a comparable incidence among cytogenetic abnormalities evolving from AA and in primary MDS, 6% to 20%.21-28 In MDS, the prevalence of patients with normal karyotypes ranges from 31% to 60%.17,21-23,25 27 Except for the evolution to leukemia or RAEBt, diagnosis of MDS with normal karyotype in the context of AA is hampered by many subjective factors and was not a subject of this study.
Cytogenetic abnormalities are strong predictors of clinical behavior and survival in acute and chronic leukemias,30,31 multiple myeloma,32 and primary MDS.18 In our series, in agreement with the IPSS classification for primary MDS,18 there was a major difference in prognosis of patients who developed defects of chromosome 7 or who had complex karyotypic abnormalities; these lesions were present in all of our patients who died or developed leukemia. In retrospect, these patients usually had had treatment-refractory and persistent pancytopenia. In contrast, trisomy 8 and other prognostically more benign abnormalities were frequent in the context of a good hematologic response to immunosuppression, and even after evolution to trisomy 8, improved blood counts were often dependent on continued CsA administration. Blood counts of patients at the time of cytogenetic evolution to trisomy 8 were significantly higher than those of patients with monosomy 7. The prognosis of trisomy 8 in primary MDS is not entirely favorable, with survival comparable to that of monosomy 7.25-28 Trisomy 8 is found in more advanced FAB subtypes, often included in the intermediate-risk group with average survival of 2.4 years (17.2 months in an analysis of 115 patients with trisomy)8,26 and time to evolution of AML of 1.6 years.18 The sharp contrast of this observation with the clinical course and prognosis of trisomy 8 evolving from AA suggests a different biology for an apparently identical cytogenetic abnormality in late AA and in primary MDS. Patients with abnormalities of chromosome 7 in AA fared as poorly as in primary MDS, with a high rate of conversion to acute leukemia.18 27
Fundamental questions in the evolution of clonal disease in AA are whether its pathophysiology is intrinsic to the natural history of AA, and now only observed as patients survive longer due to effective therapy, or secondary as a complication of, for example, immunosuppression,13 or chronic growth factor administration. However, MDS has occurred in AA treated with androgens only9,33 and though prolonged G-CSF treatment was linked by Japanese investigators to evolution of monosomy 7,11,34,35there was no increased risk observed in a randomized study of ATG and CsA with and without G-CSF36 or in an analysis of data from the European Group for Blood and Marrow Transplantation.37Many of our patients received hematopoietic growth factors, mainly as support or salvage therapy, and refractory disease itself may be the underlying risk factor for clonal evolution. Most AA patients who did not undergo BM transplantation will be treated with immunosuppression and hematopoietic growth factors, and in the absence of appropriate control groups, it is difficult to rigorously establish or exclude a pathophysiologic relationship between clonal evolution and therapy.
The relationship between AA, a disease dominated by an immune pathophysiology, and MDS, usually viewed as a premalignant process, remains unclear both clinically and pathophysiologically. In historical studies of abnormal karyotypes in AA, patients with abnormal cytogenetics and hypoplastic marrows at presentation were often included,6-10 and in some institutions, abnormal cytogenetics are compatible with a primary diagnosis of AA.7-9,20 In our series, our case definition was more stringent; cytogenetics were normal at first presentation, and most abnormal cytogenetic findings occurred after multiple normal studies. Whether more sensitive methods, such as fluorescent in situ hybridization, would have disclosed small populations of aberrant cells at earlier time points remains a moot question but a subject of current research. Were underdiagnosis due to inadequate techniques for the detection of chromosomal changes important, a more rapid evolution to MDS might be anticipated, but in our experience, chromosomal changes emerged gradually over time, more consistent with stochastic kinetics than a determinative process. Clinically, karyotypic abnormalities appear to concur with other features of typical AA such as the high incidence of HLA-DR238,39 and coexistence of an expanded PNH clone3; patients who show cytogenetic evolution do not obviously belong to a distinct nosologic subtype, as might be expected with primary misdiagnosis of MDS. Nevertheless, some of the same laboratory features have been reported in primary MDS.40For clinicians, the distinction between AA and hypocellular MDS ultimately may depend on empiric observations of the behavior associated with specific cytogenetic patterns. Monosomy 7 should continue to be regarded as itself a dire event, but for trisomy 8, the cytogenetic abnormality does not alter the desirability of an aggressive immunologic approach to improve the function of an empty BM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jaroslaw P. Maciejewski, Hematopoiesis and Experimental Hematology Section, Cleveland Clinic Taussig Cancer Center, 9500 Euclid Ave, Cleveland, OH 44195; e-mail:maciejj@cc.ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal