Abstract
Transendothelial migration of activated lymphocytes from the blood into the tissues is an essential step for immune functions. The housekeeping chemokine CXCL12 (or stroma cell–derived factor-1α), a highly efficient chemoattractant for T lymphocytes, drives lymphocytes to sites where they are highly likely to encounter antigens. This suggests that cross-talk between the T-cell receptor (TCR) and CXCR4 (the CXCL12 receptor) might occur within these sites. Here we show that the zeta-associated protein 70 (ZAP-70), a key element in TCR signaling, is required for CXCR4 signal transduction. The pharmacologic inhibition of ZAP-70, or the absence of ZAP-70 in Jurkat T cells and in primary CD4+ T cells obtained from a patient with ZAP deficiency, resulted in an impairment of transendothelial migration that was rescued by the transfection of ZAP-70. Moreover, the overexpression of mutated forms of ZAP-70, whose kinase domain was inactivated, also abrogated the migratory response of Jurkat T cells to CXCL12. In contrast, no involvement of ZAP-70 in T-cell arrest on inflammatory endothelium under flow conditions or in CXCL12-induced actin polymerization was observed. Furthermore, CXCL12 induced time-dependent phosphorylation of ZAP-70, Vav1, and extracellular signal-regulated kinases (ERKs); the latter were reduced in the absence of functional ZAP-70. However, though a dominant-negative Vav1 mutant (Vav1 L213A) blocked CXCL12-induced T-cell migration, pharmacologic inhibition of the ERK pathway did not affect migration, suggesting that ERK activation is dispensable for T-cell chemotaxis. We conclude that cross-talk between the ZAP-70 signaling pathway and the chemokine receptor CXCR4 is required for T-cell migration.
Introduction
Lymphocyte migration from the blood to the tissues is an essential step for immune surveillance. Chemokines play a central role in this process by conferring specificity in lymphocyte trafficking. Those present at the surfaces of endothelial cells are responsible for the activation of lymphocyte integrins, which, within seconds, change affinity for their ligands from a low- to a high-affinity state, allowing lymphocytes to stop on the endothelial wall. Chemokines secreted by cells within the subendothelial matrix attract lymphocytes across the vascular endothelial cell wall into the extracellular matrix, where they can exert their immune functions.1 2
Stroma cell–derived factor-1α—or CXCL12, according to the new chemokine nomenclature3—belongs to the CXC chemokine subfamily and is a highly efficient chemoattractant for numerous cell types,4,5 including T lymphocytes.6 To date, CXCL12 is the only identified ligand for CXCR4, a 7-transmembrane domain G-protein–coupled receptor7-9 that also serves as a coreceptor for T-cell tropic human immunodeficiency virus-1 strains.6 Mice lacking CXCL12 or its receptor, CXCR4, exhibit lethal defects including cardiovascular, neurologic, and vascular deficiencies and severe impairments of lymphopoiesis and bone marrow myelopoiesis.7-9 Recent reports have focused on the signal transduction pathway induced by the binding of CXCL12 to its receptor. CXCL12 induces the phosphorylation of several molecules that participate in the formation of focal adhesions and the reorganization of the cytoskeleton.10 Moreover, CXCL12 activates protein kinase B, extracellular signal-regulated kinases (ERKs),10,11 PI3 kinase,10,12 and the JAK/STAT pathway.13 More recently, CXCL12 has been shown to induce the phosphorylation of the phosphatase SHP2 and Cbl and the activation of Fyn and Lyn protein tyrosine kinases (PTKs).14
CXCL12 is a homeostatic chemokine, which means that it is produced within primary and secondary lymphoid tissues and in nonlymphoid tissues such as the skin and that it is involved in the physiologic traffic of cells of the immune system.15-17 Within these localizations, T and B cells are highly susceptible to encounter antigen. Thus an important question is whether antigen presentation to T or B cells, that is, T-cell receptor (TCR) or B-cell receptor (BCR) occupancy, might regulate chemokine receptor–induced activation signals. Recently, it was shown that CXCL12 plays a role in costimulating T cells,18 whereas TCR activation or BCR engagement inhibits CXCL12-induced chemotaxis.19-21 These data suggest that PTK signaling pathways activated by antigen receptor occupancy might cross-talk with CXCR4-dependent pathways.
In this report, we focused our work on the protein tyrosine kinase zeta-associated protein (ZAP)–70, a key signaling element in T-cell activation. The role of ZAP-70 on CXCL12 functional and biochemical effects was assessed in different T-cell lines and in CD4+T cells obtained from a patient with ZAP-70 deficiency. Here we show that ZAP-70 is required for CXCL12-induced transendothelial T-cell migration and for downstream signaling components including Vav1 and ERKs. Our results underscore the major implication of the nonreceptor PTK ZAP-70 in CXCR4 signaling in T cells.
Materials and methods
Reagents and antibodies
CXCL12 was obtained from Peprotech (Rocky Hills, NJ). Tumor necrosis factor (TNF)–α and recombinant soluble VCAM-1 were obtained from R&D Systems (Abingdon, United Kingdom). The following antibodies were used in this study: anti-CXCR4 monoclonal antibody (mAb; R&D Systems) and phycoerythrin–CXCR4 mAb (PharMingen, Torrey Pines, CA); antihemagglutinin (HA) mAb (Roche Molecular Biochemicals, Mannheim, Germany); anti-VSV mAb (Sigma-Aldrich, St Louis, MO); anti–phospho-ERK1/2 polyclonal antibody (Cell Signaling, Beverly, MA) and anti–ZAP-70 and anti-Vav1 mAbs (Upstate Biotechnology, Lake Placid, NY); and anti-ERK polyclonal antibody (Santa Cruz Biotechnology, CA). Anti–VLA-4 mAb (clone HP2/1) was a kind gift of Dr Sanchez-Madrid (Hospital de la Princessa, Madrid, Spain). Fluorescein isothiocyanate (FITC)–phalloidin, piceatannol, herbimycin, and pertussis toxin were obtained from Sigma-Aldrich. SB202190, Ly294002, and KN62 were from Calbiochem (San Diego, CA), and U0126 was from Cell Signaling. Fluorescent dyes used in this study were purchased from Molecular Probes (Eugene, OR). Green fluorescence fluorescein diacetate (CellTracker Green CMFDA) is a green fluorescent tracer with excitation at 522 nm and emission at 529 nm. Orange fluorescence tetramethylrhodamine (CellTracker Orange CMTMR) is a red fluorescent tracer with excitation at 541 nm and emission at 567 nm.
Cells
The Jurkat T cell line (JE6.1) and its CD3− variant (JRT3.T3) were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and were cultured in RPMI 1640 (Gibco Laboratories, Cergy Pontoise, France) supplemented with 5% fetal calf serum (FCS; Dutcher, Brumath, France), 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine, and 1 mM pyruvate (Merck). ZAP-70–deficient (P116) Jurkat cells were kindly obtained from Dr R. T. Abraham (Rochester, MI). To reconstitute ZAP-70 expression, P116 cells were transfected with pEFneo-HA-ZAP-70 and were selected in culture medium supplemented with 1 mg/mL geneticin (Invitrogen, Breda, The Netherlands). Jurkat cell lines stably transfected with mutated forms of VSV epitope-tagged ZAP-70 (FF18-22, in which Tyr-492 and Tyr-493 in the kinase domain were mutated to phenylalanine; KD12, in which Asp-461 was substituted by Asn; Y41-40, in which Tyr-319 in the linker domain was replaced by Phe) were kind gifts from Dr O. Acuto (Institut Pasteur, Paris, France).22 Transformed human umbilical vein endothelial cell line EA.hy926 was kindly provided by Dr Edgell (University of North Carolina) and was cultured in Dulbecco modified Eagle medium (Gibco Laboratories) supplemented with 20% FCS.
CD4+ T cells were obtained from a patient with ZAP deficiency and have been described elsewhere.23 Briefly, after institutional review board approval and informed consent, peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation. CD4+ T cells were purified using anti-CD4–coated magnetic beads as per the manufacturer's instructions (Dynal, Great Neck, NY). Cells were cultured in Yssel medium supplemented with 1% human AB+ serum and recombinant human interleukin-2 (IL-2) at 100 U/mL (Chiron, Emeryville, CA). Cells were stimulated weekly with phytohemagglutinin (0.5 μg/mL) (Murex, Dartford, England) and with irradiated feeder cells consisting of peripheral blood mononuclear cells and the Epstein-Barr virus–transformed B-cell line JY.
Plasmids and retroviral construction
The pEGFP-N1 plasmid was obtained from Clontech (Palo Alto, CA). Plasmids pEFneo-HA-ZAP-7024 and pEFneo-Vav1-myc L213A25 have been previously described. Murine 3BP2 cDNA26 was a myc epitope tagged by in-frame insertion into the pCS3 6 × myc tags vector.27 Plasmid encoding dominant-negative Rac1 (Rac1N17) was a kind gift of Dr M. Schwartz (La Jolla, CA). Construction of the LZRS-ZAP-70–epithelial growth factor protein (EGFP) retroviral vector and production of the retrovirus harboring the LZRS-ZAP-70–EGFP vector were previously described.28Briefly, a VSV epitope tagged ZAP-70 cDNA was cloned into theEcoRI and NotI sites of the polylinker in the murine leukemia virus-based LZRS retroviral vector. In this vector, the EGFP coding region is present downstream of an encephalomyocarditis-derived internal ribosomal entry site. The PG13 packaging line that produces gibbon ape leukemia virus envelope-pseudotyped retrovirus harboring the LZRS-ZAP-70/EGFP vector were identified by their autofluorescence and sorted on a FACS Vantage flow cytometer (Becton Dickinson, San Jose, CA). ZAP-70–deficient CD4+ T cells were transduced on fibronectin-coated plates and were exposed to retrovirus for 6 hours before incubation in Yssel medium overnight with IL-2. The transduction procedure was repeated once as previously described.28
Transient transfection
Simian virus 40 T-antigen–transfected human leukemic Jurkat T cells (Jurkat-TAg) (kindly provided by G. Crabtree, Stanford, CA) were electroporated as described previously.29 Briefly, 107 cells in a logarithmic growth phase were transfected with 30 μg pEFneo-Vav1-myc L213A plasmid or empty control vector, together with 5 μg pEGFP-N1 vector. Cells were cultured for 48 hours and were used in cell migration assays, as described below. Green fluorescence protein–positive (GFP+) cells were detected by flow cytometry (FACScan, Becton Dickinson).
Cell migration assay
Migration of T cells was assessed in 24-well chemotaxis chambers (6.5-mm diameter; Costar, Cambridge, MA). Polycarbonate transwell culture inserts with an 8-μm pore size were used for Jurkat cell lines, whereas inserts with a 3-μm pore sizes were used for phytohemagglutinin-activated T cells. Endothelial cells were cultured on the upper side of the membrane for 2 days before experiment and were left unstimulated. Medium alone or medium plus CXCL12 (100 ng/mL) was added in the lower chamber, and the lymphocyte suspension was added in the upper well. Lymphocyte migration in the lower well was measured after 90-minute migration. Unless mentioned, 0.5 × 106lymphocytes of each cell type were added in the upper well throughout this study. In some experiments, migration was performed through the mixture of 2 or 3 different cell lines that had been labeled with different fluorescent dyes that did not interfere with T-cell migration. Briefly, cell lines were separately incubated with CellTracker Green CMFDA (0.5 μg/mL) or CellTracker Orange CMTMR (2 μg/mL), or they were left unlabeled, for 30 minutes at 37°C in the dark. Cells were then washed 3 times, mixed, and added to the upper well. Migration of each population was quantified by using beads (Flow Count; Beckman-Coulter, Brea, CA) and flow cytometry.
Flow chamber and in vitro flow studies
The flow chamber was purchased from Immunetics (Cambridge, MA) and has been described elsewhere.30 It was designed to allow stabilized laminar flow between 0.1 and 2 dyn/cm2. T cells at a concentration of 1 × 106/mL in Hanks balanced salt solution supplemented with 1 mM CaCl2 and 1 mM MgCl2 were perfused through the chamber on a monolayer of endothelial cells or coated rsVCAM-1 using a withdrawal syringe pump (Harvard Apparatus, Boston, MA). In most experiments, T-cell lines were fluorescently labeled, washed 3 times, mixed, and perfused through the flow chamber for 5 minutes, as described.31 Medium was perfused to remove nonfirmly adherent lymphocytes before quantification on 6 random fields of 0.65 mm2 each using a laser scanning confocal microscope (Ultima Meridian; DGL Bioscience) with a 10× objective. In rolling experiments, lymphocyte–endothelial interactions in different fields were videotaped for 30 seconds using a CCD video camera and a video recorder. Images were then digitized using a Power Macintosh G3 (Apple Computer, Cupertino, CA) with video card (miroMOTION DC30; Pinnacle Systems, Germany) and commercial software (Adobe Premiere and Adobe Photoshop; Adobe Systems, San Jose, CA). Rolling velocities were measured by determining the number of frames (each 1/30thof a second length of time) it took 25 cells to cross a 350-μm long field.
Measurement of actin polymerization
Cells were stimulated with CXCL12 for the indicated periods of time at 37°C. Reactions were stopped by the addition of ice-cold phosphate-buffered saline (PBS). After centrifugation at 4°C for 3 minutes at 1500 rpm, cells were fixed for 15 minutes in 0.5 mL PBS, 3.7% paraformaldehyde. Cells were then washed 3 times in ice-cold PBS and 1% bovine serum albumin (BSA) and were incubated in PBS, 1% BSA, 0.1% azide, and 0.05% saponin for 15 minutes at 20°C. After centrifugation, cells were gently resuspended in the above buffer containing 50 ng/mL FITC-labeled phalloidin for 30 minutes at 20°C in the dark. After washing the cells 3 times in ice-cold PBS—1% BSA, polymerized actin (F-actin) was analyzed by flow cytometry.
Immunoblot analysis and immunoprecipitation
Cells (107/mL) in RPMI containing 5% FCS and 25 mM HEPES (pH 7.5) were preincubated at 37°C for different periods of time with CXCL12 (50 ng/mL). Activation was stopped by the addition of 1 mL ice-cold stop buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 100 mM NaF, 10 mM EDTA, 10 mM Na4P2O7, and 2 mM Na3VO4). After centrifugation, cells were resuspended in ice-cold lysis buffer (1% Triton X-100 in 150 mM NaCl, 50 mM HEPES, pH 7.4, 5 mM NaF, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride) for 30 minutes. Lysates were clarified by centrifugation at 15 000g for 10 minutes at 4°C, and protein concentration was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL). Cleared lysates were incubated for 3 hours at 4°C with the indicated antibodies and protein G–Sepharose beads (Sigma). Pellets were then washed 3 times with ice-cold lysis buffer containing 0.2% Triton X-100 and were resuspended in sodium dodecyl sulfate (SDS) sample buffer. Eluted immunoprecipitates or whole-cell lysates were separated by SDS–polyacrylamide gel electrophoresis (PAGE) and were analyzed by immunoblotting. Reactive proteins were visualized using enhanced chemiluminescence and autoradiography.
Results
Transendothelial T-cell migration requires the presence of the PTK ZAP-70
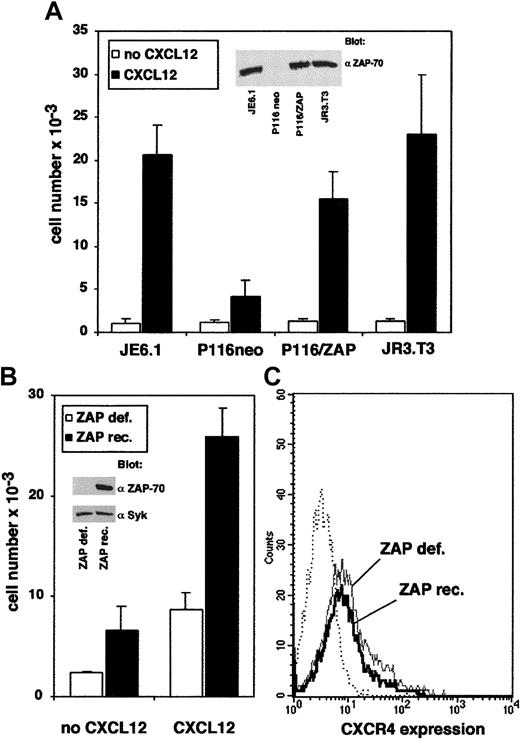
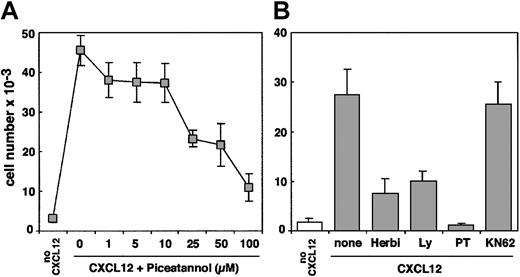
Different lines of evidence support the fact that the protein tyrosine kinase ZAP-70 is required for CXCL12-induced T-cell migration. First, we compared the transendothelial migration of Jurkat cells, P116 cells (a mutant Jurkat cell line that lacks ZAP-70) transfected with either the wild-type ZAP-70 cDNA or with the empty vector, and CD3− JRT3.T3 cells. Typically, 0.5 × 106cells of the different cell lines were added in the upper well of a Transwell chamber and were allowed to migrate through a resting endothelial cell monolayer. The absolute number of each population of migrating cells was then determined by flow cytometry using calibrated beads. As shown in Figure 1A, the migration of ZAP-70–deficient cells (P116 neo) was severely impaired compared with Jurkat cells. Stable expression of wild-type ZAP-70 in the deficient cell line strongly enhanced its migratory capability, reaching levels similar to those of wild-type Jurkat cells. In contrast, the absence of CD3 complex surface expression did not affect T-cell migration, suggesting that ZAP-70 can function in CXCR4 signaling pathways in the absence of functional TCRζ subunits (Figure1A). Consistent with a previous report,20 we also observed that T-cell activation by a CD3 mAb decreased the migration of ZAP-70+-P116 cells, but not the migration of P116 cells transfected with the empty vector (not shown). Proper expression of ZAP-70 in ZAP-70+-P116 cells was visualized by anti–ZAP-70 immunoblot analysis (Figure 1A, inset). Second, we used primary CD4+ T cells derived from a patient with severe combined immunodeficiency (SCID) previously shown to be lacking ZAP-70.23 Cells were retrovirally transduced with ZAP-70, as previously described.28 We compared the migration of untransduced ZAP-70–deficient cells with that of ZAP-70–reconstituted cells. As shown in Figure 1B, the migration of ZAP-70–deficient cells was clearly impaired compared with cells reconstituted with wild-type ZAP-70. As a control, ZAP-70 was expressed in retrovirally transduced CD4+ T cells (Figure 1B, inset). Similar expression levels of CXCR4 were detected in ZAP-70–deficient and reconstituted cells from the patient with SCID (Figure 1C), indicating that differences observed in migration levels did not result from a decreased CXCR4 surface expression. Moreover, CXCR4 expression was also similar in Jurkat cells and in the P116 cell lines (either ZAP-70–deficient or –reconstituted). Finally, we tested the effect of piceatannol, a Syk kinase inhibitor,32 33 on T-cell migration induced by CXCL12. Dose responses showed that up to 50% of migration was inhibited by piceatannol at a concentration of 25 μM (Figure2A). As a control, CXCL12-induced T-cell migration was also inhibited by inhibitors of PI3 kinase (Ly294002), PTKs (herbimycin A), or G proteins (pertussis toxin) (Figure 2B). In contrast, KN62, a calmodulin kinase inhibitor, did not affect cell migration (Figure 2B).
PTK ZAP-70 is involved in T-cell transendothelial migration induced by CXCL12.
(A) Transendothelial migration of the ZAP-70–deficient mutant Jurkat cell line (P116), transfected with either wild-type ZAP-70 cDNA (pEFneo-HA-ZAP-70) or with empty vector, was compared with the migration of Jurkat cells (JE6.1) or with CD3− JR3.T3 T cells. Cells (0.5 × 106) of each cell type were added in the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added in the lower chamber. Transendothelial migration of cells from the upper well to the lower well was measured after 90 minutes. The absolute number of each population was determined by flow cytometry using calibrated beads. (B) CXCL12-induced migration of phytohemagglutinin-activated CD4+ T cells obtained from a patient deficient in ZAP-70 expression. ZAP-70/GFP-retrovirus–mediated transduced cells (filled histograms) and untransduced cells (open histograms) from the same patient were added to the upper well. Medium alone or medium plus CXCL12 (100 ng/mL) was added in the lower chamber. Transendothelial migration and quantification of the cell number were performed as described in “Materials and methods.” (C) Same cells were analyzed for CXCR4 expression by flow cytometry. ZAP-70/GFP-retrovirus–mediated transduced cells (full black line) and untransduced cells (full gray line) were labeled using a phycoerythrin-conjugated anti-CXCR4 mAb. Phycoerythrin-conjugated, isotype-matched mAb (dotted line) was used as a negative control.
PTK ZAP-70 is involved in T-cell transendothelial migration induced by CXCL12.
(A) Transendothelial migration of the ZAP-70–deficient mutant Jurkat cell line (P116), transfected with either wild-type ZAP-70 cDNA (pEFneo-HA-ZAP-70) or with empty vector, was compared with the migration of Jurkat cells (JE6.1) or with CD3− JR3.T3 T cells. Cells (0.5 × 106) of each cell type were added in the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added in the lower chamber. Transendothelial migration of cells from the upper well to the lower well was measured after 90 minutes. The absolute number of each population was determined by flow cytometry using calibrated beads. (B) CXCL12-induced migration of phytohemagglutinin-activated CD4+ T cells obtained from a patient deficient in ZAP-70 expression. ZAP-70/GFP-retrovirus–mediated transduced cells (filled histograms) and untransduced cells (open histograms) from the same patient were added to the upper well. Medium alone or medium plus CXCL12 (100 ng/mL) was added in the lower chamber. Transendothelial migration and quantification of the cell number were performed as described in “Materials and methods.” (C) Same cells were analyzed for CXCR4 expression by flow cytometry. ZAP-70/GFP-retrovirus–mediated transduced cells (full black line) and untransduced cells (full gray line) were labeled using a phycoerythrin-conjugated anti-CXCR4 mAb. Phycoerythrin-conjugated, isotype-matched mAb (dotted line) was used as a negative control.
CXCL12-induced T-cell transendothelial migration is blocked by the ZAP-70 inhibitor piceatannol.
(A) Jurkat cells were preincubated with the indicated concentrations of piceatannol for 3 hours at 37°C and then were added to the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added to the lower chamber. Transendothelial migration and quantification of cell migration were performed as above. (B) Jurkat cells were left untreated (control) or were preincubated with the PTK inhibitor herbimycin A (10 μM), the PI3 kinase inhibitor Ly294002 (20 μM), the G-protein inhibitor pertussis toxin (10 ng/mL), or the calmodulin inhibitor KN62 (40 μM) for 3 hours at 37°C. Transendothelial migration and quantification of cell migration was performed as in panel A.
CXCL12-induced T-cell transendothelial migration is blocked by the ZAP-70 inhibitor piceatannol.
(A) Jurkat cells were preincubated with the indicated concentrations of piceatannol for 3 hours at 37°C and then were added to the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added to the lower chamber. Transendothelial migration and quantification of cell migration were performed as above. (B) Jurkat cells were left untreated (control) or were preincubated with the PTK inhibitor herbimycin A (10 μM), the PI3 kinase inhibitor Ly294002 (20 μM), the G-protein inhibitor pertussis toxin (10 ng/mL), or the calmodulin inhibitor KN62 (40 μM) for 3 hours at 37°C. Transendothelial migration and quantification of cell migration was performed as in panel A.
ZAP-70 is not required for T-cell arrest on endothelium under flow conditions
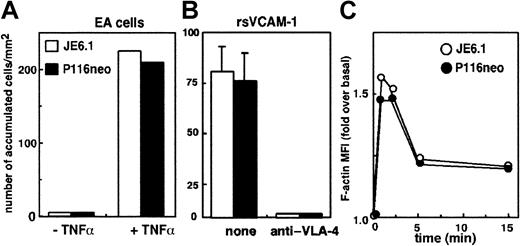
Recruitment of lymphocytes into inflammatory tissues requires the interactions of lymphocytes carried away by blood flow with the endothelium through a multistep process that involves tethering and rolling of lymphocytes on endothelial cells, rapid activation of integrins, and firm arrest of cells before diapedesis into the tissues.1,2 16 Because ZAP-70 appears to play an important role in the last step of this process, transendothelial migration, we tested its role in T-cell arrest on endothelial cells under flow. Endothelial cells were activated by TNF-α 18 hours before the experiments. Under these conditions, the expression of adhesion molecules such as VCAM-1 or ICAM-1 was optimum (not shown). ZAP-70–deficient or –expressing cells were labeled with fluorescent dyes, mixed, and perfused through a flow chamber on the endothelial cell layer under the same flow conditions. T-cell arrest was finally quantified by measuring the number of firmly adherent cells. As shown in Figure 3A, we did not observe any significant differences between Jurkat and P116 cells. A similar experiment performed on chambers coated with recombinant soluble VCAM-1 (rsVCAM-1) also showed that ZAP-70 expression did not affect T-cell arrest on VCAM-1 (Figure 3B). Measurement of rolling velocities also showed that ZAP-70 is not involved in this step (not shown).
ZAP-70 is not involved in T-cell arrest on inflammatory endothelium or in CXCL12-induced actin polymerization.
(A) Jurkat and P116 cells were labeled with different fluorescent dyes, washed, mixed, and perfused through a flow chamber at a flow rate of 40 mL/h on the monolayer of endothelial cells previously activated by TNF-α for 18 hours or left untreated. T-cell arrest was quantified by measuring the total number of firmly adherent cells arrested on 6 independent fields chosen at random. (B) A similar experiment was performed on chambers coated with recombinant soluble VCAM-1 (rsVCAM-1) with Jurkat and P116 cells that were previously incubated with a blocking anti–VLA-4 mAb or were left untreated. (C) Jurkat and P116 cells were incubated for the indicated times with CXCL12 (50 ng/mL). Cells were then fixed and permeabilized in the presence of 0.05% saponin, and FITC–phalloidin was added for 30 minutes before the final wash. Cells were then analyzed by flow cytometry. Results are expressed as the ratio of the mean fluorescence intensity (MFI) of stimulated cells to the MFI of unstimulated cells.
ZAP-70 is not involved in T-cell arrest on inflammatory endothelium or in CXCL12-induced actin polymerization.
(A) Jurkat and P116 cells were labeled with different fluorescent dyes, washed, mixed, and perfused through a flow chamber at a flow rate of 40 mL/h on the monolayer of endothelial cells previously activated by TNF-α for 18 hours or left untreated. T-cell arrest was quantified by measuring the total number of firmly adherent cells arrested on 6 independent fields chosen at random. (B) A similar experiment was performed on chambers coated with recombinant soluble VCAM-1 (rsVCAM-1) with Jurkat and P116 cells that were previously incubated with a blocking anti–VLA-4 mAb or were left untreated. (C) Jurkat and P116 cells were incubated for the indicated times with CXCL12 (50 ng/mL). Cells were then fixed and permeabilized in the presence of 0.05% saponin, and FITC–phalloidin was added for 30 minutes before the final wash. Cells were then analyzed by flow cytometry. Results are expressed as the ratio of the mean fluorescence intensity (MFI) of stimulated cells to the MFI of unstimulated cells.
Finally, we assessed the potential role of ZAP-70 in the regulation of actin polymerization induced by CXCL12 on T cells. As shown in Figure3C, CXCL12 induced similar actin polymerization on Jurkat and P116 cells, suggesting that ZAP-70 is not involved in this process.
CXCL12 induces tyrosine phosphorylation of ZAP-70 and Vav1
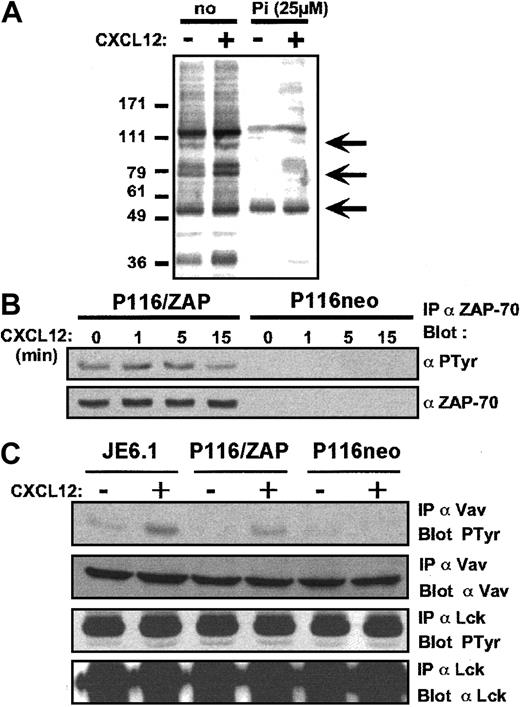
To further study the cross-talk between ZAP-70 and CXCR4 signaling, we examined tyrosine phosphorylation events induced by CXCL12 in Jurkat cells in the presence or absence of the ZAP-70 inhibitor piceatannol. Figure 4A shows a strong inhibition of tyrosine phosphorylation patterns in zones corresponding to apparent molecular weights of ZAP-70 and Vav1, whereas phosphorylation in the zone corresponding to p56lckwas unchanged (arrowed).
CXCL12 induces tyrosine phosphorylation of ZAP-70 and Vav1.
(A) Jurkat cells were preincubated for 3 hours in the presence of the carrier alone or of the ZAP-70 inhibitor piceatannol (25 μM) and were stimulated with CXCL12 (50 ng/mL) for 1 minute at 37°C. Antiphosphotyrosine immunoblot analysis was then performed on total cell lysates. (B) Immunoprecipitations with anti–ZAP-70 antibody followed by antiphosphotyrosine immunoblot analysis was performed on ZAP-70+-P116 and P116 cells activated by CXCL12 (50 ng/mL) for the indicated periods of time. The membrane was then stripped and reblotted with an anti–ZAP-70 antibody. (C) Immunoprecipitations with anti-Vav1 and anti-Lck antibodies, followed by antiphosphotyrosine immunoblot, were performed on Jurkat, ZAP-70+-P116, and P116 cells activated by CXCL12 (50 ng/mL) for 1 minute. Membranes were stripped and reblotted with anti-Vav1 and anti-Lck antibodies.
CXCL12 induces tyrosine phosphorylation of ZAP-70 and Vav1.
(A) Jurkat cells were preincubated for 3 hours in the presence of the carrier alone or of the ZAP-70 inhibitor piceatannol (25 μM) and were stimulated with CXCL12 (50 ng/mL) for 1 minute at 37°C. Antiphosphotyrosine immunoblot analysis was then performed on total cell lysates. (B) Immunoprecipitations with anti–ZAP-70 antibody followed by antiphosphotyrosine immunoblot analysis was performed on ZAP-70+-P116 and P116 cells activated by CXCL12 (50 ng/mL) for the indicated periods of time. The membrane was then stripped and reblotted with an anti–ZAP-70 antibody. (C) Immunoprecipitations with anti-Vav1 and anti-Lck antibodies, followed by antiphosphotyrosine immunoblot, were performed on Jurkat, ZAP-70+-P116, and P116 cells activated by CXCL12 (50 ng/mL) for 1 minute. Membranes were stripped and reblotted with anti-Vav1 and anti-Lck antibodies.
To confirm tyrosine phosphorylation of ZAP-70 by CXCL12, ZAP-70+-P116 cells (left side) and P116 cells (right side) were activated by CXCL12 for different periods of time, and cell lysates were subjected to anti ZAP-70 immunoprecipitations. Proteins separated by SDS-PAGE were then analyzed by immunoblotting with either antiphosphotyrosine or ZAP-70 antibodies. Figure 4B shows that in ZAP-70+-P116 cells, ZAP-70 was tyrosine phosphorylated in a time-dependent manner in the presence of CXCL12. As a control, no ZAP-70 was detected in the ZAP-70–deficient cell line P116. In addition, Figure 4C shows that CXCL12 induced tyrosine phosphorylation of Vav1 in Jurkat and ZAP-70+-P116 cells, and this phosphorylation was strongly reduced in the absence of ZAP-70. In contrast, no difference in Lck tyrosine phosphorylation was observed (Figure 4C).
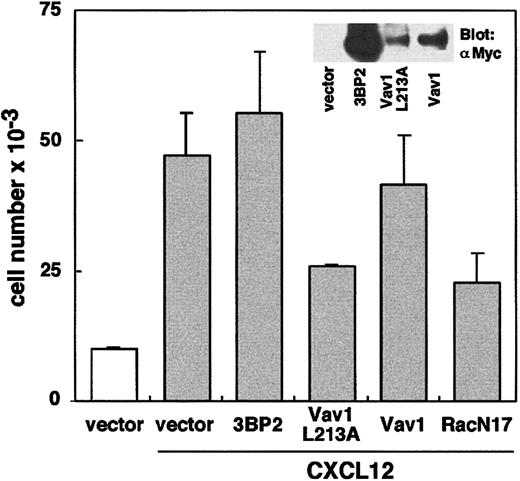
To further confirm a role of Vav1 in ZAP-70–dependent T-cell migration, we transiently expressed a dominant-negative form of Vav1 (Vav1 L213A)25 into the Jurkat-TAg cell line. Figure5 shows that T-cell migration in the presence of CXCL12 is strongly decreased by the overexpression of Vav1 L213A compared with cells transfected with vector only. In contrast, overexpression of wild-type Vav1 did not significantly modify cell migration. Consistent with previous studies,34 we also observed that T-cell migration was inhibited up to 50% by the overexpression of a dominant-negative form of Rac1 (Rac1N17). To take into account any side effects that might inhibit nonspecific T-cell migration, we observed that the overexpression of 3BP2, an unrelated cytoplasmic adapter,26 had no significant effect on migration (Figure 5). Overall, these data suggest that CXCL12 activates ZAP-70, which in turn regulates the phosphorylation of Vav1, a key enzyme in the activation of the Rac/Rho family GTPases.
Transfection of a dominant-negative form of Vav1 decreases T-cell migration in the presence of CXCL12.
Jurkat-TAg cells were transfected with 30 μg of an empty vector pEFneo (used as a control) or plasmids encoding 3BP2-myc, Vav1-myc L213A, wild-type Vav1-myc, and Rac1 N17, together with 5μg pEGFP-N1 vector. Cells were cultured for 48 hours and were used in cell migration assays, as described in Figure 1A. Transfected GFP+ cells were sorted by FACS analysis.
Transfection of a dominant-negative form of Vav1 decreases T-cell migration in the presence of CXCL12.
Jurkat-TAg cells were transfected with 30 μg of an empty vector pEFneo (used as a control) or plasmids encoding 3BP2-myc, Vav1-myc L213A, wild-type Vav1-myc, and Rac1 N17, together with 5μg pEGFP-N1 vector. Cells were cultured for 48 hours and were used in cell migration assays, as described in Figure 1A. Transfected GFP+ cells were sorted by FACS analysis.
Catalytic activity of ZAP-70 is required for CXCL12-induced T-cell migration
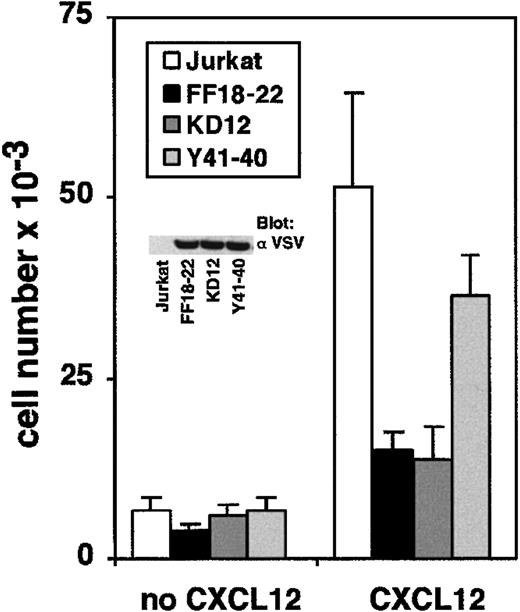
To determine which domain of ZAP-70 participates in the regulation of CXCL12 signaling, we tested the migration of Jurkat cell lines stably transfected with different ZAP-70 mutants. The FF18-22 cell line expresses a mutated form of ZAP-70 in which tyrosines 492 and 493 of the regulatory loop of its kinase domain have been replaced by phenylalanine. The KD12 cell line expresses a kinase-deficient ZAP-70 in which Asn is substituted for Asp-461, an essential amino acid for the catalytic activity. The Y41-40 cell line expresses a mutant of ZAP-70 in which Tyr-319 in the linker domain was replaced by Phe.35 Expression of ZAP-70 mutants in Jurkat cells has been shown to affect normal functions of endogenous ZAP-70 after TCR stimulation.22 Proper expression of ZAP-70 mutants in FF18-22, KD12, and Y41-40 cell lines was visualized by anti-VSV immunoblot analysis (Figure 6, inset). Migration of these cell lines was compared with that of control Jurkat cells. Without CXCL12, all the cell lines migrated at approximately the same level. In contrast, when CXCL12 was added to the lower well, a dramatic discrepancy between Jurkat cells and FF18-22 or KD12 cells was observed (Figure 6). Interestingly, the mutation of tyrosine 319, a residue involved in ZAP-70–p56lck interaction, had a limited effect on T-cell migration. The defective migration of FF18-22 and KD12 cells was not attributed to a decreased expression of CXCR4 because CXCR4 was expressed at similar levels on the different clones (not shown). Thus, inactivating the kinase domain of ZAP-70 not only interferes with TCR-induced signal transduction, it interferes with biochemical pathways activated by CXCL12.
Catalytic activity of ZAP-70 is required for CXCL12-induced T-cell migration.
Jurkat KD12, FF18-22, and Y41-40 cell lines were added to the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added to the lower chamber. Transendothelial migration of the cells was measured as above. Expression of VSV-tagged ZAP-70 mutants was visualized by anti-VSV immunoblot analysis of cell lysates.
Catalytic activity of ZAP-70 is required for CXCL12-induced T-cell migration.
Jurkat KD12, FF18-22, and Y41-40 cell lines were added to the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added to the lower chamber. Transendothelial migration of the cells was measured as above. Expression of VSV-tagged ZAP-70 mutants was visualized by anti-VSV immunoblot analysis of cell lysates.
Role of ZAP-70 in CXCL12 activation of the ERK pathway
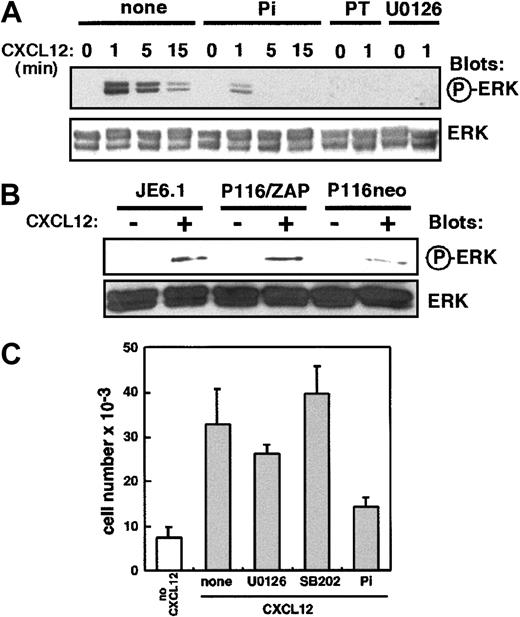
Because the stimulation of CXCR4 activates the mitogen-activated protein kinase (MAPK) signaling pathways,10 11 we investigated the potential role of ZAP-70 during ERK activation by CXCL12. Figure 7A shows that in Jurkat cell lines, CXCL12-induced phosphorylation of ERK was strongly inhibited by preincubation with piceatannol. As controls, U0126, a specific MEK inhibitor, and pertussis toxin, a G-protein inhibitor, completely blocked MAPK phosphorylation events induced by CXCL12. Next, we compared the ability of CXCL12 to induce ERK activation in P116 and ZAP-70+-P116 cells. As shown in Figure 7B, the lack of ZAP-70 resulted in a marked decrease of ERK phosphorylation induced by CXCL12. Quantification of spot intensity performed by densitometry scanning showed that ERK activation was decreased up to 50% in the absence of ZAP-70 (not shown). Finally, we measured Jurkat cell transendothelial migration after preincubation with U0126 or SB202190, a p38 MAPK inhibitor, or piceatannol. Although U0126 and SB202190 blocked ERK (Figure 7A) and p38 MAPK (not shown) activities, respectively, they did not affect T-cell transendothelial migration (Figure 7C). In contrast, and as shown before, piceatannol inhibited CXCL12-induced Jurkat cell migration. Together, these data show that ZAP-70 participates in the signaling pathways required for MAPK activation by CXCL12 in T cells. However, they also indicate that MAPK activation by CXCL12 is not required for T-cell migration.
ZAP-70 is involved in CXCL12 activation of the ERK pathway.
(A) Jurkat cells were left untreated (control) or were preincubated with piceatannol (25 μM), U0126 (50 μM), or pertussis toxin (PT, 10 ng/mL) for 3 hours at 37°C. Cells were then activated by CXCL12 (50 ng/mL) for different periods of time. Phosphorylated ERK was revealed by immunoblot analysis with an anti–phospho-ERK antibody. The amount of ERK was controlled by immunoblot analysis with anti-ERK polyclonal antibody. (B) Jurkat, ZAP-70+-P116, and P116 cells were activated by CXCL12 (50 ng/mL) for 1 minute, and ERK phosphorylation was analyzed as in panel A. (C) Jurkat cells were left untreated (control) or were preincubated with U0126 (50 μM), SB202190 (40 μM), or piceatannol (25 μM) for 3 hours at 37°C and then were added to the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added to the lower chamber. Transendothelial migration and quantification of cell migration was performed as in Figure 1A.
ZAP-70 is involved in CXCL12 activation of the ERK pathway.
(A) Jurkat cells were left untreated (control) or were preincubated with piceatannol (25 μM), U0126 (50 μM), or pertussis toxin (PT, 10 ng/mL) for 3 hours at 37°C. Cells were then activated by CXCL12 (50 ng/mL) for different periods of time. Phosphorylated ERK was revealed by immunoblot analysis with an anti–phospho-ERK antibody. The amount of ERK was controlled by immunoblot analysis with anti-ERK polyclonal antibody. (B) Jurkat, ZAP-70+-P116, and P116 cells were activated by CXCL12 (50 ng/mL) for 1 minute, and ERK phosphorylation was analyzed as in panel A. (C) Jurkat cells were left untreated (control) or were preincubated with U0126 (50 μM), SB202190 (40 μM), or piceatannol (25 μM) for 3 hours at 37°C and then were added to the upper well of a Transwell chamber. Medium alone or medium plus CXCL12 (100 ng/mL) was added to the lower chamber. Transendothelial migration and quantification of cell migration was performed as in Figure 1A.
Discussion
During the past few years, chemokines and their receptors have received considerable attention. In addition to their roles in leukocyte traffic and immune system development, their involvement in physiological and pathologic processes were found to be considerable and broader than initially thought.17,36 From this perspective, it has been shown recently that CXCL12 may play an important role in T-cell costimulation besides T-cell chemotaxis. On TCR-stimulated T cells, CXCL12 up-regulates early-activation markers and costimulates proliferation and cytokine production.18Such cross-talk between TCR- and CXCR4-dependent pathways might be of capital importance in the regulation of the immune response. This prompted us to examine how both pathways could be connected.
In this work, we demonstrate that ZAP-70, a PTK critically involved in TCR signaling, is also implicated in the regulation of CXCL12-induced T-cell migration. We show here that the absence of ZAP-70 in leukemic T-cell lines or in lymphocytes obtained from a patient with ZAP-70 deficiency resulted in reduced CXCL12-induced chemotaxis. Restoration of ZAP-70 expression in these cells, either by transfection or by retroviral transduction of ZAP-70, re-established their response to CXCL12. Our data are consistent with those of a recent report describing a role of ZAP-70 in T-cell migration on fibronectin induced by CXCL12.37 Interestingly, Soede et al38 39showed that ZAP-70 was also required in the LFA-1–dependent migration of a T-cell hybridoma in response to CXCL12, a pathway involved in the amplification of the chemokine signal. Thus, in addition to its critical role in TCR signaling, ZAP-70 also appears to play an important role in precise molecular pathways regulating T-cell chemotaxis.
We also examined how ZAP-70 interacts with the signaling pathways induced by the binding of CXCL12 to its receptor. It is well known that TCR engagement results in the activation of the nonreceptor PTKs Lck and Fyn, which phosphorylate TCRζ, leading to the recruitment of ZAP-70 through Src homology 2 (SH2) motifs, followed by its phosphorylation and activation. Next, the phosphorylation of downstream molecules such as SLP-76, LAT, PLCγ1, or Vav1 occurs, leading to calcium mobilization, activation of the MAPK and PI3 kinase pathways, and finally culminating in cell proliferation and differentiation (see reviews 40,41). In P116 cells transfected with wild-type ZAP-70, we observed that CXCL12 induces a rapid and transient tyrosine phosphorylation of ZAP-70, suggesting that this chemokine can directly activate ZAP-70. Tyrosine phosphorylation of ZAP-70 is not only critical for enhancing its catalytic activity,42,43 it also provides binding sites for SH2 domain–containing enzymes, such as Vav1, or adapter proteins.29,35,44-46 However, it is also possible that ZAP-70 could be recruited to the CXCR4 signaling complex through interactions with other signaling elements. Nevertheless, our data are consistent with the findings of Bacon et al,47who observed that RANTES induces tyrosine kinase activity of ZAP-70 in human T cells.
We also studied how mutations within ZAP-70 kinase domain might affect the migratory capabilities of leukemic T cells. In humans, most of the autosomal recessive forms of SCID induced by ZAP-70 mutations are located within this kinase domain.23,48-50 Therefore, we used 2 Jurkat cell lines stably transfected with mutated forms of ZAP-70 in which the kinase domain was inactivated. All these mutations have been shown to result in impaired ZAP-70 function during T-cell activation.22 Interestingly, neither of these 2 cell lines responded to CXCL12 in a migration assay, suggesting that expression and activity of ZAP-70 are both required for CXCL12-induced T-cell migration. In contrast, the mutation of tyrosine 319, a residue involved in ZAP-70–p56lck interaction, had a limited effect on T-cell migration.
Thus, an important point raised by these results is how defects in ZAP-70 result in the impairment of T-cell migration. Our data show that ZAP-70 is involved in at least 2 pathways, namely the activation of the MAPK pathway and the activation of the nucleotide exchange factor Vav1. Activation of the MAPK pathway by chemokines has been shown in different cell lines. In T cells, Tilton et al11 have shown that CXCL12 activates ERK2, and they suggested that CXCL12-induced ERK2 activation might play a role in embryogenesis and lymphopoiesis. Recently, Cambien et al51 showed that pharmacologic inhibition of ZAP-70–related PTK Syk affected MCP-1–induced stress-activated protein kinase SAPK1/JNK1 activation and transendothelial migration of a monocytic cell line. However, other reports obtained in Jurkat cells52 and in hematopoietic progenitor cells53 concluded that CXCL12-induced chemotaxis is not related to the ERK pathway. Obviously, our results obtained in Jurkat T cells support the latter observation. Although the discrepancy might be explained by the different cells and chemokines used in these studies, one might suggest that activation of the MAPK pathway by ZAP-70 contributes to other functions, such as the comitogenic effect of CXCL12 on TCR-stimulated T cells,18but not to chemotaxis.
ZAP-70 also appears to control tyrosine phosphorylation of the nucleotide exchange factor, Vav1, on the CXCL12 stimulation of T cells. Vav1 is phosphorylated on tyrosine in response to a wide variety of stimuli,54 and it has been involved in multiple pathways controlled by ZAP-70 in T cells.55 Several PTKs, including ZAP-70, have been shown to phosphorylate Vav1.29,56,57 In addition to its enzymatic activity as a guanine nucleotide exchange factor for the Rho family of small GTPases, Vav1 functions as an adapter protein that helps convey signals from membrane-associated complexes.55,58 Our results show that CXCL12-induced tyrosine phosphorylation of Vav1 is clearly impaired in the ZAP-70–deficient cell line P116. In contrast, we did not detect any significant modification in the phosphorylation of the Src kinase Lck, which acts upstream of ZAP-70. Vav1 phosphorylation results in an augmentation of its GDP/GTP exchange activity for the Rho family GTPases, Rac1, Cdc42, and RhoA.59 These proteins regulate signals leading to actin cytoskeleton changes60and were shown to regulate CXCL12-induced T-cell chemotaxis.34 Indeed, we observed that the expression in Jurkat cells of dominant-negative forms of Vav1 or its target, Rac1, severely impaired CXCL12-induced migration. Moreover, it has been recently shown that interactions between Wiskott-Aldrich syndrome protein and Cdc42 are critical for CXCL12-induced chemotaxis of T cells.61 Our results fit well with these data, suggesting that the activation of Rac1/Cdc42 by CXCL12 can occur after the activation of ZAP-70 and Vav1.
In conclusion, our work emphasizes the cross-talk between the TCR and the chemokine receptor, CXCR4, leading to a regulation of T-cell migration through the activation of a ZAP-70–dependent signaling pathway. This regulatory process, which results from a complex and coordinate integration of both pathways, is likely to play an important role in the immune response.
We thank O. Acuto, V. Di Bartolo, and D. Mège (Institut Pasteur, Paris) for FF18-22, KD12, and Y41-40 cell lines; R. T. Abraham (Rochester, MI) for the P116 cell line; G. Crabtree (Stanford, CA) for the Jurkat-TAg cell line; and N. Taylor (CNRS UMR 5535, Montpellier, France) for critical reading of the manuscript.
Supported by the Institut National de la Santé et de la Recherche Médicale, the Fondation pour la Recherche Médicale, and the Association pour la Recherche contre le Cancer. N.N. is supported by grants from AFM and March of Dimes.
M.T. and C.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marcel Deckert, INSERM U343, IFR 50, Hôpital de l'Archet, 151 Rte de Saint-Antoine de Ginestière, B.P. 3079, 06202, Nice Cédex 3, France; e-mail: deckert@unice.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal