Abstract
The design and evaluation of therapies for the sickle cell and β-thalassemia syndromes rely on our understanding of hemoglobin accumulation during human erythropoiesis. Here we report direct measurements of hemoglobin composition and messenger RNA (mRNA) levels in cultured CD34+ cells and correlate those measurements with studies of freshly obtained bone marrow samples. Hemoglobin levels in differentiating cells were also compared with morphologic, immunophenotypic, and cell cycle assessments. A population of large preproerythroblasts was first identified within 24 hours and became the dominant population by day 5. The transition from proerythroblast to basophilic normoblast occurred later, from days 7 to 9, and correlated with a peak of 74.1% ± 3.9% of the cells in the S phase of cell cycle. Orthochromatic normoblasts were the dominant and final cell type by day 13. High-performance liquid chromatography–based quantitation of fetal (HbF) and adult (HbA) hemoglobin and real-time polymerase chain reaction globin mRNA quantitation demonstrated a coordinate rise in the accumulation of both proteins and mRNA among these developmentally staged populations. Quantitative analyses on freshly sorted bone marrow populations demonstrated a similar rising pattern with β-globin and HbA as the dominant species at both early and late stages of differentiation. We found no evidence for HbF dominant populations or switching during differentiation in adult cells. Instead, rapid increases in both HbF (heterocellular) and HbA (pancellular) content were observed, which coincided with the apex in cell cycling and the proerythroblast-basophilic normoblast transition. Based on these measurements, we conclude that HbF and HbA content are regulated with the rate of proliferation during adult erythropoiesis.
Introduction
For decades, models of hemoglobin biosynthesis in adults have included a transition from fetal (γ-globin; HbF) to adult (β-globin; HbA) types during the differentiation process. Based on the premise that γ-globin gene activity decreases as adult erythroid cells differentiate,1 erythropoiesis in the postfetal stage of human development is postulated to recapitulate fetal-to-adult hemoglobin switching during human ontogeny.2-4Conceptually, this proposed “compressed switch” in the adult lineage has been useful for interpreting studies examining the control of HbF during normal and stressed erythropoiesis, and in response to cell cycle–specific drugs. However, the model itself is based largely on extrapolation of indirectly gathered data rather than direct measurements of globin messenger RNAs (mRNAs) and hemoglobin.5 6
Recently, we have initiated a genomic-based approach toward understanding the transcriptional basis of adult human erythropoiesis (http://hembase.niddk.nih.gov/). Developmentally staged human erythroid cells were generated using suspension culture methods. Due to the resemblance of erythroid suspension cultures with in vivo erythropoiesis, those culture methods are generally thought to be the best available in vitro models of human erythropoiesis.7 8To date, more than 5000 expressed sequence tags have been catalogued among complementary DNAs (cDNAs) from adult human erythroid cells having the developmental maturity equal to or less than that of proerythroblasts or basophilic normoblasts. The transcriptional phenotype of those cells includes a large number of cell cycle–associated and proliferation-associated genes. Despite the immaturity of the cells, we found that more than 85% of the β-globin locus–encoded sequences from 2 separate gene libraries correspond to the adult (δ or β) rather than fetal (Gγ orAγ) globin transcripts. However, the high percentage of adult globin transcripts in these immature erythroid cells is difficult to reconcile with the “switch” models of hemoglobin accumulation during adult erythropoiesis. For this reason, we decided to measure directly the fetal and adult globin mRNA and hemoglobin levels in the cultured erythroid cells as they respond to the hormone erythropoietin and differentiate. We further performed correlative studies to relate hemoglobin content with proliferative features of the differentiating erythroid cells in vitro and compare them to results obtained directly from human bone marrow aspirates.
Materials and methods
Culture and analysis of CD34+ cells from human blood
Peripheral blood CD34+ cells from healthy donors were mobilized and isolated as part of an institutional review board–approved protocol using a clinimax device (Miltenyi Biotec, Auburn, CA).9 10 All cell isolations were performed by personnel in the Dowling Clinic, Cell Processing Section of the National Institutes of Health Department of Transfusion Medicine. All the isolated cells were cultured for 14 consecutive days in Dulbecco modified Eagle medium containing 30% fetal bovine serum (Intergen, Purchase, NY), 1% bovine serum albumin (BSA), 10−5 M β-mercaptoethanol, 10−6 M dexamethasone, 0.3 mg/mL transferrin, 2 mM glutamine (Biofluids, Rockville, MD), antibiotics (penicillin/streptomycin), and 4 U/mL erythropoietin (Amgen, Thousand Oaks, CA). All reagents were from Sigma (St Louis, MO) unless otherwise stated in the text. Each day, cells in culture were enumerated using an electronic cell counter (Coulter, Hialeah, FL). Samples containing between 5 × 105 and 2 × 106 cells were collected for high-performance liquid chromatography (HPLC) analysis after washing twice in phosphate-buffered saline (PBS). Cells were also collected daily for total RNA purification, phenotyping, propidium iodide (PI) staining, and cytospin preparation. PI staining (Exalfa, Roxbury, MA) and immunostaining with monoclonal antibodies labeled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) and directed against CD34, CD71, glycophorin A (GPA; Beckman Coulter, Miami, FL), HbF (Caltag Laboratories, Burlingame, CA), HbA (Perkin Elmer Wallac, Norton, OH), and with isotypic controls (Beckman Coulter) were performed according to manufacturers' procedures and analyzed by flow cytometry.
CD34+ cells from 7 donors were cultured separately for this study. CD34+ cells from 3 separate donors were initially analyzed every day by HPLC, cytospin quantitation, cell cycle analysis, and phenotyping with antibodies against CD34, CD71, and GPA. For the remaining analyses, HPLC, cytospins, and surface immunophenotyping were performed every 2 to 6 days for confirmation of the same temporal pattern of maturation.
Analysis of mononuclear cells from human bone marrow
Approximately 108 mononuclear cells from purchased bone marrow (Poietics, Walkersville, MD) were labeled with anti-CD71–PE and anti-GPA–FITC monoclonal antibodies (Beckman Coulter), 1 mL each, in 8 mL Hanks balanced salt solution supplemented with 5 mM EDTA and 0.5% BSA, for 30 minutes at 4°C. Following a wash with the same buffer, flow cytometry was used for sorting CD71+GPA− versus CD71+GPA+ cells. Sorted cells were washed twice with PBS, analyzed by light microscopy of cytospin preparations, counted, aliquoted, and collected by centrifugation. Morphology-based counting and assessment of the cytospin preparations were performed without knowledge of the sample origin by one of the authors (P.N.). Cell samples stored at −80°C were subsequently used for HPLC analyses of hemoglobin proteins and for purification of total RNA used in quantitative polymerase chain reaction (PCR).
Flow cytometry
Flow cytometric analyses were performed using an EPICS ELITE ESP flow cytometer (Coulter). In each experiment, at least 10 000 cells were analyzed using argon laser excitation and bandpass emission filters: 675 nm for PI, 525 nm for FITC, and 575 nm for PE.
HPLC of hemoglobins
The cells were lysed in deionized sterile water, by repeated freezing and thawing. Cell debris was pelleted by brief centrifugation and the supernatants were filtered through Ultrafree-MC devices (Millipore, Bedford, MA) before cation-exchange chromatography. Hemoglobin species from cell lysates were separated on a 20 × 4-mm POLYCATA column (PolyLC, Columbia, MD) fitted to a Gilson HPLC system (Gilson, Middleton, WI). The hemoglobins were eluted during 4 minutes 8% to 40% gradient of buffer B (20 mM Bis-Tris, 2 mM KCN, 200 mM NaCl, pH 6.55) in buffer A (20 mM Bis-Tris, 2 mM KCN, pH 6.96) according to the manufacturer's protocol. Hemoglobin proteins were detected by absorbance measurements at 415 nm. Direct quantitation of hemoglobin was done by integration of the areas under the HbF and HbA peaks using software supplied by the manufacturer. Confirmation of HbF and HbA retention times in experimental samples was performed by electrospray mass spectroscopy (Hewlett-Packard Series 1100). Purified HbF and HbA (Perkin-Elmer Wallac) were used for reference. The purified HbF and HbA standards of known concentration were used for preparation of the standard curves. A linear calibration was generated from duplicate titrations covering the range from 0.023 to 18.5 μg. The standard curve linear correlation coefficient was calculated as R2 = 0.994.
Quantitative real-time PCR assay
Total RNA was isolated using TRIzol (Life Technologies, Rockville, MD) and quantitated by absorbance at 260 nm. In all samples, cDNA was synthesized using SuperScript II reverse transcriptase (Life Technologies) from the same amount of total RNA. Quantitative real-time PCR assays were carried out in a 7700 Sequence Detection System using TaqMan master mix and the protocol of the manufacturer (PE Applied Biosystem, Foster City, CA). The TaqMan master mix contained the AmpliTaq Gold DNA polymerase with 5′-3′ nuclease activity, which hydrolyzes a dual fluorescently labeled, target-specific oligonucleotide (TaqMan probe). On the intact probe, emission of the reporter dye (6-carboxyfluorescein, FAM) at the 5′ end is quenched by resonance energy transfer to the quencher dye at the 3′ end (tetramethylrhodamine, TAMRA). During hydrolysis, the reporter was released and separated from the quencher causing increase in the fluorescence (emission intensity) in the real time. The γ-globin and β-globin specific primers and probes were designed as described earlier,11 synthesized by PE Applied Biosystem and quantitated by absorbance at 260 nm. To prevent amplification of contaminating genomic DNA, probes were designed to span exon junctions in the fully processed mRNA. Absolute quantitation of nucleic acid templates was based on the inversely proportional relationship between the number of cycles required to reach the threshold emission intensity level (Ct) and the initial number of template molecules. Standard curves were prepared based on accurately determined dilutions of plasmids containing cDNA of γ-globin or β-globin as a template. Plasmid dilutions covered a dynamic range of 5 logarithmic orders or greater. For all standard curves linear correlation coefficients R2 ≥ 0.99. The number of molecules per nanogram of template was calculated using constant threshold levels and the standard curves.
Results
Semisynchronous appearance of erythroid cells in erythropoietin-supplemented culture
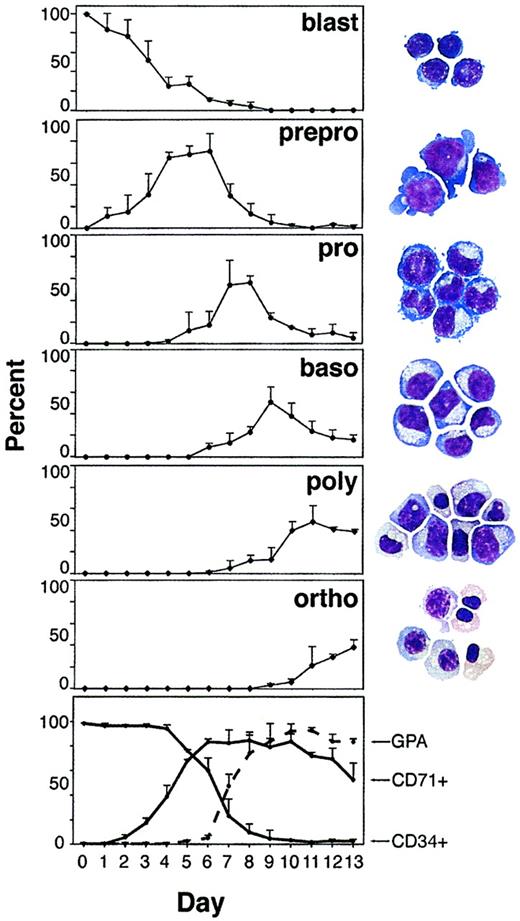
CD34+ cells were cultured for 2 weeks in media supplemented with high levels of erythropoietin for hemoglobin synthesis.12 The CD34+ cells initially placed in culture were morphologically recognizable as a homogeneous population of small blasts (Figure 1). Other cell types were not observed. Within 24 hours, a population of large erythroid blasts, referred to here as preproerythroblasts, began to appear. The preproerythroblasts cell size varied from 25 to 35 μm. Their nuclei were round and contained prominent nucleoli. The chromatin was fine, uncondensed, and uniformly dispersed. The cytoplasm was intensely basophilic. A prominent Golgi area and cytoplasmic vacuoles were present. Broad-based cytoplasmic projections or buds were characteristically present. Occasionally, budding of the nuclear envelope was identified as shown in Figure 1. Preproerythroblasts became the predominant population on days 4 to 6 (68% on day 5). Erythroid precursors appeared later and in a sequential manner during the culture period (Figure 1). Proerythroblasts, basophilic normoblasts, polychromatic normoblasts, and orthochromatic normoblasts were identified as the major populations on days 7, 9, 11, and 13, respectively. Enucleation was rarely observed.
Erythropoiesis in vitro: daily determination and quantitation of cell types and expression of surface proteins.
The 6 upper panels show daily percentage of a given cell type among all cells in culture calculated in a blinded fashion from cytospin preparations; blast indicates undifferentiated blast; prepro, preproerythroblasts; pro, proerythroblasts; baso, basophilic normoblasts; poly, polychromatic normoblasts; ortho, orthochromatic normoblasts. Stained cytospins performed on days 1, 5, 7, 9, 11, and 13 are shown on the right. The bottom panel demonstrates surface phenotype of cells in culture stained for glycophorin A (GPA), transferrin receptor (CD71), and CD34.
Erythropoiesis in vitro: daily determination and quantitation of cell types and expression of surface proteins.
The 6 upper panels show daily percentage of a given cell type among all cells in culture calculated in a blinded fashion from cytospin preparations; blast indicates undifferentiated blast; prepro, preproerythroblasts; pro, proerythroblasts; baso, basophilic normoblasts; poly, polychromatic normoblasts; ortho, orthochromatic normoblasts. Stained cytospins performed on days 1, 5, 7, 9, 11, and 13 are shown on the right. The bottom panel demonstrates surface phenotype of cells in culture stained for glycophorin A (GPA), transferrin receptor (CD71), and CD34.
The differentiation of erythroid cells in the presence of erythropoietin was also reflected in the differential expression of characteristic surface membrane proteins (Figure 1, bottom panel). CD71 (the transferrin receptor) and GPA were chosen as parameters because they have been extensively studied previously.13 Even though CD71 is not an erythroid-specific marker, a combination of lower light scatter and high-level expression of this protein is generally reserved for erythroid cells.14 CD34 was also examined because the cultured cells were originally isolated on the basis of this marker of hematopoietic progenitor cells. During maturation, the expression of CD34 gradually decreased to undetectable levels after 9 days. The preproerythroblast population identified early in the culture period was detected by flow cytometry as expressing high levels of CD71. Importantly, during culture days 2 to 5, a distinct population of immature erythroid cells was identified that expressed CD71 at high levels in the absence of high-level GPA expression. Expression of CD71 was maintained at relatively high levels until late in the culture period. Expression of GPA at high levels was delayed relative to CD71 and correlated with the transition from proerythroblast to basophilic normoblast on days 7 to 9 in culture.
Erythroid maturation correlates with a rapid rise and fall in the rate of cell proliferation
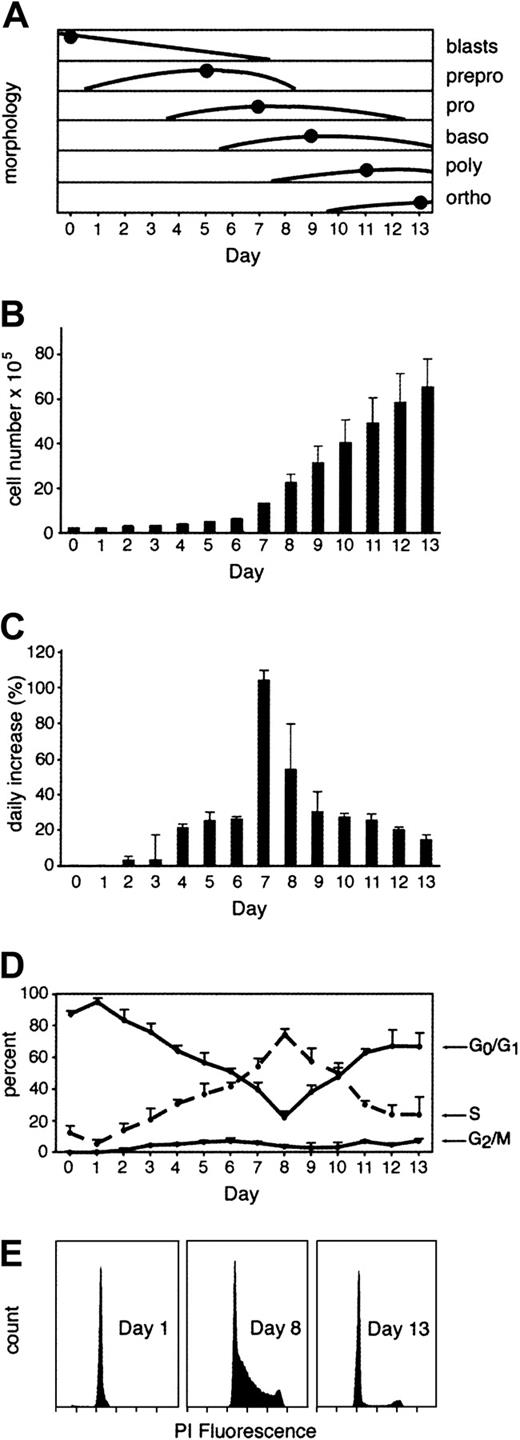
In addition to phenotypic maturation and hemoglobin quantitation, the proliferation status of the cells was measured. Cell counts and cell cycle analyses were performed daily (Figure2). Although an increase in cell numbers was detected after 24 to 48 hours, the total cell counts remained relatively low during the first week in culture (Figure 2B). More rapid increase in cell counts was observed on days 7 and 8 with an increase from 6.25 × 105 cells/mL on day 6 up to 22.5 × 105 cells/mL on day 8. Cell counts continued to increase reaching levels on day 13 of 71.2 × 105cells/mL compared with 2.5 × 105 cells/mL originally placed in the culture medium. The increase in cell number was also calculated as a daily rate or percentage increases (Figure 2C) to demonstrate changes in proliferation rate according to the developmental stage of the population. As shown, a rise in the rate of proliferation was measured during the first week with the maximal daily proliferation increase detected on days 7 to 8 (around 100%). This phase of increasing proliferation was followed by a decreased rate of proliferation to levels of less than 20% by the end of the culture period. The dominant cell type on the day of maximum proliferation (day 7) was the proerythroblast.
Proliferation rates and cell cycle stages during erythroid differentiation in vitro.
(A) Schematic representation of different erythroid stages during erythropoiesis as a percentage of the whole cell population. The dots mark the peak day for each cell type. Data collection and cell name abbreviations are as described in Figure 1. (B) Daily cell counts per 1 mL in culture. (C) Daily increase in cell counts measured as a percentage increase from the cell count on the previous day. (D) Flow cytometry measurements of cells at S, G2/M and G0/G1 stages of the cell cycle based on PI staining. (E) Representative cell cycle histograms performed on culture days 1, 8, and 13.
Proliferation rates and cell cycle stages during erythroid differentiation in vitro.
(A) Schematic representation of different erythroid stages during erythropoiesis as a percentage of the whole cell population. The dots mark the peak day for each cell type. Data collection and cell name abbreviations are as described in Figure 1. (B) Daily cell counts per 1 mL in culture. (C) Daily increase in cell counts measured as a percentage increase from the cell count on the previous day. (D) Flow cytometry measurements of cells at S, G2/M and G0/G1 stages of the cell cycle based on PI staining. (E) Representative cell cycle histograms performed on culture days 1, 8, and 13.
Assessments of cell cycle were consistent with the proliferation rates determined by cell enumeration
Eighty to 90% of the CD34+ cells were in the G0/G1 phase at the beginning of the culture period. After 24 hours the G0/G1 phase population began to decrease, and the S-phase population began to rise (Figure 2D). A rapid increase in the percentage of cells in S phase corresponded with the rise in cell numbers shown in Figure 2C. The S-phase percentage peaked on day 8 at 74.1% ± 3.9% of the population. As the basophilic normoblasts became the dominant population, a rapid fall in both the proliferation rate and percentage of cells in S phase was detected. The apex in the rate of proliferation was therefore associated morphologically with a transition from proerythroblast to basophilic normoblast morphology (compare panels A and D of Figure 2). Thereafter, the proliferation of cells as reflected by cell cycle analyses decreased consistent with the cell enumeration studies described above. The reduced level of proliferation correlated also with progressive nuclear condensation known as pyknosis and corresponded with increased GPA expression (compare Figures 1 and 2). It is noteworthy that this rapid rise and fall in the S-phase cells around culture day 8 was mirrored in the G0/G1pattern, but no corresponding rise and fall in the proportion of cells in G2/M was detected during the same period. One possible interpretation of this pattern is that some degree of S-phase prolongation relative to G0/G1 occurs during this highly proliferative stage of erythroid differentiation.
HbF and HbA share a similar pattern of accumulation during erythropoiesis
Determination of mean hemoglobin protein production per cell in culture was performed daily by HPLC analysis of cell pellets (Figure3A-B). Mean values for the HbF and HbA in the erythrocytes in the donors' peripheral blood at the time of CD34+ cell purification were 0.23 pg/cell and 30 pg/cell, respectively. CD34+ cells analyzed within 24 hours of purification showed no significant hemoglobin amounts above the background. By day 4, HPLC revealed a mean HbA content of 0.27 pg/cell compared with 0.023 pg/cell HbF. Although levels of both hemoglobin species remained relatively low until day 6, a rapid rise in the production of HbA as well as HbF was measured between days 7 and 9. Compared with culture day 4, the levels of HbA and HbF both increased significantly (paired t test P < .05) by culture days 5 and 7, respectively. After day 9, the levels of both hemoglobin types reached a plateau. Although HbF and HbA shared the same S-shaped pattern of accumulation, HbA expression stabilizes at around 20 to 25 pg/cell. In contrast, the HbF also reached a plateau value after day 9, but the level reached was about 0.2 pg/cell (approximately 100 times lower level than the HbA plateau). When the HbF/HbA ratio was calculated from directly measured protein quantities over the time of erythropoiesis (Figure 3C), the ratio dropped from 8.5% on day 4 to levels below 1% (average 0.9%) after day 9. By comparing the direct measurements of hemoglobin with the ratios, it was determined that the mean decrease in HbF/HbA ratio was due to the rapid rise in HbA content. No evidence suggested that HbF synthesis occurred at any stage before HbA, or that the mean cellular content of HbF was dominant during the maturation process.
HbA and HbF and mRNA content during the course of erythropoiesis in vitro.
(A-C) Hemoglobin protein levels were quantitated by HPLC. (A) Mean cellular content of HbF. (B) Mean cellular content of HbA. (C) HbF/HbA ratios. (D-F) Globin transcript levels (molecules/1 ng total RNA) were quantitated by real-time PCR. (D) Mean content of γ-globin mRNA transcript. (E) Mean content of β-globin mRNA transcripts. (F) Mean mRNA ratios of γ/β globin. The initial levels above background for hemoglobin (panels A and B) and globin mRNA (panels D and E) are shown with arrows. The asterisks and P values are noted on the days when increases above the initial hemoglobin and globin mRNA values (shown with arrows) became statistically significant.
HbA and HbF and mRNA content during the course of erythropoiesis in vitro.
(A-C) Hemoglobin protein levels were quantitated by HPLC. (A) Mean cellular content of HbF. (B) Mean cellular content of HbA. (C) HbF/HbA ratios. (D-F) Globin transcript levels (molecules/1 ng total RNA) were quantitated by real-time PCR. (D) Mean content of γ-globin mRNA transcript. (E) Mean content of β-globin mRNA transcripts. (F) Mean mRNA ratios of γ/β globin. The initial levels above background for hemoglobin (panels A and B) and globin mRNA (panels D and E) are shown with arrows. The asterisks and P values are noted on the days when increases above the initial hemoglobin and globin mRNA values (shown with arrows) became statistically significant.
In addition to protein quantitation, the transcriptions of γ-globin and β-globin mRNA were analyzed daily by fluorescence-based, real-time PCR (Figure 3D-F). The γ-globin and β-globin transcripts were detected at background levels at the beginning of the culture period. The sensitivity of PCR enabled measurements earlier than HPLC with levels above baseline in both transcripts detected after 2 days. Both γ-globin and β-globin mRNA reached plateau levels after day 9. Although the overall pattern of γ-globin and β-globin mRNA growth mirrored that of HbA and HbF, a more monotonous rise in globin mRNA was detected from days 3 to 9 when compared to the abrupt rise in hemoglobin from days 6 to 9. Compared with culture day 3, significant increases in γ-globin were detected on day 6, and a significant rise in β-globin on day 5 (paired t testP < .05). The γ-globin mRNA levels reached plateaus about 100 times lower than the β-globin mRNA levels (12 × 106 versus 8 × 108 molecules/1 ng, respectively). When the ratios of γ/β transcripts were compared, values measured at the beginning of erythropoiesis were higher (5.4% on day 3). The ratio then gradually decreased to less than 2% late in the culture period. As previously shown for HbF and HbA proteins, this decrease in the γ/β-globin ratio did not reflect a higher mean level of γ-globin expression at the early stages of erythropoiesis in comparison to the later stages. Instead, a much greater increase in β-globin transcripts relative to the small absolute increase in γ-globin transcripts during erythroid maturation was responsible.
Immunostaining suggests HbF shifts from a pancellular to heterocellular distribution as the cells mature
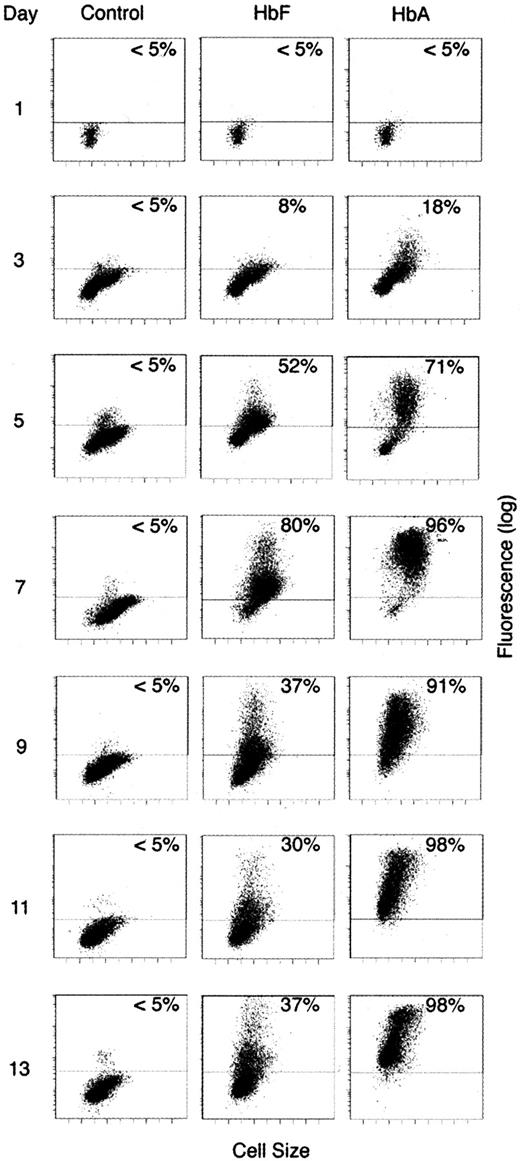
To estimate the percentage of cells expressing HbF or HbA within each population, we immunostained cells throughout the culture period and monitored the patterns of HbF-based and HbA-based fluorescence using flow cytometry. As shown in Figure4, the staining patterns of cells incubated with anti-HbF or anti-HbA were compared with isotype staining every 48 hours over the 2 weeks in culture. On culture day 1, neither HbF nor HbA staining was detected above background levels. Although detection of HbF-stained cells remained near background levels on day 3, a population of large, HbA-expressing cells was seen. By day 5, staining with both antibodies was detected above background levels in a subpopulation of cells with HbA-based fluorescence greater than that of HbF. By day 7, 80% or more of the cells stained above background levels with both antibodies. The low level of HbF-based fluorescence in the major population made detection in some of the cells difficult to distinguish from the high-level autofluorescence. No population with HbF intensities greater than HbA was identified among populations stained for both HbA and HbF (not shown). The distribution of HbF-based fluorescence also appeared variegated with some cells having relatively high levels of HbF-based fluorescence on day 7. During the second week, HbA staining above background levels remained detectable in more than 90% of the cells. Unexpectedly, the HbA-based fluorescence became lower as the cells became smaller during the second week in culture. Whereas the pattern of HbF-based fluorescence remained variegated during the second week, the staining pattern also became heterocellular with more than half of the population demonstrating background levels of fluorescence. The pattern of hemoglobin-based fluorescence over the culture period was reproduced among cells from a separate donor cultured under identical conditions (not shown).
Flow cytometric analysis of HbF and HbA.
Distributions of fluorescence versus size for cells stained with isotypic control antibodies (control) versus antibodies directed against HbF and HbA (stained separately) are shown according to the culture day on the left. The level of fluorescence demonstrated by at least 95% of the control-stained population (horizontal bars) was used to define the positive population. The percentage of hemoglobin-stained cells with fluorescence above that level is shown on the upper right of each panel. Nearly identical staining was detected with isotypic control antibodies for HbF and HbA; the HbF control panels are shown.
Flow cytometric analysis of HbF and HbA.
Distributions of fluorescence versus size for cells stained with isotypic control antibodies (control) versus antibodies directed against HbF and HbA (stained separately) are shown according to the culture day on the left. The level of fluorescence demonstrated by at least 95% of the control-stained population (horizontal bars) was used to define the positive population. The percentage of hemoglobin-stained cells with fluorescence above that level is shown on the upper right of each panel. Nearly identical staining was detected with isotypic control antibodies for HbF and HbA; the HbF control panels are shown.
The pattern of mean HbF and HbA accumulation during erythropoiesis in bone marrow is the same as in cultured CD34+cells
In culture, the increase in the expression of GPA mirrored the exponential rise in HbA accumulation on days 7 through 9. High-level expression of CD71 preceded the rise in the expression of GPA and marked earlier stages of erythroid differentiation. These phenotypic correlates of CD71 and GPA were subsequently used to sort the corresponding developmental stages of bone marrow cells for measurement of hemoglobin expression in vivo. Freshly aspirated bone marrow cells were sorted according to the expression of CD71 and GPA (Figure5A). The phenotype of the sorted cells was confirmed by flow cytometry, and cytospin preparations of the CD71+GPA− pool revealed populations of blasts and proerythroblasts like those shown in Figure 5. Differentiation beyond the proerythroblast stage was not identified with the exception of a rare basophilic normoblast (< 2%). The CD71+GPA+ population consisted of more mature erythroid cells. Differential counting revealed approximately 80% erythroid cells in both populations, with granulocytic precursors contaminating the CD71+GPA− pool, and cells having a monocytic appearance within the CD71+GPA+ pool. Among those sorted populations, HbF and HbA levels were measured by HPLC and levels of γ-globin and β-globin messages were estimated by quantitative PCR (Figure 5B-C).
HbA and HbF and mRNA content among sorted erythroid human bone marrow populations.
(A) Bone marrow mononuclear cells were stained and sorted using the flow cytometric gates of CD71+ , GPA− cells versus CD71+, GPA+ (rectangles). Stained cytospins of the postsort populations are shown on the right. (B) HPLC analyses of the control HPLC (top) showing the elution times of HbF and HbA versus sorted GPA+ cells (middle) and GPA−cells. Note differences in millivolt (mV) scales. (C) Quantitative PCR analyses of globin mRNA. Amplification curves of standard plasmid dilutions containing γ-globin (upper panel) and β-globin (lower panel) are shown in black. Amplification curves from total RNA extracted from the GA+CD71− cells are shown in blue, while GA+CD71+-derived curves are shown in red. Increases in the amount of template mRNA molecules were detected by a decrease in the number of PCR cycles required to reach the emission intensity threshold level of 0.02 (bold horizontal line). ΔRn = fluorescence emission intensity.
HbA and HbF and mRNA content among sorted erythroid human bone marrow populations.
(A) Bone marrow mononuclear cells were stained and sorted using the flow cytometric gates of CD71+ , GPA− cells versus CD71+, GPA+ (rectangles). Stained cytospins of the postsort populations are shown on the right. (B) HPLC analyses of the control HPLC (top) showing the elution times of HbF and HbA versus sorted GPA+ cells (middle) and GPA−cells. Note differences in millivolt (mV) scales. (C) Quantitative PCR analyses of globin mRNA. Amplification curves of standard plasmid dilutions containing γ-globin (upper panel) and β-globin (lower panel) are shown in black. Amplification curves from total RNA extracted from the GA+CD71− cells are shown in blue, while GA+CD71+-derived curves are shown in red. Increases in the amount of template mRNA molecules were detected by a decrease in the number of PCR cycles required to reach the emission intensity threshold level of 0.02 (bold horizontal line). ΔRn = fluorescence emission intensity.
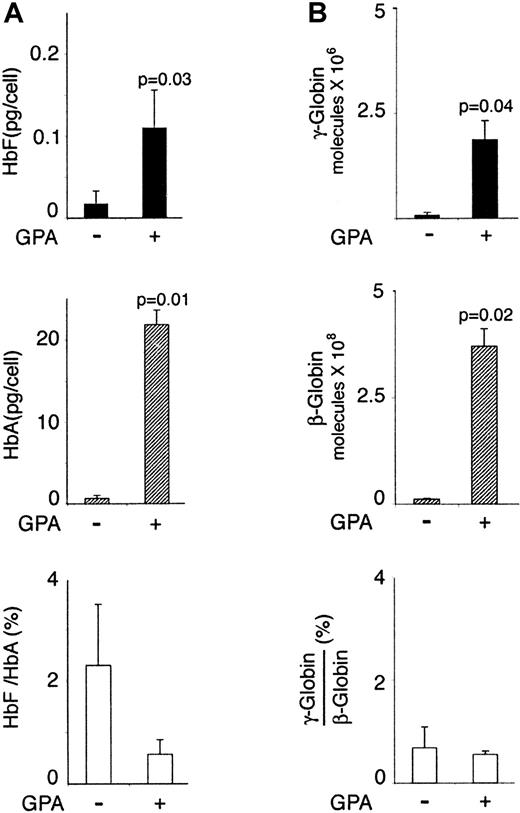
In fresh bone marrow aspirated from 3 adults, the HbF was measured at 0.023 ± 0.02 pg/cell in the CD71+GPA−population, and at 0.11 ± 0.05 pg/cell in the CD71+GPA+ cells (Figure6, left panel). HbA was detected at 0.61 ± 0.4 pg/cell level in the less mature cells and 21.8 ± 1.8 pg/cell in the more mature cells. Due again to the rapid rise in HbA during erythropoiesis, the HbF/HbA ratio dropped from 2% ± 1% in the less mature cells to 0.6% ± 0.3% in the more mature cells. Quantitative PCR assays revealed a similar pattern of protein and globin transcript synthesis during erythropoiesis in bone marrow. In more mature CD71+GPA+ cells, the mean level of γ-globin mRNA was about 23-fold higher than in the immature CD71+GPA− population. The mean level of β-globin mRNA in more mature CD71+GPA+ cells was about 33 times higher than in the less mature CD71+GPA− population. Despite the presence of nonerythroid cells (approximately 20% of both sorted populations), these coordinate increases in hemoglobin and globin mRNA in bone marrow cells are consistent with those values calculated in the in vitro setting. When the ratios of γ-globin and β-globin message were compared within each of the sorted populations, the γ-globin message was 100-fold lower than that of β-globin message among both CD71+GPA− and CD71+GPA+ cells. Once again, we detected no evidence suggesting HbF dominance at any maturation stage of adult human erythropoiesis.
HbF and HbA in bone marrow erythropoiesis.
HbA and HbF (left panel) and mRNA (right panel) content during erythropoiesis in vivo. Bone marrow mononuclear cells from 3 healthy adults were labeled and sorted in the independent experiments as shown on Figure 5. CD71+GPA− cells versus CD71+GPA+ were compared with respect to HbF and HbA protein content (A) (HPLC measurements), and γ-globin mRNA and β-globin mRNA levels (B) (quantitative PCR). The mean values, SD bars, and paired t test values are shown.
HbF and HbA in bone marrow erythropoiesis.
HbA and HbF (left panel) and mRNA (right panel) content during erythropoiesis in vivo. Bone marrow mononuclear cells from 3 healthy adults were labeled and sorted in the independent experiments as shown on Figure 5. CD71+GPA− cells versus CD71+GPA+ were compared with respect to HbF and HbA protein content (A) (HPLC measurements), and γ-globin mRNA and β-globin mRNA levels (B) (quantitative PCR). The mean values, SD bars, and paired t test values are shown.
Discussion
Although the pattern of hemoglobin accumulation and the mechanisms of erythroid differentiation in the bone marrow are fundamental for development of genetic and cellular therapies for the hemoglobinopathies and thalassemias, these questions are far from being resolved. Several experimental approaches in this regard have been derived from the use of erythroid colony techniques.15Colony assays provide an elegant method for the enumeration and study of clonal erythropoiesis. However, the interpretation of mechanisms of differentiation related to hemoglobin ontogeny from serum-supplemented colony assays has remained controversial due to the general finding that cultured erythroid colonies produce significantly more HbF than erythroid cells present in the donor's bone marrow and blood.16 Thus, extrapolation of most colony-based data to model hemoglobin accumulation within adult humans is inherently difficult. In contrast, more recently developed suspension culture models produce cells that undergo morphologic changes parallel to those in marrow and have hemoglobin levels equivalent to those found in vivo.7 Unfortunately, the relatively low number of precursor cells present in the 2-phase suspension culture system has prevented direct quantitation of fetal and adult hemoglobin during the earlier stages of differentiation hemoglobin.17 In addition, the presence of nonerythroid cell populations in bulk cultures prevents hemoglobin quantitation on a per cell basis. To overcome these problems, we cultured sufficient numbers of nonhemoglobin-producing CD34+ cells in single-phase suspension culture to evaluate the process of differentiation-associated hemoglobin accumulation from its onset. Our data include the very early transition from cells that do not produce appreciable hemoglobin into hemoglobin-producing cells that have not yet expressed classical markers of erythroid maturation like GPA. In addition, the CD34+ suspension culture system generates cells with hemoglobin contents closely resembling both early and late stage cells obtained directly from human marrow and peripheral blood. Therefore, this report represents direct and quantitative measurement of globin gene transcripts and hemoglobin accumulation in vitro and in vivo among a full range of differentiating adult human erythroid cells.
The close correlation between HPLC and quantitative PCR data (Figure 3) suggests the level of hemoglobin present in cells is directly correlative with globin mRNA synthesis. As expected, a significant rise in globin mRNA levels was detected prior to that of hemoglobin. Control over globin mRNA transcription and posttranscriptional stability have been extensively studied.18,19 Promoter and other regulatory elements including a control region located upstream of the globin genes on chromosome 11 are thought to coordinate and “open” the locus for the initiation of high-level gene transcription. Insulator elements have more recently been identified, which maintain a transcriptional sanctuary.20 The concept of chromatin “opening” and “closing” during erythropoiesis becomes more complex when considering the physical changes in chromatin associated with genomic duplication and mitosis during each cell cycle.21 Genome duplication may be a requirement for erythroid differentiation.22 Theoretically, the loss of the nuclear envelope during mitosis may also contribute to differentiation by providing physical access of maternally produced trans-acting factors with the genomes of the daughter cells. Our data suggest globin gene expression begins in the preproerythroblast. Extremely high levels of globin gene transcription are then achieved and maintained after the proerythroblast stage of development. Those cells are apparently opening the globin locus chromatin just as the genome itself is closing with the onset of pyknosis. Therefore, control over globin gene expression during the remaining differentiation cycles must involve mechanisms for genetic memory that maintain a transcriptionally active globin locus coincidentally with progressive and global genomic condensation. Epigenetic mechanisms may also be relevant for regulating γ/β globin ratios as the cells proliferate. Methylation patterns as well as replication timing may be important in this regard.23
Particular attention must be given to the interpretation of the fluorescence-based studies of HbF and HbA presented in Figure 4. During the first week in culture, the expressions of both HbF and HbA appear to increase with a pattern similar to the expression of the transferrin receptor (Figure 1). By day 7, a pancellular distribution of both hemoglobin species is identified. A relatively high and uniform level of HbA-based fluorescence expression is present compared with the generally lower and variegated levels of HbF-based fluorescence among those cells. After culture day 7, interpretation of the fluorescence-based assays regarding HbF became confounded by the intriguing loss of HbA-based fluorescence. The data demonstrate generally lower levels of HbA-based fluorescence that coincide with the dramatic rise in hemoglobin concentration as the cells mature and become smaller during terminal differentiation. Because identical staining, fixation, and isotypic controls were used for all the samples, we reasoned that an intrinsic property of the cells may be responsible for the paradoxical loss of HbA-based fluorescence during erythroid maturation. In reviewing the absorption spectra of hemoglobin,24 we noted the presence of absorption peaks in the regions of the antibody-based fluorescence used to detect HbA and HbF (FITC 525 nm; PE 575 nm). Based on these spectra, a loss or quenching of fluorescence from the antibodies may be expected. Therefore, the heterocellular distribution of HbF-based fluorescence in this study during the second week in culture may be due either to a loss of HbF or quenching of low-level HbF-based signals by HbA. Others have reported similar problems with fluorescence-based quantitation of hemoglobin levels especially at more advanced stages of erythropoiesis due to limited antibody access to binding sites or fluorescence quenching.25 Of note, other single cell hemoglobin assays are incapable of detecting low-level HbF expression among mature cells due to sensitivity thresholds around 3 pg/cell.26
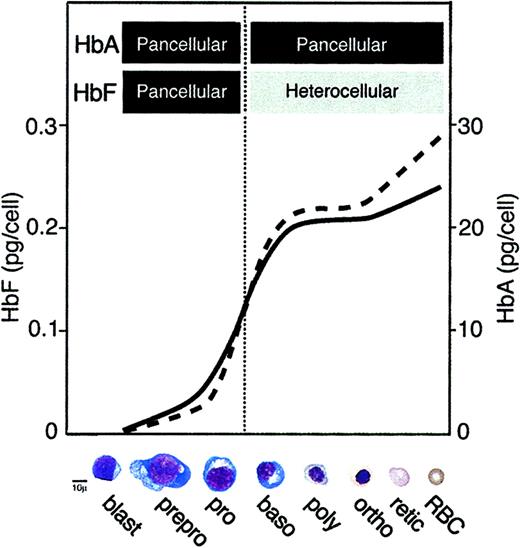
Based on our measurements of changes in mRNA and protein levels during in vitro maturation of cultured cells, and the concordance of data from uncultured bone marrow cells, we propose a model for the accumulation of hemoglobin during adult erythropoiesis. The model suggests that both HbF and HbA accumulate with similar patterns within a population of differentiating erythroid cells as shown in Figure7. The mean HbF and HbA values according to differentiation stage are shown on the left and right vertical axes with the HbF scale 100-fold lower than that of HbA. The images of the maturing cells are provided on the x-axis to emphasize the concept presented more than 50 years ago by Cartwright: erythroid cell maturation and hemoglobin production are inseparable.27Differentiation begins within immature blasts that have not begun to produce hemoglobin and ends, of course, with mature erythrocytes. The predominant feature of this model is the similarity between the shape of HbF (solid) and HbA (dashed) curves. Both assume a sigmoid shape. The most rapid hemoglobin ascent coincides with the extremely high proportion of cells in S phase seen in proerythroblasts and basophilic normoblasts (Figure 2). Compared with mRNA levels, the rise in hemoglobin is delayed and more abrupt. After this ascent, both mean hemoglobin and globin mRNA approach a plateau prior to the cessation of cell cycling. Unlike the pancellular distribution of HbA throughout erythropoiesis, the sigmoidal rise in the mean level of HbF appears to result from a heterocellular and variegated distribution of HbF within the population after the proerythoblast stage. Beyond the final cell division, the mean hemoglobin content again rises as distribution to daughter cells no longer occurs. A slight divergence in the postmitotic accumulation of fetal and adult hemoglobins was measured here and has been reported previously.28 Thus, the total level of both fetal and adult hemoglobins rises as populations of erythroblasts proliferate and mature, with the steepest rise occurring during the peak phase of cell proliferation.
Model of hemoglobin production during erythroid differentiation in adults.
Patterns for the accumulation of mean HbF (solid line) and HbA (dashed line) are shown with corresponding scales on the left and right y-axes, respectively, and cell types on the x-axis. The vertical dotted line identifies the transition from a rising to falling rate of proliferation. Bars at the top of figure denote the apparent fluorescence-based distribution of hemoglobin within the populations (see text for details). Hemoglobin levels attributed to the mature erythrocytes were obtained from HPLC analyses using peripheral blood from normal, healthy donors.
Model of hemoglobin production during erythroid differentiation in adults.
Patterns for the accumulation of mean HbF (solid line) and HbA (dashed line) are shown with corresponding scales on the left and right y-axes, respectively, and cell types on the x-axis. The vertical dotted line identifies the transition from a rising to falling rate of proliferation. Bars at the top of figure denote the apparent fluorescence-based distribution of hemoglobin within the populations (see text for details). Hemoglobin levels attributed to the mature erythrocytes were obtained from HPLC analyses using peripheral blood from normal, healthy donors.
Several differences exist between the model for hemoglobin accumulation proposed here and elsewhere.4 We found no evidence that γ-globin mRNA or HbF accumulate before β-globin mRNA or HbA. HbF was instead found at relatively low levels in a pancellular distribution at very early stages of maturation. Of note, when examined as HbF/HbA ratios, our data are consistent with the results of others,5,29,30 in suggesting a slight degree of asynchrony that results in a fall in the HbF/HbA ratio as the cells mature. However, our results suggest that this asynchrony is due to the very large, pancellular increases in HbA accumulation compared with the lower magnitude and variegated increase in HbF. The pattern of coordinately rising levels of HbF (heterocellular, variegated) and HbA (pancellular) challenges the hypothesis that a switch in the dominant hemoglobin from HbF to HbA occurs during adult erythropoiesis. Our inability to identify even subpopulations of HbF-dominant cells early in the maturation process may also be relevant with regard to the hypothesis that premature commitment is responsible for the generation of F cells in the setting of stress erythropoiesis.4 31 We propose an alternative hypothesis that the regulation of variegated HbF levels manifest at the peak proliferative stage of adult erythroid differentiation provides a mechanism for stress-related increases of HbF.
Our model provides a new perspective for the investigation of erythroid cells producing higher levels of HbF. Among mature cells in the blood of people with normal and increased levels of HbF, stability in the total hemoglobin content is maintained by the reduction of HbA.32 By monitoring hemoglobin accumulation, we may now explore if a similarly reciprocal relationship between HbF and HbA is present in less mature cells from those people. Erythropoiesis in the setting of increased HbF should also be examined to determine whether the kinetics of differentiation and proliferation are the same as those demonstrated here. Of note, we identified the most intense increase in total hemoglobin within a single definitive cell cycle that coincided with a dramatic shift in the rate of proliferation. However, globin gene transcription appears to increase significantly at least one cell cycle earlier in the preproerythroblasts. Hence, a clear association between cell cycling, cell morphology, and the modulation of globin transcription and total hemoglobin content likely exists during normal human erythropoiesis. Increases in HbF content and F-cell percentages during stress erythropoiesis suggest modulation of the HbF/HbA ratio is also associated with the rate of proliferation. Significant changes in HbF parameters have also been observed with the use of cell cycle–specific drugs like hydroxyurea, butyrates, and 5-azacytidine.33 34 Perhaps those agents as well as stress erythropoiesis are modulating HbF by perturbing relationships between hemoglobin production and mitosis at critical stages during erythroid differentiation.
The authors wish to thank Dr Regginald Smith for providing TaqMan probes used in the first set of quantitative PCR assays, Dr Lewis Panell and Sharon Gambino for performing mass spectroscopy analyses, and Dr Alan Schechter for several helpful discussions and critical reading of this manuscript. We also recognize assistance in obtaining the cells used for this study from the National Institutes of Health Clinical Center (Department of Transfusion Medicine) and the National Heart, Lung, and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jeffery L. Miller, Laboratory of Chemical Biology, Bldg 10, Rm 9B17, National Institutes of Health, Bethesda, MD 20892; e-mail: jm7f@nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal