Abstract
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived CD5+ B lymphocytes. TPA (12-O-tetradecanoylphorbol 13- acetate) and interleukin-4 (IL-4) inhibit apoptosis of B-CLL lymphocytes ex vivo. We used specific inhibitors of protein kinase C (PKC), extracellular-regulated kinase (ERK), and phosphatidylinositol 3–kinase (PI3-kinase) to study their involvement in TPA- and IL-4–induced survival of B-CLL lymphocytes. BisI, a specific inhibitor of PKC, induced apoptosis and inhibited the antiapoptotic activity of TPA and IL-4. B-CLL cells have a basal PKC activity that was increased by TPA but not by IL-4. TPA, but not IL-4, induced ERK activation. However, the inhibition of ERK activation did not affect the viability of B-CLL lymphocytes, demonstrating that this pathway is not involved in their survival. Inhibition of PI3-kinase by LY294002 induced apoptosis of B-CLL cells and inhibited the survival effect of IL-4 and TPA. In addition, Akt, a downstream effector of PI3-kinase activity, was phosphorylated by TPA and IL-4 in B-CLL cells, though PI3-kinase had no effect on PKC-dependent phosphorylation of Akt. Furthermore, the inhibition of PKC or PI3-kinase increased dexamethasone- and fludarabine-induced apoptosis ex vivo in the presence of survival factors. These results demonstrate that PKC and PI3-kinase are involved in the survival of B-CLL cells and suggest that inhibitors of these pathways could be combined with the drugs used in the treatment of B-CLL.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of monoclonal CD5+ B lymphocytes.1,2 Most circulating cells appear to be nondividing, and the clonal excess of B cells results from decreased apoptosis rather than increased proliferation.3
Apoptosis of B-CLL lymphocytes is regulated by several cytokines.4 When B-CLL lymphocytes are placed in culture medium, they undergo apoptosis,5 probably triggered by the absence of survival factor(s) that are present in vivo. Candidate survival factors that prevent apoptosis of B-CLL lymphocytes ex vivo include interleukin-4 (IL-4),6,7 interferon γ (IFN-γ),8 IFN-α,9 IL-2,10IL-6,11 IL-8,12 IL-13,13 and stromal cell–derived factor 1 (SDF-1).14 Interestingly, increased serum levels of IFN-γ8 and IL-815and increased production of IL-4 by T cells16 have been described in B-CLL. IL-4 is the most studied interleukin in preventing apoptosis of B-CLL lymphocytes ex vivo. Thus, in addition to its effects on spontaneous apoptosis, IL-4 inhibits apoptosis induced by glucocorticoids,6,17 IL-5,18IL-10,19 chlorambucil,20 and fludarabine.21
Protein kinase pathways involved in the survival of several cell types include protein kinase C (PKC), extracellular-regulated kinase (ERK), and phosphatidylinositol 3–kinase (PI3-kinase) pathways.22-24 The phorbol ester TPA (12-O-tetradecanoylphorbol 13-acetate) and bryostatin, 2 structurally unrelated activators of PKC, have been described as inhibitors of spontaneous and chemotherapy-induced apoptosis in B-CLL lymphocytes.17,25-29 Activation of PKC triggers the ERK pathway,30,31 and IL-4 induces PI3-kinase in various cell types, including B lymphocytes.32
PI3-kinase is an important mediator of survival factors, protecting many cell types from multiple apoptosis-inducing stimuli.23,33 PI3-kinase phosphorylates the D-3 position of phosphatidylinositol, phosphatidylinositol 4-phosphate, and phosphatidylinositol 4,5-diphosphate. Kinases such as 3-phosphoinositide–dependent kinase 1 (PDK1) and Akt bind to these phosphorylated intermediates through their pleckstrin homology domain. PDK1, in turn, phosphorylates and activates Akt, which has an important role in cell survival.23
The role of PKC, ERK, and PI3-kinase pathways in the control of the apoptosis of B-CLL lymphocytes has not been reported. Here we used specific inhibitors of these kinases to study their involvement in TPA- and IL-4–induced survival of B-CLL lymphocytes. Our results indicate that PKC and PI3-kinase pathways regulate survival in B-CLL cells.
Patients, materials, and methods
Patients
Twelve patients (7 men, 5 women) who ranged in age from 48 to 89 years (median age, 76 years) and who had not received treatment were studied. B-CLL was diagnosed according to standard clinical and laboratory criteria. Written informed consent was obtained from all patients according to the Declaration of Helsinki, and approval was obtained from the Comité de Bioètica of the Universitat de Barcelona for these studies.
Cytokines and chemical reagents
TPA, phenylmethylsulfonyl fluoride (PMSF), and propidium iodide (PI) were from Sigma Chemical (St Louis, MO). Dexamethasone was from Merck KGaA (Darmstadt, Germany). Fludarabine was from Schering AG (Berlin, Germany). Recombinant human IL-4 was from R&D Systems (Minneapolis, MN). Bisindolylmaleimide I (BisI), U0126, and LY294002 were from Calbiochem-Novabiochem (San Diego, CA).
Isolation of B-CLL cells
Peripheral blood lymphocytes from patients with B-CLL were obtained from the Hematopathology Unit at the Hospital Clinic, Barcelona, Spain. Mononuclear cells from heparinized peripheral blood were isolated by centrifugation on a Ficoll-Hypaque (Seromed, Berlin, Germany) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide.
The purity of B-CLL samples was evaluated by flow cytometry. Briefly, 5 × 105 cells were washed in phosphate-buffered saline (PBS) and were incubated in 50 μL PBS with allophycocyanin (APC)–conjugated anti-CD3 and phycoerythrin (PE)–conjugated anti-CD19 (PharMingen, Becton Dickinson, Mountain View, CA) for 10 minutes in the dark. Cells were then diluted with PBS to a volume of 250 μL and analyzed with a FACSCalibur (Becton Dickinson). Data analysis was performed with CellQuest software (Becton Dickinson). To analyze sufficient numbers of cells, a live-gate in forward scatter versus CD3 was drawn, and at least 5 × 103 CD3+ cells were acquired. The percentage of CD3+ T cells in B-CLL samples ranged from 0.4% to 5% (mean, 2.2% ± 1.5%).
Cell culture
B-CLL lymphocytes were cultured immediately after thawing at a concentration of 0.5 to 5 × 106 cells/mL in RPMI 1640 culture medium supplemented with 2 mM glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin (Biological Industries, Kibbutz Beit Haemek, Israel), and 10% heat-inactivated fetal bovine serum (Gibco BRL, Paisley, Scotland) at 37°C in a humidified atmosphere containing 5% carbon dioxide.17 The viability of B-CLL cells before commencing the incubation ranged from 62% to 91% (mean, 77% ± 9%). Factors were added 2 hours after the beginning of the culture, and cells were incubated for the indicated periods of time.
Flow cytometry analysis of phosphatidylserine exposure and cell membrane permeability
Cell viability and the rate of apoptosis were determined simultaneously by double staining with fluorescein isothiocyanate (FITC)–conjugated annexin V (Bender MedSystem, Vienna, Austria) and PI, as described previously.34 Briefly, 2 × 105 B-CLL cells were washed in PBS and resuspended in 100 μL annexin V–binding buffer (10 mM HEPES, pH 7.4, 2.5 mM CaCl2, 140 mM NaCl) containing 0.2 μL (1 μg/mL) FITC–annexin V. After 15 minutes of incubation in the dark at room temperature, cells were diluted with 100 μL annexin V–binding buffer containing 1 μg/mL PI and were analyzed with a FACSCalibur (Becton Dickinson). Data analysis was performed with CellQuest software (Becton Dickinson). Cell viability was measured as the percentage of annexin V– and PI-negative cells.
Protein kinase C activity
Protein kinase C activity was analyzed in particulate fraction containing membrane-associated PKC, as previously described,35 with minor modifications. Briefly, B-CLL cell pellets were homogenized in 200 μL of 25 mM Tris-HCl, containing 4 mM EGTA, 2 mM EDTA, 5 mM dithiothreitol, 1 mM PMSF, 1 μg/mL pepstatin, 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 1 mM benzamidine, at pH 7.5 (buffer A). After 20 minutes incubation at 4°C, B-CLL cells were homogenized by 20 strokes with a dounce homogenizer. After centrifugation at 100 000g for 1 hour at 4°C, the supernatant (representing the cytosolic fraction) was collected, and the pellet was resuspended in 150 μL buffer A supplemented with 1% Triton X-100. After incubating for 1 hour at 4°C, the suspension was centrifuged at 100 000g for 1 hour at 4°C. This second supernatant (particulate fraction) contained the membrane-associate PKC. The distribution of α-tubulin was used to estimate the purity of particulate fractions.
PKC activity was assayed in both fractions by measuring the incorporation of 32P from (γ-32P)ATP (Amersham, Buckinghamshire, United Kingdom) into a highly selective peptide substrate based on neurogranin (28-43)36 (Promega, Madison, WI) at 30°C. The assay reaction mixture contained 2 μg protein from each fraction in assay buffer containing 50 mM HEPES (pH 7.5), 0.25 mM EDTA, 0.5 mM EGTA, 0.125 mM dithiothreitol, 12.5 mM MgCl2, 100 μM ATP (specific activity, 200 cpm/pmol), 0.75 mM CaCl2, 280 μg/mL phosphatidylserine, 10 μM, TPA and 50 μM peptide substrate in a final volume of 50 μL. The reaction was stopped after 10 minutes on ice, and the sample (25 μL) was applied to Whatman P-81 paper (2 × 2 cm) (Whatman, Maidstone, United Kingdom). Papers were washed 4 times in 5% orthophosphoric acid with gentle agitation for 15 minutes. Radioactivity bound to the washed papers was determined by liquid scintillation counting. PKC activity was determined after subtracting the incorporation of 32P in the absence of CaCl2, phosphatidylserine, and TPA. Proteins were determined using the Micro BCA Protein assay reagent kit (Pierce, Rockford, IL) and with bovine serum albumin as standard.
Phosphorylation in intact cells
Phosphorylation of PKC substrates in intact cells was analyzed as previously described.37 Briefly, B-CLL cells (5 × 106 cells for each condition) were washed 3 times with RPMI 1640 medium without phosphate (Biological Industries) and resuspended at 10 × 106 cells/mL in the same medium supplemented with 32P-Orthophosphate (Amersham) (200 μCi/mL [7.4 MBq/mL]) for 5 hours at 37°C. Free phosphorus was eliminated by washing 3 times with ice-cold PBS, and the cells were then incubated for the indicated times in the presence of different factors. Cells were harvested and immediately extracted with 100 μL of 100 mM Tris-HCl, pH 6.8, 0.5% Triton X-100, 2 mM EGTA, 10 mM sodium fluoride, 1 mM PMSF, 1 μg/mL pepstatin, 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 1 mM benzamidine for 20 minutes at 4°C. The extract was heated at 100°C for 10 minutes and centrifuged at 13 000 rpm for 10 minutes. The same amount of cell extract was analyzed by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). 32P-labeled proteins were detected by autoradiography.
Western blot analysis of protein phosphorylation
B-CLL lymphocytes were incubated at a density of 5 × 106 cells/mL with factors for the indicated period of time, washed in ice-cold PBS, resuspended in lysis buffer containing 50 mM Tris-HCl pH 7.5, 1% Triton X-100, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 5 mM EDTA, 40 mM β-glycerophosphate, 100 mM NaCl, 1 μg/mL pepstatin, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 mM benzamidine, and 1 mM PMSF, and incubated on ice for 5 to 10 minutes. After a 1-minute vortex, cell debris was eliminated by centrifugation at 13 000 rpm for 15 minutes, supernatants were collected, and protein concentrations were determined using the Micro BCA Protein assay reagent kit (Pierce). For the detection of phosphorylated forms of ERK-1/ERK-2 (ERK) and Akt, whole lysates (50 μg) were resolved by 10% SDS-PAGE, transferred to Immobilon-P membranes (Millipore, Bedford, MA), and analyzed by Western blotting with specific antibodies against phospho-ERK (Thr202/Tyr204) and phospho-Akt (Ser473) (New England BioLabs, Beverly, MA). As a confirmation of equal loading and transfer proteins, blots were stripped and reproved with antibodies against ERK (Upstate Biotechnology, Lake Placid, NY). After binding with horseradish peroxidase–conjugated secondary antibodies, blots were visualized with the enhanced chemiluminescence detection system (Amersham).
Statistical analysis
Levels of significance between samples were assessed by analysis of variance (Fisher PLSD test).
Results
PKC inhibition blocks the survival effect of TPA and IL-4
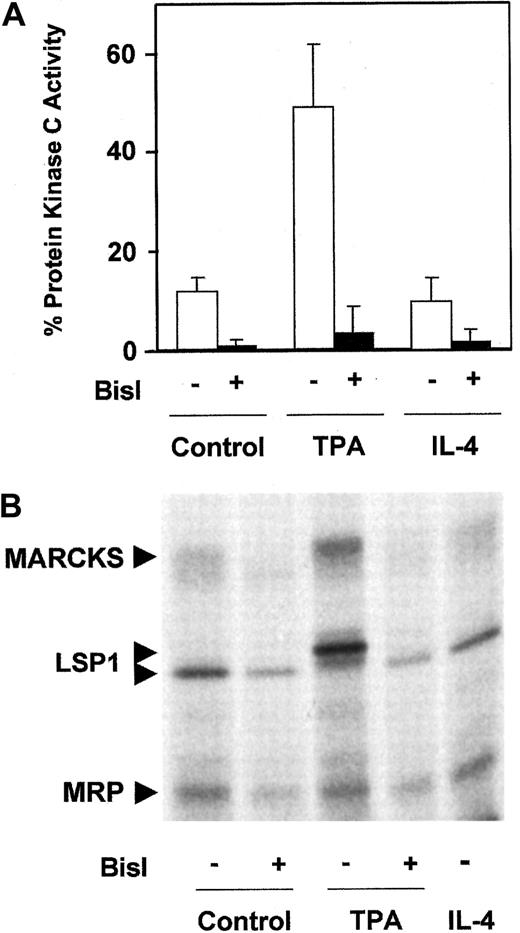
To study the involvement of PKC in the survival of B-CLL lymphocytes, we used Bisindolylmaleimide I (BisI), a specific inhibitor of PKC.38 Treatment of B-CLL cells with 5 μM BisI induced apoptosis in 7 of 10 patients analyzed (patients 3, 4, 6, 7, 9, 11, and 12) and inhibited the survival effect of TPA and IL-4 (Table1). The induction of apoptosis by BisI in the absence of TPA or IL-4 suggested that in most cases basal PKC activity contributed to the survival of B-CLL cells. Therefore, we measured PKC activity in B-CLL cells. As shown in Figure1A, B-CLL cells have a basal membrane-associated PKC activity that corresponds to 13% of the total PKC activity. This activity was completely blocked in the presence of 1 μM BisI, corroborating that it corresponds to PKC activity. Membrane-associated PKC was increased by TPA but not by IL-4. Furthermore, we analyzed the phosphorylation level of the 3 major PKC substrates in B-CLL cells: myristoylated alanine-rich PKC substrate (MARCKS), MARCKS-related protein (MRP), and leukocyte-specific protein 1.37 39 B-CLL cells have a basal level of phosphorylation of these substrates that was inhibited by BisI, increased by TPA, and not affected by IL-4 (Figure 1B).
Effect of BisI on survival of B-CLL lymphocytes
| Patient . | Viability (%) . | |||||
|---|---|---|---|---|---|---|
| Control . | BisI . | TPA . | BisI + TPA . | IL-4 . | BisI + IL-4 . | |
| 2 | 40 ± 7 | 36 ± 19 | 49 ± 0.9 | 42 ± 1 | 39 ± 5 | 38 ± 10 |
| 3 | 38 ± 7 | 19 ± 14 | 77 ± 4 | 46 ± 20 | 64 ± 4 | 30 ± 10 |
| 4 | 82 ± 0.4 | 59 ± 3 | 85 ± 1 | 77 ± 2 | 76 ± 1 | 56 ± 2 |
| 5 | 78 ± 6 | 68 ± 6 | 68 ± 16 | 80 ± 8 | 84 ± 8 | 80 ± 11 |
| 6 | 59 ± 1 | 47 ± 1 | 61 ± 0.1 | 51 ± 0.7 | ND | ND |
| 7 | 79 ± 1 | 37 ± 7 | 71 ± 3 | 57 ± 17 | 77 ± 0.4 | 29 ± 2 |
| 9 | 68 ± 0.8 | 25 ± 3 | 66 ± 4 | 54 ± 30 | 82 ± 0.1 | 46 ± 1 |
| 10 | 72 ± 6 | 66 ± 11 | 66 ± 2 | 67 ± 5 | 69 ± 7 | 65 ± 9 |
| 11 | 34 ± 2 | 23 ± 4 | 53 ± 2 | 31 ± 3 | ND | ND |
| 12 | 48 ± 9 | 15 ± 8 | 71 ± 4 | 52 ± 7 | 71 ± 2 | 24 ± 2 |
| Mean ± SD | 60 ± 18 | 39 ± 19* | 67 ± 11 | 56 ± 15† | 70 ± 14 | 46 ± 20‡ |
| Patient . | Viability (%) . | |||||
|---|---|---|---|---|---|---|
| Control . | BisI . | TPA . | BisI + TPA . | IL-4 . | BisI + IL-4 . | |
| 2 | 40 ± 7 | 36 ± 19 | 49 ± 0.9 | 42 ± 1 | 39 ± 5 | 38 ± 10 |
| 3 | 38 ± 7 | 19 ± 14 | 77 ± 4 | 46 ± 20 | 64 ± 4 | 30 ± 10 |
| 4 | 82 ± 0.4 | 59 ± 3 | 85 ± 1 | 77 ± 2 | 76 ± 1 | 56 ± 2 |
| 5 | 78 ± 6 | 68 ± 6 | 68 ± 16 | 80 ± 8 | 84 ± 8 | 80 ± 11 |
| 6 | 59 ± 1 | 47 ± 1 | 61 ± 0.1 | 51 ± 0.7 | ND | ND |
| 7 | 79 ± 1 | 37 ± 7 | 71 ± 3 | 57 ± 17 | 77 ± 0.4 | 29 ± 2 |
| 9 | 68 ± 0.8 | 25 ± 3 | 66 ± 4 | 54 ± 30 | 82 ± 0.1 | 46 ± 1 |
| 10 | 72 ± 6 | 66 ± 11 | 66 ± 2 | 67 ± 5 | 69 ± 7 | 65 ± 9 |
| 11 | 34 ± 2 | 23 ± 4 | 53 ± 2 | 31 ± 3 | ND | ND |
| 12 | 48 ± 9 | 15 ± 8 | 71 ± 4 | 52 ± 7 | 71 ± 2 | 24 ± 2 |
| Mean ± SD | 60 ± 18 | 39 ± 19* | 67 ± 11 | 56 ± 15† | 70 ± 14 | 46 ± 20‡ |
B-CLL cells were incubated for 48 hours in medium alone (control), with 10 nM TPA or 10 ng/mL IL-4, in the absence or presence of 5 μM BisI. Cell viability was determined by flow cytometry. Data are shown as the mean ± SD of 2 independent experiments performed in duplicate.
ND indicates not determined.
P < .005 versus control.
P < .005 versus TPA.
P < .005 versus IL-4.
Analysis of PKC activity in B-CLL cells.
(A) Membrane-associated PKC activity in particulate fraction. B-CLL cells were incubated for 10 minutes in the presence of 10 nM TPA for 20 minutes in the presence of 10 ng/mL IL-4 or in medium alone. After that time, cells were harvested and lysed, and the distribution of the enzyme between cytosolic fraction and particulate fraction was evaluated. PKC activity analysis was performed as described in “Patients, materials, and methods.” The cell extract was preincubated with 1 μM BisI for 20 minutes at room temperature before reaction was initiated. Values are mean ± SD for 6 different patients. Results are shown as the percentage of PKC activity present in the particulate fraction with respect to total PKC activity (cytosolic fraction plus particulate fraction). (B) Phosphorylation of PKC substrates in intact B-CLL cells. B-CLL cells were labeled with32P as described in “Patients, materials, and methods,” in the absence or the presence of 5 μM BisI. Then cells were incubated with 10 nM TPA or 10 ng/mL IL-4 for 10 and 20 minutes, respectively. The figure shows the results corresponding to 1 representative patient from 4 patients analyzed who had similar results.
Analysis of PKC activity in B-CLL cells.
(A) Membrane-associated PKC activity in particulate fraction. B-CLL cells were incubated for 10 minutes in the presence of 10 nM TPA for 20 minutes in the presence of 10 ng/mL IL-4 or in medium alone. After that time, cells were harvested and lysed, and the distribution of the enzyme between cytosolic fraction and particulate fraction was evaluated. PKC activity analysis was performed as described in “Patients, materials, and methods.” The cell extract was preincubated with 1 μM BisI for 20 minutes at room temperature before reaction was initiated. Values are mean ± SD for 6 different patients. Results are shown as the percentage of PKC activity present in the particulate fraction with respect to total PKC activity (cytosolic fraction plus particulate fraction). (B) Phosphorylation of PKC substrates in intact B-CLL cells. B-CLL cells were labeled with32P as described in “Patients, materials, and methods,” in the absence or the presence of 5 μM BisI. Then cells were incubated with 10 nM TPA or 10 ng/mL IL-4 for 10 and 20 minutes, respectively. The figure shows the results corresponding to 1 representative patient from 4 patients analyzed who had similar results.
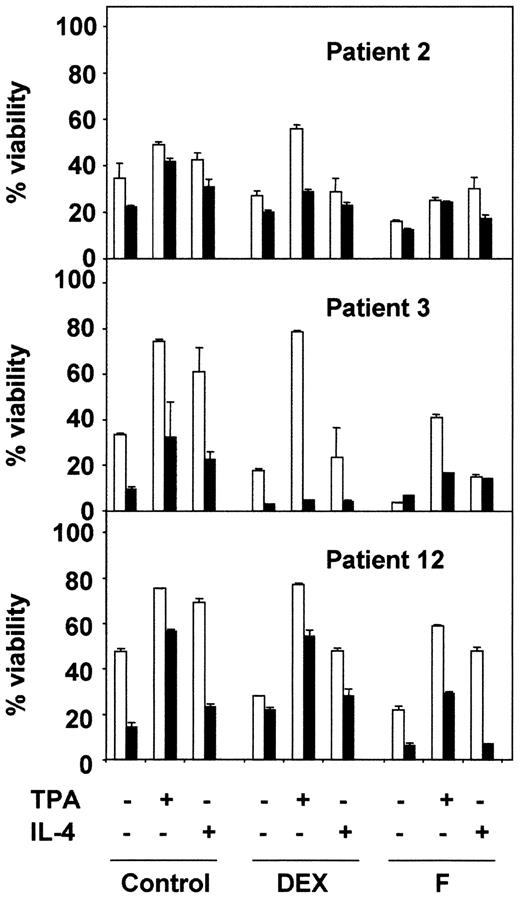
Next, we analyzed the effect of BisI on the survival effect of TPA and IL-4 in combination with 2 drugs used in the chemotherapy of B-CLL: dexamethasone (DEX) and fludarabine (F). The results shown in Figure2 correspond to 3 representative patients from 10 analyzed. BisI slightly increased the dexamethasone- or fludarabine-induced apoptosis of B-CLL cells, and it inhibited the protective effect of TPA and IL-4. Taken together, these results demonstrate that PKC activity is involved in the survival of B-CLL lymphocytes.
PKC inhibition blocks the antiapoptotic effect of TPA and IL-4.
B-CLL cells from 3 representative patients were incubated for 1 hour in the presence (▪) or the absence (■) of 5 μM BisI. After that time, drugs were added to the culture, incubation continued for another 48 hours. At the end of the culture, cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
PKC inhibition blocks the antiapoptotic effect of TPA and IL-4.
B-CLL cells from 3 representative patients were incubated for 1 hour in the presence (▪) or the absence (■) of 5 μM BisI. After that time, drugs were added to the culture, incubation continued for another 48 hours. At the end of the culture, cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
Analysis of ERK signaling cascades in the survival of B-CLL cells
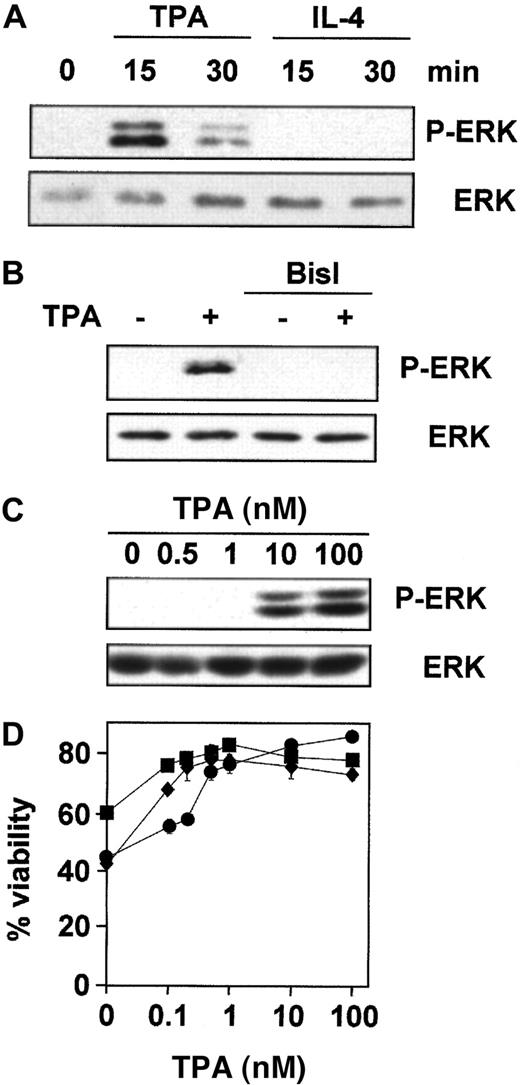
ERK is involved in the inhibition of apoptosis in response to survival factors in various cell types.22 40 Thus, we examined the role of ERK in TPA- and IL-4–mediated survival of B-CLL cells. We first studied whether TPA and IL-4 induced the activation of ERK using specific antibodies against its phosphorylated form. ERK was phosphorylated after 15 minutes of incubation with TPA in the 7 patients analyzed (Figure 3A), and this effect was dependent on PKC activation because it was blocked by preincubation with BisI (Figure 3B). In contrast, IL-4 did not induce ERK-phosphorylation in the 7 patients analyzed. Then we analyzed the correlation between the effect of TPA on ERK phosphorylation and B-CLL survival. The dose-response for TPA-induced ERK phosphorylation showed that the phosphorylated forms of ERK were detected when cells were incubated with 10 nM TPA in the 4 patients analyzed (Figure 3C). In contrast, the protective effect of TPA on dexamethasone-induced apoptosis reached plateau levels at 1 nM (Figure 3D). Similar results were obtained on spontaneous and fludarabine-induced apoptosis (data not shown). Thus, much higher doses of TPA were needed to induce ERK phosphorylation than for survival of B-CLL cells.
TPA, but not IL-4, induces PKC-dependent phosphorylation of ERK.
Purified peripheral blood B-CLL cells from patient 1 were incubated (A) for different times with 100 nM TPA and 10 ng/mL IL-4, (B) for 20 minutes with 100 nM TPA after preincubation for 1 hour with 5 μM BisI, and (C) with different concentrations of TPA for 20 minutes. Then cells were harvested and lysed, and phosphorylated forms of ERK (P-ERK) were analyzed by Western blot. (D) Cells from patients 1 (●), 2 (■), and 3 (⧫) were incubated for 48 hours with different doses of TPA in the presence of 10 μM dexamethasone, and cell viability was determined by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
TPA, but not IL-4, induces PKC-dependent phosphorylation of ERK.
Purified peripheral blood B-CLL cells from patient 1 were incubated (A) for different times with 100 nM TPA and 10 ng/mL IL-4, (B) for 20 minutes with 100 nM TPA after preincubation for 1 hour with 5 μM BisI, and (C) with different concentrations of TPA for 20 minutes. Then cells were harvested and lysed, and phosphorylated forms of ERK (P-ERK) were analyzed by Western blot. (D) Cells from patients 1 (●), 2 (■), and 3 (⧫) were incubated for 48 hours with different doses of TPA in the presence of 10 μM dexamethasone, and cell viability was determined by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
To further investigate whether the ERK cascade plays a role in TPA-induced B-CLL lymphocyte survival, we used a specific inhibitor of MEK (mitogen of extracellular regulated kinase) activation, U0126,41 42 which at a dose of 1 μM inhibited ERK activation (Figure 4A) but did not alter the survival effect of TPA (Figure 4B). Similar results were obtained using PD98059, a structurally unrelated inhibitor of ERK cascade. As expected, neither U0126 nor PD98059 inhibited IL-4–induced survival in the 7 patients analyzed (data not shown). Taken together, these results indicate that ERK is not involved in the antiapoptotic effect of TPA and IL-4 in B-CLL cells.
Inhibition of MEK/ERK-pathway did not affect the protective effect of TPA.
(A) B-CLL cells from 4 representative patients were incubated with or without 1 μM U0126 for 1 hour before the addition of 10 nM TPA for 20 minutes. ERK phosphorylation was analyzed by Western blot. (B) B-CLL cells were incubated for 1 hour in the presence (▪) or absence (■) of 1 μM U0126. After that time, drugs were added to the culture, and incubation continued for another 48 hours. At the end of the culture, cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD of 7 patients analyzed.
Inhibition of MEK/ERK-pathway did not affect the protective effect of TPA.
(A) B-CLL cells from 4 representative patients were incubated with or without 1 μM U0126 for 1 hour before the addition of 10 nM TPA for 20 minutes. ERK phosphorylation was analyzed by Western blot. (B) B-CLL cells were incubated for 1 hour in the presence (▪) or absence (■) of 1 μM U0126. After that time, drugs were added to the culture, and incubation continued for another 48 hours. At the end of the culture, cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD of 7 patients analyzed.
PI3-kinase pathway is involved in the survival of B-CLL cells
PI3-kinase plays an important role in the suppression of apoptosis in many cell types.23 To study the role of PI3-kinase in the survival of B-CLL cells, we used LY294002, a specific inhibitor of this kinase.43 B-CLL cells from 8 patients were cultured for 48 hours with medium alone (control), TPA, or IL-4 in the presence or absence of 20 μM LY294002, a concentration that inhibits PI3-kinase,42 and the percentage of nonapoptotic cells was analyzed by flow cytometry. LY294002 triggered apoptosis in B-CLL lymphocytes in all the patients studied except for patient 8 (Table2). In most cases, the viability of cells treated with TPA or IL-4 in the presence of LY294002 was higher than that corresponding to cells incubated with LY294002 alone, but it was lower than the viability of cells incubated with TPA or IL-4, except in patient 8, in whom viability in the presence of these factors was not decreased by LY294002, and in patient 1, in whom viability in the presence of IL-4 was not affected by LY294002 (Table 2).
Effect of LY294002 on survival of B-CLL lymphocytes
| Patient . | Viability (%) . | |||||
|---|---|---|---|---|---|---|
| Control . | LY294002 . | TPA . | LY294002 + TPA . | IL-4 . | LY294002 + IL-4 . | |
| 1 | 46 ± 7 | 22 ± 2 | 64 ± 12 | 38 ± 14 | 77 ± 1 | 66 ± 1 |
| 2 | 50 ± 11 | 20 ± 8 | 54 ± 17 | 34 ± 9 | 35 ± 1 | 26 ± 0.4 |
| 3 | 49 ± 12 | 12 ± 5 | 77 ± 2 | 42 ± 11 | 62 ± 2 | 32 ± 19 |
| 6 | 70 ± 1 | 35 ± 2 | 75 ± 0.3 | 45 ± 0.5 | 78 ± 1 | 60 ± 1 |
| 8 | 72 ± 1 | 60 ± 15 | 69 ± 1 | 57 ± 15 | 78 ± 1 | 70 ± 0.3 |
| 9 | 75 ± 6 | 35 ± 20 | 72 ± 6 | 67 ± 7 | 80 ± 3 | 67 ± 10 |
| 10 | 76 ± 11 | 35 ± 12 | 72 ± 10 | 49 ± 4 | 72 ± 10 | 49 ± 20 |
| 12 | 43 ± 6 | 5 ± 0.4 | 72 ± 4 | 45 ± 0.3 | 69 ± 2 | 24 ± 2 |
| Mean ± SD | 60 ± 5 | 28 ± 6* | 69 ± 3 | 47 ± 4† | 69 ± 5 | 49 ± 7‡ |
| Patient . | Viability (%) . | |||||
|---|---|---|---|---|---|---|
| Control . | LY294002 . | TPA . | LY294002 + TPA . | IL-4 . | LY294002 + IL-4 . | |
| 1 | 46 ± 7 | 22 ± 2 | 64 ± 12 | 38 ± 14 | 77 ± 1 | 66 ± 1 |
| 2 | 50 ± 11 | 20 ± 8 | 54 ± 17 | 34 ± 9 | 35 ± 1 | 26 ± 0.4 |
| 3 | 49 ± 12 | 12 ± 5 | 77 ± 2 | 42 ± 11 | 62 ± 2 | 32 ± 19 |
| 6 | 70 ± 1 | 35 ± 2 | 75 ± 0.3 | 45 ± 0.5 | 78 ± 1 | 60 ± 1 |
| 8 | 72 ± 1 | 60 ± 15 | 69 ± 1 | 57 ± 15 | 78 ± 1 | 70 ± 0.3 |
| 9 | 75 ± 6 | 35 ± 20 | 72 ± 6 | 67 ± 7 | 80 ± 3 | 67 ± 10 |
| 10 | 76 ± 11 | 35 ± 12 | 72 ± 10 | 49 ± 4 | 72 ± 10 | 49 ± 20 |
| 12 | 43 ± 6 | 5 ± 0.4 | 72 ± 4 | 45 ± 0.3 | 69 ± 2 | 24 ± 2 |
| Mean ± SD | 60 ± 5 | 28 ± 6* | 69 ± 3 | 47 ± 4† | 69 ± 5 | 49 ± 7‡ |
B-CLL cells were incubated for 48 hours in medium alone (control), with 10 nM TPA or 10 ng/mL IL-4, in the absence or presence of 20 μM LY294002. Cell viability was determined by flow cytometry. Data are shown as the mean ± SD of 2 or 3 independent experiments performed in duplicate.
P < .005 versus control.
P < .005 versus TPA.
P < .01 versus IL-4.
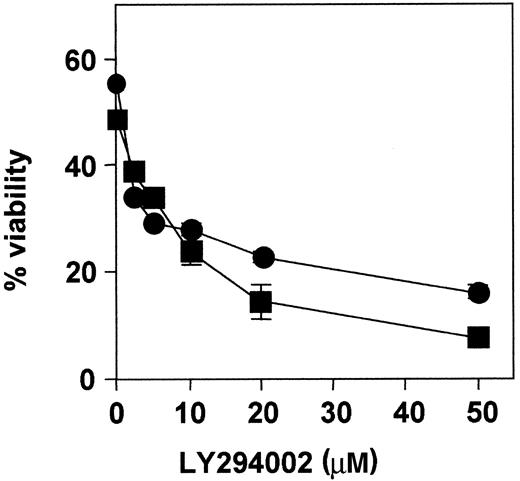
In addition, dose-response experiments were performed in cells from patients 1 and 3 (Figure 5). Cells were incubated with increasing concentrations of LY294002, ranging from 2 to 50 μM for 48 hours. Cell cytotoxicity was dose-dependent, and the IC50 was approximately 3 μM and 6 μM, respectively. Analysis of caspase-3 activation and PARP cleavage by Western blot showed that LY294002 activated caspase-3 in B-CLL cells (data not shown).
Dose-response effect of LY294002.
B-CLL cells from patients 1 (●) and 3 (■) were incubated with increasing doses of LY294002 for 48 hours, and cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
Dose-response effect of LY294002.
B-CLL cells from patients 1 (●) and 3 (■) were incubated with increasing doses of LY294002 for 48 hours, and cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
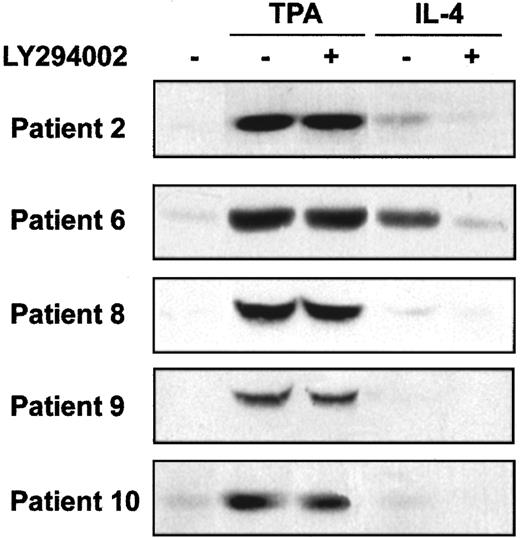
PI3-kinase activates several downstream signal transduction pathways, including Akt and p70S6-kinase. We analyzed the involvement of these kinases on the survival of B-CLL cells. First, we studied Akt phosphorylation in B-CLL cells and whether TPA and IL-4 induced its phosphorylation. Akt phosphorylation was analyzed in samples obtained from 8 patients. In all patients, phosphorylated Akt was not detected in nonstimulated B-CLL cells. TPA (8 of 8 patients) and IL-4 (4 of 8 patients) induced Akt phosphorylation. Five representative cases are shown in Figure 6. Furthermore, the inhibition of PI3-kinase by LY294002 blocked the effect of IL-4 but did not alter the effect TPA. Rapamycin, a specific inhibitor of the activation of p70S6K, had no effect on the antiapoptotic activity of TPA or IL-4 (data not shown), indicating that p70S6K and rapamycin-dependent pathways are not involved in the survival of B-CLL cells.
Effect of TPA and IL-4 on Akt phosphorylation.
Cells from 5 representative patients were incubated with 20 μM LY294002 for 1 hour before the addition of 10 nM TPA or 10 ng/mL IL-4 to the culture. Akt phosphorylation in Ser473 was analyzed by Western blot 20 minutes after the addition of drugs.
Effect of TPA and IL-4 on Akt phosphorylation.
Cells from 5 representative patients were incubated with 20 μM LY294002 for 1 hour before the addition of 10 nM TPA or 10 ng/mL IL-4 to the culture. Akt phosphorylation in Ser473 was analyzed by Western blot 20 minutes after the addition of drugs.
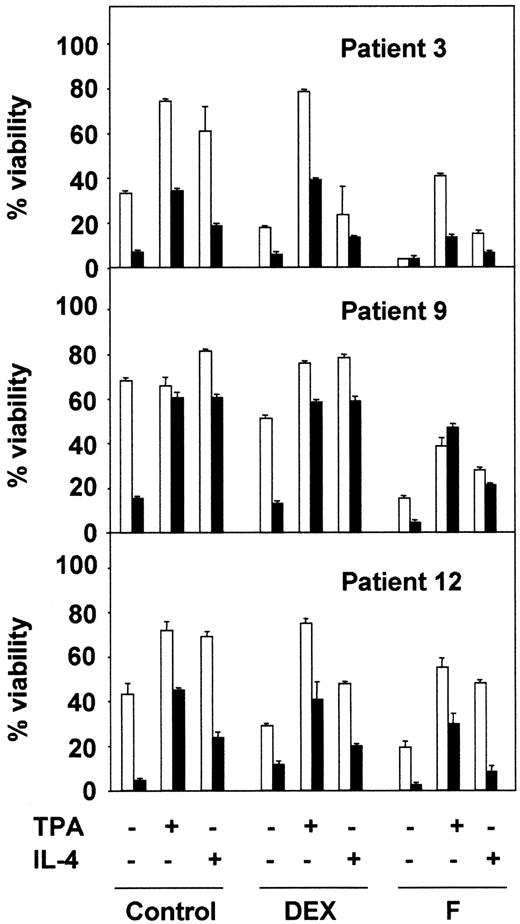
Next, we analyzed the effect of PI3-kinase inhibition on chemotherapy-induced apoptosis of B-CLL cells. LY294002 had an additive effect on the apoptotic activity of fludarabine in 3 of the 4 patients analyzed (Figure 7). However, the inhibition of PI3-kinase had no effect on dexamethasone-induced apoptosis (8 of 8 patients analyzed; data not shown). LY294002 also reduced the viability of cells treated with dexamethasone or fludarabine, in the presence of TPA or IL-4, except in patient 9, in whom TPA blocked the apoptosis induced by LY294002 (Figure 7). The effect of LY294002 was more pronounced for IL-4–induced survival than for the antiapoptotic effect of TPA in most of the patients analyzed.
Effect of LY294002 on TPA- and IL-4–induced survival on B-CLL lymphocytes.
B-CLL lymphocytes from 3 representative patients were preincubated in the presence (▪) or absence (■) of 20 μM LY294002 for 1 hour before the addition of drugs. After 48 hours of cell culture, cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
Effect of LY294002 on TPA- and IL-4–induced survival on B-CLL lymphocytes.
B-CLL lymphocytes from 3 representative patients were preincubated in the presence (▪) or absence (■) of 20 μM LY294002 for 1 hour before the addition of drugs. After 48 hours of cell culture, cell viability was analyzed by flow cytometry. Results are represented as the mean ± SD from one experiment performed in duplicate.
Discussion
We examined the signal transduction pathways involved in the survival of B-CLL cells. Two of the most studied survival factors for B-CLL cells are TPA17,21,25-29 and IL-4.6,7,13 17-19 We used specific inhibitors of kinases involved in the control of apoptosis to analyze their role in the survival of B-CLL cells.
The PKC inhibitor BisI induced apoptosis in B-CLL cells. It has been reported that UCN-01, another PKC inhibitor, induces apoptosis in these cells.44 These findings are consistent with the observation that B-CLL cells have a basal level of PKC activity. In addition, the survival activity of TPA depends on PKC activity because BisI inhibits its antiapoptotic effect. Although IL-4 did not activate PKC, BisI inhibited its survival effect, indicating that the basal PKC activity present in B-CLL cells is necessary for the protective effect of IL-4. The downstream elements of PKC-mediated survival pathway in B-CLL cells may involve activation of the transcription factor NF-κB45 and increased expression of the antiapoptotic genes Bcl-XL, Mcl-1, andXIAP.29
TPA, but not IL-4, activated ERK in B-CLL cells. This activation was mediated by PKC, because it was completely blocked by BisI. However, the inhibition of ERK did not affect the survival effect of TPA. The activation of this protein kinase may have other roles in B-CLL lymphocytes. For instance, it may be involved in TPA-induced differentiation.46 The inability of IL-4 to induce ERK activation has been reported in other hematopoietic cells, and it could be attributed to the fact that IL-4 does not activate the Ras pathway in these cells.47
Interestingly, the PI3-kinase–specific inhibitor LY294002 induced apoptosis in B-CLL cells. In agreement with these results B-CLL cells have basal PI3-kinase activity (manuscript in preparation). Furthermore, the apoptotic effect of LY294002 was not completely prevented by IL-4 and TPA in many patients with B-CLL. PI3-kinase activity is important in IL-4–induced survival, which was almost blocked by LY294002 in most of the patients analyzed. Our results indicate that PI3-kinase is an important survival pathway for B-CLL cells, and they are consistent with the finding that peritoneal CD5+ B cells from mice with targeted disruption of p85α, a regulatory subunit of PI3-kinase, show decreased survival after incubation with IL-4.48,49 Several cytokines that induce survival of B-CLL cells, including IL-4, IL-2, tumor necrosis factor-α, IL-6, and IL-13, induce the activation of PI3-kinase in B lymphocytes and other cell types.50-53 Thus, the PI3-kinase pathway could contribute to the survival mediated by these cytokines in B-CLL cells. The antiapoptotic effect of TPA was not impaired in some cases by LY294002, suggesting that PKC may act in a parallel pathway or may be a downstream effector of PI3-kinase, as described in other cells.54
One downstream effector of PI3-kinase is Akt/PKB, which is a major participant in cell survival.23 Akt phosphorylates and inactivates at least 2 proteins involved directly in the control of apoptosis, Bad55,56 and Caspase-9.57 Bad is not expressed in most B-CLL samples,58 and the phosphorylation of caspase-9 has not been analyzed in B-CLL cells. In addition to inactivating apoptosis-regulating proteins, Akt may control the expression of genes involved in cell survival. Thus, Akt phosphorylates 3 members of the Forkhead family of transcription factors,23 which are retained in the cytoplasm, thus impairing the transcription of proapoptotic genes such asFasL59 and Bim.60 We found that TPA and IL-4 induce phosphorylation of Akt in B-CLL cells. Although TPA induced Akt phosphorylation, this effect was independent of PI3-kinase. This suggests that PI3-kinase–dependent activation of Akt does not contribute to PKC-induced survival in B-CLL cells. Thus, we propose that the effect of LY294002 in basal conditions or in cells stimulated with TPA was caused by the inhibition of PI3-kinase and a downstream PI3-kinase–dependent signaling pathway but that it was likely independent of Akt activity. However, we cannot rule out the involvement of Akt in TPA-induced B-CLL cell survival. IL-4 induced Akt phosphorylation in 50% of the cases analyzed, and this phosphorylation depends on PI3-kinase, suggesting that this is one of the mechanisms by which the activation of PI3-kinase induces survival of these cells, as described in many other systems.23 In addition, several protein kinases downstream of PI3-kinase involved in cell survival have been described, including several isotypes of PKC54 and CISK/SGK3.61 The role of these pathways in the survival of B-CLL cells will be analyzed in the future.
In conclusion, our results demonstrate that PI3-kinase and PKC play important roles in the survival of B-CLL cells. Because inhibition of these kinases increases chemotherapy-induced apoptosis ex vivo in the presence of survival factors, this finding could contribute to the design of new therapeutic strategies. For example, combinations of drugs used in the treatment of B-CLL with inhibitors of PKC or PI3-kinase should be considered. Finally, the elucidation of the downstream components of the PI3-kinase and PKC pathways in the survival of B-CLL cells may suggest new pharmacologic targets for B-CLL therapy.
We thank Dra M. Dalmau for technical support in flow cytometry analysis. We also thank Dra E. Castaño, Dra M. Piqué, Dr F. Vinyals, Dr J. M. López-Blanco, D. Iglesias, and Dra N. Villamor for comments, helpful discussions, and suggestions, and R. Rycroft for language assistance.
Supported by grants from the Ministerio de Ciencia y Tecnologı́a (SAF 98-0100 and SAF2001-3026) (J.G.). M.B., B.B., and C.C. are recipients of a research fellowship from the Ministerio de Ciencia y Tecnologı́a, the Instituto de Salud Carlos III, and the Fundación Ramón Areces, respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joan Gil, Departament de Ciències Fisiològiques II, Campus de Bellvitge, Universitat de Barcelona, c/Feixa Llarga s/n, E-08907 L'Hospitalet de Llobregat, Spain; e-mail:joangil@bellvitge.bvg.ub.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal