Abstract
In this study, the finding that a significant proportion of all dendritic cells (DCs) resident in vivo in the human postnatal thymus displayed a myeloid-related phenotype prompted us to re-examine the developmental origin of thymic DCs, a cell type hitherto considered to represent a homogeneous lymphoid-derived population. We show here that these novel intrathymic DCs are truly myeloid, as they arise from CD34+ early thymic progenitors through CD34lointermediates which have lost the capacity to generate T cells, but display myelomonocytic differentiation potential. We also demonstrate that phenotypically and functionally equivalent myeloid precursors devoid of T-cell potential do exist in vivo in the postnatal thymus. Moreover, although interleukin 7 (IL-7) supports the generation of such myeloid intermediates, we show that their developmental branching from the main intrathymic T-cell pathway is linked to the up-regulation of the myelomonocytic granulocyte macrophage–colony-stimulating factor (GM-CSF) receptor, to the down-regulation of the IL-7 receptor and to the lack of pre–T-cell receptor α (pTα) gene transcriptional activation. Taken together, these data challenge the current view that the thymus is colonized by a lymphoid-restricted progenitor and provide evidence that a more immature precursor population with lymphoid and myelomonocytic potential is actually seeding the human postnatal thymus.
Introduction
Dendritic cells (DCs) are hematopoietic-derived highly specialized antigen-presenting cells (APCs) that display potent ability to induce both specific immune responses and deletion of potentially autoreactive T cells.1-3 These nonoverlapping functions have been proposed to result from the actions of 2 major DC populations which have been characterized as myeloid and lymphoid DCs, respectively, on the basis of their anatomical localization and cell-surface phenotypes and, ultimately, of their distinct developmental origin.1-5 In mice, DCs bearing the myeloid marker CD11b (Mac-1), but lacking CD8α, derive efficiently from myeloid precursors and represent the major DC subset in the periphery.6,7 Murine DCs with the reciprocal CD8α+CD11b− phenotype, although present at various levels in all lymphoid organs, represent the prototype of the DC population resident in the postnatal thymus.8 As these latter DCs derive from an intrathymic lymphoid-restricted progenitor able to generate also T, B, and NK cells, but not myeloid cells, upon transfer in vivo,5,9,10 they have hitherto been considered of lymphoid origin. This proposal is further supported by the finding that development of thymic DCs and T cells is linked via a common precursor at an early stage of thymocyte development.5 11
There are 2 subsets of DCs with distinct phenotype, localization, and function which have been described also in humans.12,13One subset of CD11c+ DCs, also termed DC1, which expresses myeloid markers and high levels of CD1a, can be generated from peripheral blood monocytes in response to granulocyte macrophage–colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4)14,15 and therefore is considered of myeloid origin. DCs with a DC1-like phenotype can also develop in response to GM-CSF and tumor necrosis factor alpha (TNF-α) from cord blood CD34+ stem cells along 2 independent pathways which involve separate intermediate DC progenitors: either myelomonocytic CD14+ precursors or CD1a+ CD14−precursors.16
A second subset of CD11c− DCs, also termed DC2, has recently been shown to correspond to the enigmatic population of “plasmacytoid” T cells.17 DC2 cells lack myeloid markers and are characterized by a unique CD4+CD1a− CD45RA+ phenotype, with high expression levels of IL-3Rα, but a sparse expression of GM-CSF receptor (GM-CSFR). Unlike DC1, DC2 generation from CD34+hematopoietic precursors does not require the myelomonocytic cytokine GM-CSF.18,19 Interestingly, DCs equivalent to peripheral DC2 have been identify by Spits and coworkers in the human thymus, and were shown to express significant transcriptional levels of the pre–T-cell receptor α (pTα) gene,20 a finding that supports their developmental linkage to T cells, as proposed for CD8α+ thymic DCs in mice,11 and, eventually, their lymphoid origin. Accordingly, the development of DC2 from human CD34+ hematopoietic precursors can be blocked by ectopic expression of inhibitor of DNA binding (Id)2 or Id3 proteins, which also blocks the development of T and B lymphocytes, but not of myeloid cells and DC1.21
Despite the proposed lymphoid origin of intrathymic DCs, our knowledge about their immediate precursors in humans is limited. We have previously reported that IL-7, a conventional “lymphoid” cytokine, is able to support the generation in vitro of mature functional DCs from the earliest CD34+ intrathymic precursors, which display both T cell and natural killer (NK) cell precursor potential as well.22-25 Such thymocyte-derived DCs were shown to develop through a predominant CD1a+pathway22-24 equivalent to that reported for DCs derived from a common T/NK/DC lymphoid precursor (CLP) resident in bone marrow,26 a fact that further supports their lymphoid origin. However, regardless of their thymic origin, in vitro–derived DCs displayed a myeloid-related phenotype similar to that of peripheral DC1, but fully distinct from that of the recently identified intrathymic DC2 subset. Whereas the myeloidlike phenotype displayed by in vitro–derived thymic DCs could be attributed to the particular differentiation/cytokine assay used, evidence has recently been provided that, besides DC2, DCs bearing a myeloid phenotype similar to that of peripheral DC1 can be found in the human thymus.27 28 However, the ontogeny of such DCs remains to be clarified. Therefore, the aim of the present study has been to re-examine the developmental origin of human intrathymic DCs. Based on precursor-product relationships, we have characterized the intrathymic differentiation pathway of these novel myeloid-related DCs and provided evidence that they are authentic myeloid DCs in developmental terms. The possible meaning of the existence of early intrathymic progenitors able to develop through a myeloid DC pathway is discussed in the context of the current view that the thymus is seeded by a lymphoid-restricted progenitor derived from the bone marrow.
Materials and methods
Flow cytometry
Fluorescein isothiocyanate (FITC)–labeled monoclonal antibodies (MoAbs) against human CD7, CD8, CD11b, CD11c, CD19, CD40, CD44, and human leukocyte antigen (HLA)–DR were purchased from Caltag Laboratories (South San Francisco, CA); CD14 from BD Biosciences (Erembodegem, Belgium); CD86 from Serotec (Kidlington, Oxford, United Kingdom); TCRγδ and TCRαβ from Endogen (Woburn, MA); and CD1c from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). Phycoerythrin (PE)–labeled MoAbs against CD3, CD5, CD13, CD33, CD34, CD56, and IL-3Rα were from BD Biosciences; CD8, CD11c, CD45RA, CD45RO, CD83, and HLA-DR were from Caltag; CD1a, CD2, and GM-CSFRα were from Beckman Coulter (Marseille, France). PE-Cy5–labeled MoAbs against CD4, CD13, and CD56 were from Caltag; and CD34 was from Beckman Coulter. Unlabeled MoAbs against CD3, CD14, CD19, CD56, and CD54 were from BD Biosciences, and anti-CD80 was from Caltag. FITC-, PE-, or PE-Cy5–conjugated goat anti–mouse F(ab2)' IgGs were from Caltag. Single-, 2-, or 3-color stained cells were analyzed in a flow cytometer (EPICS Profile; Coulter Electronics, Hialeah, FL). Data were collected on 1 × 104 to 3 × 104 viable cells. Isotype-matched irrelevant antibodies from Caltag were used as controls to define background fluorescence.
Isolation of thymocyte subsets and cell sorting
Human postnatal thymocytes were isolated from thymus fragments removed during corrective cardiac surgery of patients aged 1 month to 4 years after informed consent was provided according to the Declaration of Helsinki. After Lymphoprep (Nycomed Pharma, Oslo, Norway) centrifugation, recovered viable thymocytes (hereafter referred to as total thymocytes) were depleted of monocytes, T, B, and NK cells (Lin+) by MoAb-coupled magnetic bead treatment (Dynabeads; Dynal, Oslo, Norway) as described.23CD34hiCD33lo and CD34hiCD33− progenitors (up to 0.04% and 0.15% of total thymocytes, respectively23) were then isolated from the recovered Lin− population by cell sorting with a FACStar plus (BD Biosciences).23 Primary CD34loCD44hi (CD5−/lo) and CD34loCD44lo (CD5+) thymocytes were isolated from Lin− thymocytes by fluorescence activated cell sorting (FACS) after anti-CD44–FITC and anti-CD34–PE-Cy5 labeling. The equivalent populations generated in vitro from CD34+CD33lo progenitors were sorted by FACS from the CD34lo (> 98%) progeny recovered by day 3.5 in multicytokine-supported cultures (see below), after labeling with anti-CD44–FITC and anti-CD5–PE. Sorted cells were more than or equal to 98% pure as determined by postsort analysis.
For the isolation of thymic DCs, Lin− thymocytes were labeled with anti-HLA-DR–FITC, and incubated with anti-FITC Multisort MicroBeads, and HLA-DR+ cells were then selected using an autoMACS magnetic cell sorter (Miltenyi Biotec). Sorted cells (> 98% pure) were then labeled with anti-IL-3Rα–PE, and IL-3Rα+ cells were sorted with anti-PE MicroBeads. CD13+ DCs were sorted from the IL-3Rα− cell fraction with anti-CD13–PE and anti-PE MicroBeads. For quantitative analysis on the relative contribution of CD13−IL-3Rα+ and CD13+IL-3Rα− DC subsets in vivo, HLA-DR+ cells were directly sorted from total thymocytes, and 3-color flow cytometry analyses were then performed on electronically gated large Lin− HLA-DR+ cells (0.2% of total thymocytes).
CD14+ and CD14− cells derived in vitro from CD34hiCD33lo intrathymic precursors were autoMACS sorted after labeling with anti-CD14–PE and anti-PE MicroBeads. Both FACS and magnetic cell sorting procedures were performed at 0°C to 4°C in phosphate-buffered saline (PBS) containing 5 mM ethylenediaminetetraacetic acid (EDTA) and 0.5% bovine serum albumin (BSA) to disrupt cell complexes.
Cell cultures
DCs were generated either from CD34+CD33lo early thymic progenitors or from CD34loCD44hi and CD34loCD44lo intermediates cultured (5 × 105/mL) for the indicated time periods in 24-well (5 × 105 cells/well) or 96-well (105cells/well) plates (Costar, Cambridge, MA) in RPMI 1640 medium (Gibco, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS) (Gibco) and the following cytokines: 100 IU/mL recombinant human interleukin-7 (rhIL-7), 60 IU/mL rhIL-1α, 50 IU/mL rhIL-6, 100 IU/mL recombinant human stem cell factor (rhSCF), and 100 IU/mL recombinant human GM-CSF (rhGM-CSF) from the National Institute of Biological Standards and Controls (NIBSC, Potters Bar, Hertfordshire, United Kingdom). These cultures are referred to as multicytokine-supported cultures throughout the text. For the simultaneous generation of NK cells and DCs, these cultures were supplemented with 50 IU/mL rhIL-2 (Hoffman La Roche, Basel, Switzerland).
To assess the generation CD14+CD1a−myelomonocytes, progenitor cells (5 × 105/mL) were set up in cultures containing 100 IU/mL rhIL-7, 100 IU/mL rhSCF, and 250 IU/mL rhM-CSF (NIBSC), and analyzed by flow cytometry after 10 to 15 days. Generation of T lymphocytes was assessed in a hybrid human/mouse fetal thymic organ culture (hu/moFTOC) system, as previously described.29
Real-time quantitative polymerase chain reaction
Total RNA from thymocyte subsets was isolated using standard procedures. We used 10 ng to 50 ng of RNA per polymerase chain reaction (PCR) after reverse transcription into cDNA with an oligo-dT primer (Gibco). Real-time quantitative reverse transcriptase–PCR (RT-PCR) was carried out with a Taqman assay. The primers and Taqman probe for RT-PCR were designed using Primer Express software (Applied Biosystems, Foster City, CA). The sense 5′-GTGTCCAGCCCTACCCAC-3′ and antisense 5′-ATCCACCAGCAGCATGATTG-3′ primers were used in combination with the 5′-TGTGGGCGGCACACCCTTTC-3′ Taqman (6-FAM–labeled) probe (Applied Biosystems). Primers and probes were used at a final concentration of 300 nM and 200 nM, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplifications were carried out with the Pre-Developed Taqman Assay Reagent specific for human GAPDH gene expression quantification (Applied Biosystems), according to the manufacturer's instructions. All PCR reactions were set in triplicates using the TaqMan Universal PCR Master Mix (Applied Biosystems). Amplifications, detections, and analyses were performed in an ABI PRISM 7700 system (Applied Biosystems). pTα quantitative values were obtained after interpolation in a standard curve constructed by amplification of serial dilutions of total human thymocyte cDNA, followed by normalization to GAPDH.
Results
DCs with a myeloidlike phenotype are resident in vivo in the human postnatal thymus
We have previously shown that the earliest CD34hiCD33lo intrathymic progenitors in man can generate mature functional DCs in multicytokine-supported cultures including IL-7, IL-1α, SCF, IL-6, and GM-CSF.23 As summarized in Table 1 and Figure1A, an extensive phenotypic analysis of such in vitro–derived DCs revealed a prominent expression of the myeloid-related antigens CD11b, CD11c, CD13, and CD33, as well as high expression of several markers characteristic of peripheral DC1 but absent on DC2, such as CD1a, CD1c, and CD45RO; but a sparse expression of the IL-3 receptor (IL-3Rα, CD123) expressed on DC2. In addition, we found high expression of CD44, CD54, major histocompatibility complex (MHC) class II (HLA-DR), and CD4, and variable surface levels of CD40, CD80, and CD86 costimulatory markers and the mature DC marker CD83. Therefore, these in vitro–derived DCs displayed a surface phenotype that seemed closer to that of myeloid-derived DC1 than to lymphoid-derived DC2.
Cell-surface markers expressed by dendritic cells generated in vitro from CD34+ human intrathymic precursors
| CD1a | +++ |
| CD1c | + |
| CD4 | ++ |
| CD5 | − |
| CD7 | +/− |
| CD8 | − |
| CD11b | ++ |
| CD11c | ++ |
| CD13 | +++ |
| CD14 | +/− |
| CD33 | ++ |
| CD40 | ++ |
| CD44 | ++ |
| CD45RA | + |
| CD45RO | ++ |
| CD54 | +++ |
| CD80 | + |
| CD83 | ++ |
| CD86 | + |
| CD116 (GM-CSFR) | + |
| CD123 (IL-3Rα) | +/− |
| HLA-DR | +++ |
| CD1a | +++ |
| CD1c | + |
| CD4 | ++ |
| CD5 | − |
| CD7 | +/− |
| CD8 | − |
| CD11b | ++ |
| CD11c | ++ |
| CD13 | +++ |
| CD14 | +/− |
| CD33 | ++ |
| CD40 | ++ |
| CD44 | ++ |
| CD45RA | + |
| CD45RO | ++ |
| CD54 | +++ |
| CD80 | + |
| CD83 | ++ |
| CD86 | + |
| CD116 (GM-CSFR) | + |
| CD123 (IL-3Rα) | +/− |
| HLA-DR | +++ |
Flow cytometry analyses were performed by day 10 on in vitro–derived dendritic cells generated from CD34hiCD33lo human thymic progenitors in multicytokine-supported cultures.
GM-CSFR indicates granulocyte macrophage–colony-stimulating factor receptor; IL-3Rα, interleukin 3 receptor alpha.
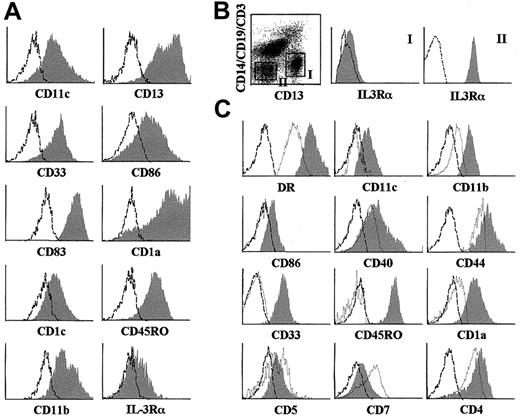
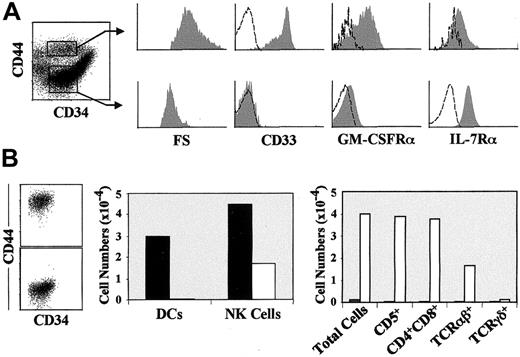
Phenotype of DCs derived in vitro from CD34hiCD33lo intrathymic precursors or resident in vivo in the human postnatal thymus.
(A) Representative flow cytometry profile of DCs derived by day 10 from sorted CD34hiCD33lo human thymic precursors set up in multicytokine-supported cultures (shaded histograms). (B) Identification of 2 distinct subsets of DCs resident in the human postnatal thymus based on reciprocal expression of CD13 and IL-3Rα. Electronic gates were set on sorted HLA-DR+ large-sized thymocytes as shown in the biparametric plot, and then IL-3Rα expression was analyzed on gated Lin−CD13+(gate I) or Lin−CD13−(gate II) cells (shaded monoparametric histograms). (C) Representative flow cytometry profile of Lin−CD13+ IL-3Rα− (shaded histograms) or Lin−CD13−IL-3Rα+ (unshaded histograms) HLA- DR+ thymic DCs gated as shown in (B). Background fluorescence (unshaded histograms in A and B, and dashed histograms in C) was determined with isotype-matched irrelevant MoAbs.
Phenotype of DCs derived in vitro from CD34hiCD33lo intrathymic precursors or resident in vivo in the human postnatal thymus.
(A) Representative flow cytometry profile of DCs derived by day 10 from sorted CD34hiCD33lo human thymic precursors set up in multicytokine-supported cultures (shaded histograms). (B) Identification of 2 distinct subsets of DCs resident in the human postnatal thymus based on reciprocal expression of CD13 and IL-3Rα. Electronic gates were set on sorted HLA-DR+ large-sized thymocytes as shown in the biparametric plot, and then IL-3Rα expression was analyzed on gated Lin−CD13+(gate I) or Lin−CD13−(gate II) cells (shaded monoparametric histograms). (C) Representative flow cytometry profile of Lin−CD13+ IL-3Rα− (shaded histograms) or Lin−CD13−IL-3Rα+ (unshaded histograms) HLA- DR+ thymic DCs gated as shown in (B). Background fluorescence (unshaded histograms in A and B, and dashed histograms in C) was determined with isotype-matched irrelevant MoAbs.
To investigate whether equivalent DC1-like cells are resident in vivo in the human thymus, HLA-DR+ cells were magnetically sorted from total thymocytes. As shown in Figure 1B, electronic gates set independently on Lin−CD13+ and Lin−CD13− large-sized HLA-DR+thymocytes revealed that DCs, defined as HLA-DR+Lin− large-sized cells (0.2% of total thymocytes), comprised 2 distinct populations based on their differential expression of CD13 and IL-3Rα. Lin−CD13+ cells were homogeneously negative for IL-3Rα, whereas all Lin−CD13− cells expressed high levels of IL-3Rα. As shown in Figure 1C, both thymic populations displayed reciprocal antigenic profiles. CD13−IL-3Rα+cells were CD4+CD1a− DCs lacking CD11c and CD33 myeloid markers, but expressing the lymphoid markers CD5 and CD7, suggesting that they corresponded to the population of intrathymic DC2.20 In contrast, CD13+IL-3Rα− cells showed a weak expression of CD5 and CD7, but were highly positive for CD11c, CD33, HLA-DR, CD44, and CD11b and coexpressed CD4 and CD1a, and CD40 and CD86 costimulatory DC markers. Strikingly, CD13+IL-3Rα−DCs, in contrast to CD13−IL-3Rα+ DCs, were CD45RO+. Therefore, besides DC2, the human thymus contains a subset of DCs which, like DCs generated in vitro from CD34hiCD33lo progenitors, resemble myeloid-derived DC1 in phenotypic terms. The relevance of this finding is strengthened by the fact that both DC subsets are found in vivo at equivalent absolute numbers, indicating that about half of all DCs resident in the human postnatal thymus belong to the DC1-like type (0.1% of total thymocytes).
Thymic DC1 cells develop from CD34hiCD33lopro–T cells through a CD34loCD44hiintermediate precursor that has lost the capacity to generate T cells
The surface phenotype of the intrathymic DC1-like population would suggest an immediate myeloid origin of these DCs. This prompted us to investigate their pathway of differentiation from the earliest thymic progenitors, with the final aim of defining precursor-product relationships that could help to conclusively establish their lineage derivation. To this end, CD34hiCD33lo thymic precursors were set up in the multicytokine culture system previously reported by Márquez et al23 to support the development of NK cells, DCs, and T-lineage cells. T/NK/DC progenitors were shown to differentiate in this system along 2 independent pathways that progressed through separate CD34lo intermediate progenitors of distinct cell size and opposite expression of CD5, CD33, and CD44,22 23 whose developmental potential has not been directly addressed. Therefore, we next evaluated the developmental outcome of both pathways, focusing on the lymphoid and myeloid potential of the 2 CD34lo intermediate progenitors, as compared with that of their immediate CD34hiCD33lo precursors. As shown in Figure2A, CD34lo intermediates derived from CD34hiCD33lo precursors by day 3.5 of culture (> 98% of total viable cells) were sorted on the basis of CD44 and CD5 expression levels. As expected, sorted CD44hiCD5−/lo cells were homogeneously large-sized CD33+CD34lo thymocytes, whereas CD5+CD44lo precursors were small-sized CD33−CD34lo cells. Both intermediate CD34lo cell subsets (hereafter referred to as CD34loCD44hi and CD34loCD44lo, respectively) were then assayed for their potential to generate lymphoid and/or myeloid cells in the appropriate in vitro assays. Freshly isolated CD34hiCD33lo intrathymic precursors were analyzed as well for comparison.
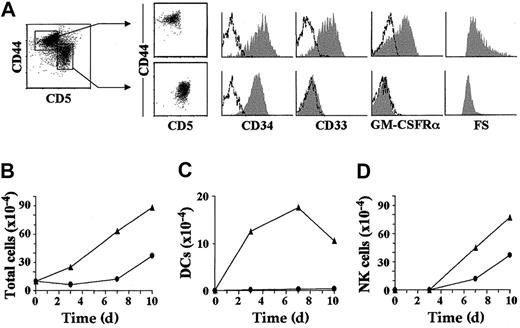
Characterization of the NK cell and DC potential of 2 separate intermediate CD34lo precursors, CD44hi or CD44lo, derived from the earliest intrathymic progenitors.
(A) Representative cell size (forward scatter, FS) and flow cytometry profile (shaded monoparametric histograms) of sorted CD44hi (CD5lo) and CD44lo(CD5+) intermediate CD34lo progenitors (biparametric plots) derived by day 3.5 from CD34hiCD33lo precursors set up in multicytokine-supported cultures. Background fluorescence determined with isotype-matched irrelevant MoAbs is shown (unshaded histograms). Absolute numbers of total viable cells (B), DCs (CD7loCD13+CD1a+) (C), and NK cells (CD7++CD13−CD56+) (D) recovered from either CD44hi (▴) or CD44lo (●) intermediate CD34lo progenitors derived in vitro and sorted as shown in (A), upon reculture in IL-2–supplemented multicytokine-supported conditions. Absolute numbers are referred to 105 input progenitors. Results are presented as the mean of 5 independent experiments.
Characterization of the NK cell and DC potential of 2 separate intermediate CD34lo precursors, CD44hi or CD44lo, derived from the earliest intrathymic progenitors.
(A) Representative cell size (forward scatter, FS) and flow cytometry profile (shaded monoparametric histograms) of sorted CD44hi (CD5lo) and CD44lo(CD5+) intermediate CD34lo progenitors (biparametric plots) derived by day 3.5 from CD34hiCD33lo precursors set up in multicytokine-supported cultures. Background fluorescence determined with isotype-matched irrelevant MoAbs is shown (unshaded histograms). Absolute numbers of total viable cells (B), DCs (CD7loCD13+CD1a+) (C), and NK cells (CD7++CD13−CD56+) (D) recovered from either CD44hi (▴) or CD44lo (●) intermediate CD34lo progenitors derived in vitro and sorted as shown in (A), upon reculture in IL-2–supplemented multicytokine-supported conditions. Absolute numbers are referred to 105 input progenitors. Results are presented as the mean of 5 independent experiments.
As shown in Table 2, CD34loCD44hi intermediates simultaneously generated DCs and NK cells (defined respectively as CD7loCD56−CD13+ and CD7+CD56+ CD13− cells) in multicytokine-supported cultures supplemented with IL-2. As expected of their more immature developmental status, CD34hiCD33lo precursors were enriched (about 3-fold) in NK and DC precursor potential. In contrast, no DCs were derived from CD34loCD44lo intermediates which were, however, capable of generating NK cells. Kinetic studies showed that generation of DCs from the CD34loCD44hiintermediates involved cellular proliferation. In fact, a 3-fold increase of total cell numbers was observed by day 3 of culture (Figure2B), when DCs represented about 50% of the whole culture (Figure 2C), but no NK cell production was yet observed (Figure 2D). DC numbers grew modestly thereafter up to day 7, but DC viability declined steadily afterward (Figure 2C). These studies also confirmed that CD34loCD44lo precursors were devoid of DC potential, as no DC production could be recorded along the whole culture period (10 days). In contrast to DC production, NK cell production was found to proceed with similar kinetics in both cultures (Figure 2D). However, CD34loCD44hi precursors were consistently more efficient (2- to 3-fold) than CD34loCD44lo thymocytes in generating NK cells, which concurs with their higher proliferation capacity.
Absolute and relative frequencies of myelomonocytes, DCs, NK cells, and T cells generated from CD34hi early thymic precursors and from their in vitro–derived progenies
| Progenitors . | DCs* (%) . | NK cells* (%) . | TCRαβ+ T cells† (%) . | Myelomonocytes‡ (%) . |
|---|---|---|---|---|
| CD34hiCD33lo | 650 (44) | 780 (53) | 14.3 (43) | 33.6 (15) |
| CD34loCD44hi | 210 (35) | 390 (65) | < 0.05 (4) | 10 (17) |
| CD34loCD44lo | < 1 (1) | 104 (95) | 9 (60) | < 0.05 (< 1) |
| Progenitors . | DCs* (%) . | NK cells* (%) . | TCRαβ+ T cells† (%) . | Myelomonocytes‡ (%) . |
|---|---|---|---|---|
| CD34hiCD33lo | 650 (44) | 780 (53) | 14.3 (43) | 33.6 (15) |
| CD34loCD44hi | 210 (35) | 390 (65) | < 0.05 (4) | 10 (17) |
| CD34loCD44lo | < 1 (1) | 104 (95) | 9 (60) | < 0.05 (< 1) |
Absolute numbers (× 10−3) and percentages (in parentheses) of DCs, NK cells, T cells, and myelomonocytes generated either from freshly isolated CD34hiCD33lothymic progenitors or from their CD34loCD44hiand CD34loCD44lo progenies derived by day 3.5 in multicytokine-supported cultures.
Generation of NK cells and DCs per 105 input cell progenitors was determined in IL-2–supplemented multicytokine-supported cultures by day 9.
Absolute numbers and percentages of TCRαβ+T cells recovered per fetal thymic lobe reconstituted with 2 × 104 progenitors were determined after 25 days in hu/moFTOC.
Generation of monocytes (CD14+CD1a−) per 105 input progenitors was determined by day 7 in cultures supplemented with M-CSF, IL-7, and SCF.
DCs indicates dendritic cells; NK, natural killer; TCR, T-cell receptor; hu/moFTOC, hybrid human/mouse fetal thymic organ culture; M-CSF, macrophage–colony-stimulating factor; IL-7, interleukin 7; SCF, stem cell factor.
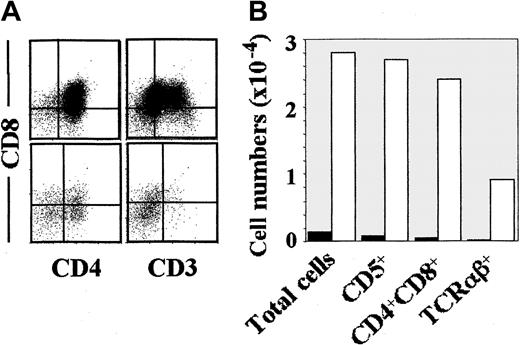
When the same subsets were analyzed for their T-cell potential in a hu/moFTOC, early CD34hiCD33loprogenitors and CD34loCD44lointermediates were found to behave as efficient pro–T cells, although pro–T-cell activity was consistently higher in the former population. Surprisingly, however, CD34loCD44hicells were devoid of pro–T-cell activity (Table 2). As shown in Figure3, CD34loCD44lointermediates were capable of generating up to 35% of CD3+TCRαβ+ cells by day 17 of culture, when all cells (> 98%) expressed CD5, and most of them (85%) had already acquired a CD4+CD8+ DP phenotype. In contrast, TCRαβ+ DP thymocytes were essentially undetectable (< 2%) in thymic lobes reconstituted with CD34loCD44hi precursors, which showed a 25-fold reduced cellular recovery (Figure 3). Interestingly, most (up to 70%) cells recovered from those lobes displayed morphologic and phenotypic features of DCs (large-sized CD3−CD4+CD5− cells), indicating that the hu/moFTOC supports the development of DCs from CD34loCD44hiprecursors. However, in absolute terms, this latter subset was about 400-fold less efficient than CD34loCD44loprecursors in the generation of DP TCRαβ+ thymocytes (Figure 3). These dramatic differences provided evidence that CD34loCD44hi cells derived from CD34hiCD33lo progenitors have lost the potential to generate T cells.
Characterization of the T-cell potential of 2 separate intermediate CD34lo precursors, CD44hi or CD44lo, derived from the earliest intrathymic progenitors.
Phenotypic analysis (A) of the cellular progeny generated in hu/moFTOC from CD44lo (upper histograms) or CD44hi (lower histograms) in vitro–derived CD34lo progenitors obtained as described in Figure 2A. Absolute cell numbers (B) of the indicated populations recovered per thymic lobe after 17 days of reconstitution with either CD44lo (empty bars) or CD44hi (filled bars) CD34lo progenitors (2 × 104/lobe).
Characterization of the T-cell potential of 2 separate intermediate CD34lo precursors, CD44hi or CD44lo, derived from the earliest intrathymic progenitors.
Phenotypic analysis (A) of the cellular progeny generated in hu/moFTOC from CD44lo (upper histograms) or CD44hi (lower histograms) in vitro–derived CD34lo progenitors obtained as described in Figure 2A. Absolute cell numbers (B) of the indicated populations recovered per thymic lobe after 17 days of reconstitution with either CD44lo (empty bars) or CD44hi (filled bars) CD34lo progenitors (2 × 104/lobe).
CD34hiCD33lo early thymic precursors can develop along a myelomonocytic pathway of differentiation that proceeds through CD34loCD44hi intermediates
Although early progenitors seeding the human postnatal thymus are currently envisaged as lymphoid-restricted precursors devoid of myeloid potential,5,10,30 generation of CD14+monocytes has been reported to occur in vitro from human CD34+ thymocytes when the myelomonocytic cytokine macrophage–colony-stimulating factor (M-CSF) is provided to the cultures.24 Therefore, we next analyzed the myeloid potential of early CD34hiCD33lo intrathymic progenitors and their CD34lo progenies under culture conditions favoring monocytic generation (ie, IL-7, SCF, and M-CSF). We found that, after 7 days, cultures derived from ex vivo–isolated CD34hiCD33lo immature thymocytes contained an unexpectedly large number (up to 40%; 15% on average) of cells that coexpressed the myelomonocytic antigen CD14, together with CD11b, CD13, and CD33 (Table 2, and data not shown). These cells lacked expression of CD1a and maintained their CD14+CD1a−phenotype during the whole culture period (18 days), suggesting that they represented differentiated monocytes. Supporting this possibility, we identified monocytic colony formation from CD34hiCD33lo precursors in methylcellulose cultures supplemented with IL-7, SCF, GM-CSF, and IL-3 (data not shown).
Strikingly, CD34loCD44hi intermediates generated in multicytokine-supported cultures also displayed the capacity to generate CD14+CD1a− monocytes, although at a 3-fold reduced frequency, in the myelomonocytic assay; whereas no CD14+ cells were derived from the CD34loCD44lo cell subset, which is thus devoid of myeloid potential (Table 2). These data indicate that the earliest CD34hiCD33lo progenitors seeding the human thymus are not irreversibly committed to the lymphoid lineage, but can develop along a myelomonocytic pathway of differentiation through CD34loCD44hi intermediates. Taken together, our results provide evidence that differentiation of DC1-like cells from early CD34hiCD33lo intrathymic pro–T cells occurs through intermediate progenitors that display myelomonocytic potential and are still able to generate NK cells, but have lost the potential to generate T cells.
CD14+CD1a− myelomonocytes derived from CD34hi CD33lo thymic progenitors display DC1 precursor potential
The above findings would suggest that development of thymic DC1 is closely linked to that of myeloid cells rather than T cells. Supporting this possibility, we found that CD14+ monocytes and DCs develop simultaneously in vitro, even under culture conditions devoid of M-CSF. Indeed, although CD14−CD1a+ cells represented a major progeny, CD14+CD1a− monocytes were also generated from CD34hiCD33lo early precursors as well as from CD34loCD44hi intermediates (up to 35% by day 6) in multicytokine-supported cultures (Figure4A, and data not shown). Interestingly, CD14+CD1a− monocytes decreased steadily in those cultures devoid of M-CSF, whereas CD14−CD1a+ cells with a mature CD83+CD80+CD86+ DC1 phenotype increased concurrently (data not shown). These results would suggest that growth and/or terminal differentiation of CD14+monocytes could not be supported in the absence of M-CSF, thus allowing the outgrowth of DC1. Alternatively, DC1 would stem from CD14+ myelomonocytes that lose CD14 and acquire CD1a.
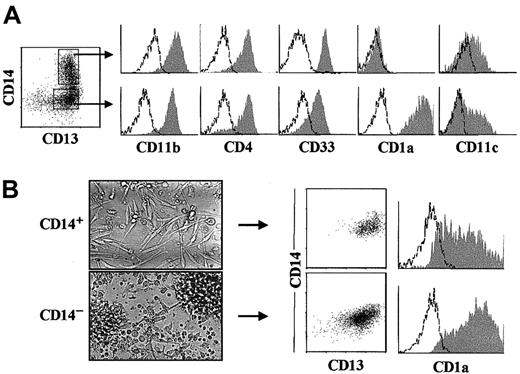
CD14+ myelomonocytes derived from intrathymic CD34hiCD33lo pro–T cells are DC1 precursors.
(A) Representative flow cytometry profile (shaded monoparametric histograms) of electronically gated CD13+CD14+and CD13+CD14−/lo cell subsets (biparametric plot) derived by day 6 from CD34hiCD33loprecursors set up in multicytokine-supported cultures. Background fluorescence determined with isotype-matched irrelevant MoAbs is shown (unshaded histograms). (B) Bright-field images of CD14+(upper panel) and CD14−/lo (lower panel) subsets sorted as shown in (A) after overnight culture (original magnification × 40), and flow cytometry analysis for the correlated expression of CD14 and CD13 (biparametric plots), and CD1a (shaded histograms) on their cellular progenies derived upon reculture for 3 additional days.
CD14+ myelomonocytes derived from intrathymic CD34hiCD33lo pro–T cells are DC1 precursors.
(A) Representative flow cytometry profile (shaded monoparametric histograms) of electronically gated CD13+CD14+and CD13+CD14−/lo cell subsets (biparametric plot) derived by day 6 from CD34hiCD33loprecursors set up in multicytokine-supported cultures. Background fluorescence determined with isotype-matched irrelevant MoAbs is shown (unshaded histograms). (B) Bright-field images of CD14+(upper panel) and CD14−/lo (lower panel) subsets sorted as shown in (A) after overnight culture (original magnification × 40), and flow cytometry analysis for the correlated expression of CD14 and CD13 (biparametric plots), and CD1a (shaded histograms) on their cellular progenies derived upon reculture for 3 additional days.
To discriminate between both possibilities, CD14+ and CD14− cells derived from CD34hiCD33lo precursors by day 6 were sorted, and their respective progenies were analyzed upon reculturing for 3 additional days. As shown in Figure 4A, CD14+ and CD14− sorted populations were both CD4+ and shared the coexpression of CD33, CD13, CD11c, and CD11b, but differed in the expression of CD1a which was prominent on CD14−cells but negative on CD14+ cells. Besides these phenotypic differences, a strikingly distinct morphology was observed, as CD14− cells were round or stellate-shaped, nonadherent, and prone to form aggregates, whereas CD14+ cells were adherent cells that displayed an elongated epithelial-like morphology (Figure 4B). Drastic phenotypic changes could be observed upon reculturing CD14+ cells, as most of them (82%) down-regulated CD14 expression, and simultaneously acquired high CD1a expression levels (50%) characteristic of DC1 by day 3. In contrast, CD14− cells retained a typical DC1 myeloid phenotype upon reculture (Figure 4B). We thus concluded that CD34hiCD33lo intrathymic precursors give rise to myeloid DC1-like cells through a CD34loCD44hi intermediate population which differentiates along 2 independent pathways through separate precursors: either CD14+CD1a− monocytes or CD14−CD1a+ pre-DC1 cells.
Constitutive expression of the myelomonocytic receptor for GM-CSF on myeloid DC1 precursors resident in the human thymus
Our findings above are compatible with the possibility that early thymic precursors retain a myeloid-lineage differentiation activity that, as proposed for bone marrow hematopoietic stem cells, can be triggered by signaling through cytokine pathways that drive myeloid cell development, such as the GM-CSF pathway.31 This would imply that GM-CSFR is expressed on thymic precursors able to generate DC1. Confirming this possibility, expression of GM-CSFRα was prominent on CD34loCD44hi DC1 precursors, but was essentially undetectable on CD34loCD44loprecursors arising from CD34hiCD33lo precursors in multicytokine-supported cultures (Figure 2A). This supports the lymphoid-restricted potential of the latter subset.
To assess the physiologic relevance of this finding, we investigated whether expression of GM-CSFRα also occurs in vivo. First, CD34loCD44hi and CD34loCD44lo intermediates were identified among Lin− freshly isolated thymocytes based on their CD44 and CD34 expression levels (Figure 5A), and phenotypic analyses were then performed upon electronic gating on both cell subsets. Close similarities were found among CD34lo intermediates identified in vivo (Figure 5A) and their in vitro–derived counterparts (Figure 2A), particularly in terms of cellular size and differential expression of CD33. Again, CD34loCD44hi precursors were homogeneously large-sized CD33+ cells, whereas CD34loCD44lo intermediates were CD33− small-sized thymocytes. More importantly, both intermediate progenitors could be traced ex vivo by their reciprocal expression of GM-CSFRα, so that essentially all CD34loCD44hi precursors displayed high GM-CSFRα surface density, whereas GM-CSFRα expression was barely detectable on CD34loCD44lo precursors. Strikingly, differential expression of GM-CSFRα concurred with a reciprocal expression of the receptor for IL-7 (IL-7Rα), so far considered as a marker of lymphoid commitment,31 which was brightly expressed on GM-CSFRα− precursors, but was weakly detectable on GM-CSFRα+ cells (Figure 5A).
The receptor for GM-CSF is expressed in vivo on thymic DC1 precursors, which have lost the potential to generate T cells.
(A) Representative cell size (FS) and flow cytometry profile (shaded monoparametric histograms) of CD44hi and CD44lointermediate CD34lo progenitors electronically gated (biparametric plots) on Lin− freshly isolated thymocytes. Background fluorescence determined with isotype-matched irrelevant MoAbs is shown (unshaded histograms). (B) DC, NK cell, and T-cell precursor potential of CD44lo (empty bars) and CD44hi (filled bars) intermediate CD34lo thymic progenitors sorted from freshly isolated Lin− thymocytes as shown in (A), and reanalyzed as shown in the biparametric plot (left panel). Generation of DCs and NK cells per 105 input progenitors was assessed as described in Figure 2 after 11 days of culture (middle panel). Generation of T cells was assessed in hu/moFTOC by day 19. Absolute numbers of cells of the indicated phenotypes recovered per thymic lobe after reconstitution with 2 × 104 progenitors are shown (right panel).
The receptor for GM-CSF is expressed in vivo on thymic DC1 precursors, which have lost the potential to generate T cells.
(A) Representative cell size (FS) and flow cytometry profile (shaded monoparametric histograms) of CD44hi and CD44lointermediate CD34lo progenitors electronically gated (biparametric plots) on Lin− freshly isolated thymocytes. Background fluorescence determined with isotype-matched irrelevant MoAbs is shown (unshaded histograms). (B) DC, NK cell, and T-cell precursor potential of CD44lo (empty bars) and CD44hi (filled bars) intermediate CD34lo thymic progenitors sorted from freshly isolated Lin− thymocytes as shown in (A), and reanalyzed as shown in the biparametric plot (left panel). Generation of DCs and NK cells per 105 input progenitors was assessed as described in Figure 2 after 11 days of culture (middle panel). Generation of T cells was assessed in hu/moFTOC by day 19. Absolute numbers of cells of the indicated phenotypes recovered per thymic lobe after reconstitution with 2 × 104 progenitors are shown (right panel).
To next investigate whether reciprocal expression of GM-CSFR and IL-7R on intermediate intrathymic precursors paralleled functional differences in terms of their developmental outcome, sorted CD34loCD44hi and CD34loCD44lo thymocytes were analyzed for their lymphoid and myeloid precursor potential as described above. As shown in Figure 5B, both subsets, representing 0.02% and 0.8% of total thymocytes, respectively, behaved as their in vitro–derived counterparts in developmental terms, as both displayed NK cell precursor potential, but only CD34loCD44hiprecursors were able to generate DC1 (Figure 5B). However, the proliferation and DC/NK differentiation capacities of the latter were consistently lower than those of the equivalent in vitro–derived population, probably reflecting a decreased cell viability during their isolation procedure. Also, generation of TCRαβ+ T cells occurred in fetal thymic lobes reconstituted with CD34loCD44lo but not with CD34loCD44hi cells (Figure 5B). The lack of T-cell precursor potential of the CD34loCD44hisubset could not be attributed to delayed differentiation kinetics; rather, cellular viability decreased gradually along these organ cultures up to day 25 (data not shown). Therefore, based on both phenotypic and functional results, we concluded that alternative expression of GM-CSFRα and IL-7Rα serves to trace, respectively, separate myeloid- and lymphoid-lineage committed intermediate progenitors present in the human thymus in vivo.
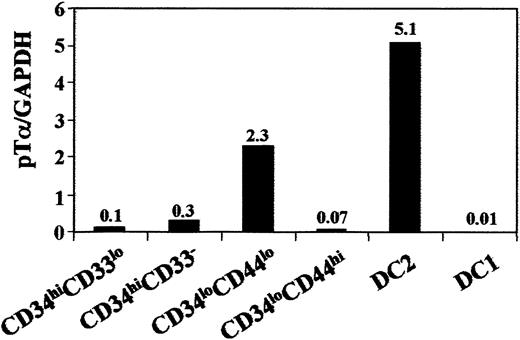
Selective lack of pTα gene transcriptional activation serves to trace the myeloid pathway of intrathymic DC development in vivo
As IL3Rα+ thymic lymphoid DC2 express high levels of pTα transcripts,20 it would be informative to know whether pTα transcription is selective of DC2 or if it is shared by the intrathymic IL3Rα− myeloid DC1 pathway. This was assessed by real-time quantitative RT-PCR, which provides a unique means to quantify transcription accurately. Sample-to-sample variations were corrected by normalization to GAPDH expression, used as endogenous control. Therefore, cDNA samples were derived from CD13−IL3Rα+ and CD13+IL3Rα− thymic DCs, as well as from both CD34loCD44hi and CD34loCD44lo intermediate precursors isolated ex vivo. In addition, CD34hiCD33loearly precursors and downstream CD34hiCD33−thymocytes were included in the study for comparison. cDNA samples were amplified in parallel for GAPDH and pTα, and quantitative values were obtained by interpolation in standard curves of total thymocyte cDNA. Final quantitative data were the result of calculating pTα/GAPDH ratios for each sample.
As shown in Figure 6, pTα transcription was very low in the most immature CD34hiCD33lointrathymic progenitors, but increased about 3-fold in downstream CD34hiCD33-precursors, and 20-fold in CD34loCD44lo pro–T cells devoid of DC1 potential. High pTα mRNA levels (50-fold higher than those of CD34hiCD33lo precursors) were detected in ex vivo–isolated CD13−IL3Rα+ thymic DC2. In contrast, pTα transcription was essentially undetectable in CD34loCD44hi intrathymic DC1 precursors as well as in the CD13+IL3Rα− DC1 subset (500-fold lower levels than those of DC2), supporting the distinct developmental origin of both DC populations. Therefore, these results provide additional evidence that intrathymic DC1 cells, unlike DC2 cells, are developmentally dissociated from the T-cell lineage.
Real-time quantitative RT-PCR analysis of pTα mRNA expression along the intrathymic DC1 differentation pathway.
Real-time RT-PCR analysis of pTα mRNA expression in the indicated intrathymic cell subsets sorted from the same thymus sample. Sample-to-sample variations were corrected by normalization to GAPDH expression, used as endogenous control.
Real-time quantitative RT-PCR analysis of pTα mRNA expression along the intrathymic DC1 differentation pathway.
Real-time RT-PCR analysis of pTα mRNA expression in the indicated intrathymic cell subsets sorted from the same thymus sample. Sample-to-sample variations were corrected by normalization to GAPDH expression, used as endogenous control.
Discussion
The finding that DCs with a myeloid-related phenotype represent a significant proportion of all DCs resident in vivo in the postnatal human thymus (0.1% of total thymic cells) prompted us to re-examine in this study the developmental origin of thymic DCs,5 9 a cell type currently considered to be lymphoid derived. Based on precursor-product relationships, we have traced the developmental pathway of such DCs from their intrathymic CD34hiprecursors, and provide evidence that, besides their surface phenotype, they are authentic myeloid DCs in terms of their lineage origin.
Our previous studies showed that T-lineage cells and functional DCs could arise simultaneously from the earliest CD34+postnatal thymocytes in response to IL-7.22 DCs with a similar phenotype were reported by others26 to arise from a bone marrow subset of CD34+ CLP, distinct from stem cells, which displayed the potential to generate T, B, and NK cells as well, but not myeloid cells. This finding led to the general view that the thymus was seeded by a CLP subset coming from the bone marrow and, hence, thymic DCs have hitherto been considered as a unique population of lymphoid-derived DCs.
More recently, the characterization of “plasmacytoid” T cells as DCs (DC2) and their identification in the human thymus have offered new clues on the phenotypic and functional features of putative lymphoid DCs. Accumulating evidence supports that DC2 represent the population of lymphoid-derived DCs in man.12 This concept relies not only on their phenotypic differences with myeloid-derived DCs (DC1), but also on functional differences including their GM-CSF–independent generation, in contrast to the GM-CSF dependence of DC1 precursors.16,17,32 More importantly, recent findings support the notion of shared molecular cues for the development of DC2 and lymphoid cells.21 In addition, DC2 and T cells share high pTα transcription, suggesting the developmental linkage of both cell types.20 Therefore, our finding that only about half of all human intrathymic DCs displayed the phenotypic features of DC2 was somewhat unexpected. More surprising was the finding that the other half corresponded to a DC type that, like monocyte-derived peripheral DC1,13,14 32 expressed CD1a and myeloid markers including GM-CSFR, but lacked lymphoid markers and showed a sparse expression of IL-3Rα.
Recently, DCs bearing myeloid-associated antigens have been found by others in the human thymus although at lower frequencies than those observed by us, perhaps due to different isolation techniques.27,28 However, whether such myeloid-related DCs represent DCs that have entered the thymus directly from the bloodstream or are produced in situ from early intrathymic progenitors is a question that was not resolved. Also, definitive conclusions on the lineage derivation of such DCs could not be drawn based on their phenotypic features.33,34 Therefore, we sought to characterize their ontogeny in this study with the final aim of defining their lineage affiliation. Precursor-product relationship studies based on multicytokine-supported cultures23 have allowed us to trace the developmental pathway of intrathymic DC1-like cells, and to identify intermediate DC1 progenitors with myeloid potential, but devoid of T-cell potential, within the human postnatal thymus.
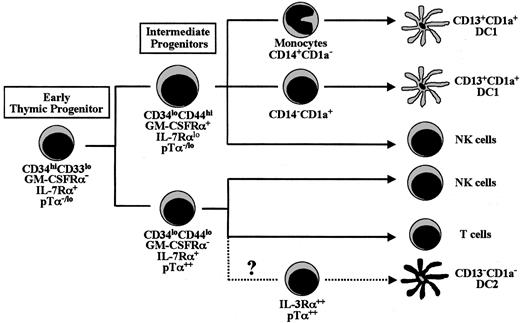
Collectively, our results allowed us to propose a model (summarized in Figure 7), supporting that the most primitive CD34hiCD33lo progenitors in the human thymus differentiate along 2 independent pathways that progress through separate CD34lo intermediate precursors, which are placed at a critical branch point of lineage decision along intrathymic development. Both intermediates, which display reciprocal phenotypes in terms of myeloid and lymphoid markers, represent progenitors which have definitively lost the potential to generate either T cells (CD34loCD44hi) or DC1-like cells (CD34loCD4lo), respectively. Strikingly, we show here that this developmental T/DC1 decision correlates in vivo with the acquisition of reciprocal cytokine receptor profiles; so that upregulation of the receptor for GM-CSF occurs on DC1 precursors which show down-regulated levels of IL-7Rα, whereas GM-CSFRα is down-regulated on T-cell progenitors expressing IL-7Rα. This finding has important implications regarding a recent report on the instructive action of cytokines in myeloid-lineage versus lymphoid-lineage commitment.31 Down-regulation of cytokine receptors which drive and support myeloid differentiation, such as the GM-CSFR, seems to be a critical step in lymphoid commitment, while up-regulation of such receptors results in myeloid-lineage induction. As this pattern of cytokine receptor expression matches that found in vivo in the intermediate progenitors identified in this study, we postulate that such intermediates represent the thymic counterparts of the lymphoid- and myeloid-committed bone marrow precursors which give rise to each of the major branches of the hematopoietic system.
Proposed model of DC, NK cell, and T-cell differentiation pathways from early CD34hiCD33lo precursors in the human postnatal thymus.
Proposed model of DC, NK cell, and T-cell differentiation pathways from early CD34hiCD33lo precursors in the human postnatal thymus.
While this possibility has to be confirmed at the clonal level when multilineage clonal assays are available, our results provide unequivocal evidence that myeloid development takes place within the human postnatal thymus. The existence of human CD34+precursor thymocytes endowed with a latent myelomonocytic capacity has been suggested before by independent in vitro studies from our group and others.22,24 However, the physiologic relevance of such intrathymic myeloid precursors has not been directly addressed, nor have the precursors been tested for their full developmental potential. Here, the identification of downstream CD34loCD44hi progenitors with increased ability to generate myeloid cells, but devoid of T-cell precursor activity, provides direct evidence that a myeloid differentiation pathway dissociated from that of lymphoid cells does physiologically exist in the human thymus. Furthermore, the potential of such myeloid intermediates to generate DC1-like cells, either directly from their CD1a+ progeny, or indirectly from CD14+monocytes, demonstrates that intrathymic DC1 cells have an immediate myeloid origin. It is thus likely that the myeloid pathway of intrathymic DC development identified in this study is equivalent to that previously reported for CD34+ cord blood stem cells, which can give rise to epidermal LCs and dermal DCs through separate CD14−CD1a+ and CD14+CD1a− intermediate precursors, respectively.16
Besides CD34loCD44hi myeloid intermediates, we identify CD34loCD44lo lymphoid-committed progenitors which lack myeloid potential and are unable to generate DC1, but behave as efficient lymphoid precursors with T and NK cell differentiation potential. Although the latter subset may include bipotential T/NK progenitors equivalent to those identified previously,35 a more attractive possibility is that they are multipotent lymphoid precursors able to generate thymic DC2 as well. This possibility is fully consistent with our finding that up-regulation of pTα transcription marks in vivo a divergence between such CD34loCD44lo lymphoid precursors and their CD34loCD44hi myeloid counterparts. This, in turn, would explain the finding that DC2, like T cells, but not DC1, express pTα transcripts. DC2 could therefore be the human counterparts of murine intrathymic DCs shown to be developmentally linked to T cells.11
Our model, however, is difficult to reconcile with our finding that NK cells can be derived also from sorted GM-CSFRα+ myeloid CD34loCD44hi intermediates (present study and data not shown), likely through a common DC/NK progenitor.23 Although we cannot rule out that such GM-CSFRα+ CD34loCD44hiprogenitors still retain a latent, but not physiologic, lymphoid potential that results in NK cell generation in vitro, a more attractive possibility is that such NK cell progeny represent a novel subset of NK cells developmentally dissociated from T (and lymphoid) cells but linked to myeloid cells. Supporting this possibility, Spits and coworkers21 have recently shown that ectopic expression of Id2 and Id3, which inhibits development of CD34+ stem cells into lymphoid T cells, B cells, and DC2, does not affect generation of NK cells and myeloid DCs.
According to the proposed model, it has to be concluded that the postnatal thymus, at least in humans, is colonized by progenitors which still have myeloid potential, regardless of whether these progenitors are truly multipotent stem cells (HSC) or intermediate lymphoid/myelomonocytic precursors equivalent to those identified in mouse fetal liver.36 As such precursor thymocytes, like HSC,31 display low GM-CSFRα transcription (data not shown), it is possible that they retain a myeloid differentiation potential that can be triggered by signaling through the GM-CSF/GM-CSFR pathway. Since GM-CSF is produced in vivo by thymic epithelial cells,37 this myelomonocytic pathway might be physiologically relevant. One would thus expect that a similar pathway could be functional in vivo in mice. Supporting this possibility, murine CD8α− thymic DCs have been identified38 whose development in γc chain–deficient mice was dissociated from that of lymphoid cells.39Moreover, Lee and coworkers have recently shown that early T-cell progenitors present in the murine fetal thymus are capable of generating macrophages in a single-cell clonal assay.40Although no clonal assays for multipotent precursors are yet available in humans, it is very likely that the myeloid intrathymic precursors identified in the present study represent the human counterparts of the T-cell/myeloid progenitors identified by Lee et al40 in mice. Supporting this possibility, IL-7 was involved in the development of such a myeloid intrathymic pathway in humans22,23 and also in mice.40 As discussed by Lee et al,40this finding may reflect the dependency of early thymocytes, but not stem cells, on survival signals provided by IL-7. Because early thymic precursors naturally express IL-7R, and IL-7 is produced by the thymic epithelium, it is likely that the IL-7/IL-7R pathway is involved in the development of intrathymic myeloid cells in vivo. Therefore, it would be interesting to determine whether up-regulation of GM-CSFR serves to trace a myeloid-related, not yet identified, intrathymic DC differentiation pathway also in mice.
Finally, a relevant question is the functional meaning of the intrathymic myeloid DC1 subset. Since the only known function of thymic DCs is to mediate negative selection of self-reactive T cells,1-3 it is an upcoming challenge to identify the physiologic roles of the 2 ontogenically distinct DC subsets identified in the human thymus.
We thank Drs J. L. San Millán and C. Hernández for advice on real-time PCR, Drs A. Alvarez and J. C. Segovia for invaluable help with cell sorting, and the pediatric cardiosurgery units from Centro Especial Ramón y Cajal and Ciudad Sanitaria La Paz (Madrid) for the thymus samples.
Supported by grants from the Comisión Interministerial de Ciencia y Tecnologı́a (SAF 97-0161), Dirección General de Enseñanza Superior (PB97-1194), Comunidad Autónoma de Madrid (08.3/0015.1/99 and 08.3/0021/2000), and Fondo de Investigación Sanitaria (FIS 00/1044), and by an institutional grant from the Fundación Ramón Areces.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marı́a L. Toribio, Centro de Biologı́a Molecular “Severo Ochoa,” Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain; e-mail:mtoribio@cbm.uam.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal