Abstract
Immune reconstitution is a critical component of recovery after treatment of human immunodeficiency virus (HIV) infection, cancer chemotherapy, and hematopoietic stem cell transplantation. The ability to enhance T-cell production would benefit such treatment. We examined the effects of exogenous interleukin-7 (IL-7) on apoptosis, proliferation, and the generation of T-cell receptor rearrangement excision circles (TRECs) in human thymus. Quantitative polymerase chain reaction demonstrated that the highest level of TRECs (14 692 copies/10 000 cells) was present in the CD1a+CD3−CD4+CD8+stage in native thymus, suggesting that TREC generation occurred following the cellular division in this subpopulation. In a thymic organ culture system, exogenous IL-7 increased the TREC frequency in fetal as well as infant thymus, indicating increased T-cell receptor (TCR) rearrangement. Although this increase could be due to the effect of IL-7 to increase thymocyte proliferation and decrease apoptosis of immature CD3− cells, the in vivo experiments using NOD/LtSz-scid mice given transplants of human fetal thymus and liver suggested that IL-7 can also directly enhance TREC generation. Our results provide compelling evidence that IL-7 has a direct effect on increasing TCR-αβ rearrangement and indicate the potential use of IL-7 for enhancing de novo naı̈ve T-cell generation in immunocompromised patients.
Introduction
It has been reported that T-cell numbers are maintained in adults predominantly through the expansion of postthymic, memory T cells, whereas in infants, T cells are predominantly maintained through the production of new naı̈ve T cells by the thymus.1 However, others and we have recently demonstrated that the adult thymus is still capable of thymopoiesis and can contribute to T-cell reconstitution in adults.2,3 Several methods have been used to measure thymopoietic capacity. Thymic size as measured by radiographic imaging1 and volumetric computed tomography measurements4,5 have been correlated with numbers of CD4+CD45RA+ naı̈ve T cells, and the number of phenotypically naı̈ve T cells after transplantation has been shown to correlate with antigen-specific function.6 However, there are concerns about limitations of estimating thymic function based on naı̈ve T-cell phenotype alone. T cells expressing a naı̈ve phenotype are not necessarily accurate surrogate markers of thymic function. Following thymic emigration, CD45RA+ naı̈ve T cells can have a long quiescent life span,7 may proliferate in an antigen-independent manner,8 or may rapidly convert to CD45RO+ memory/effector phenotype T cells.9Furthermore, naı̈ve T-cell markers may be acquired by memory T cells (especially CD8+ T cells),9,10 further compounding the difficulty in accurately enumerating naı̈ve T cells.11 12
To measure thymic function more directly in humans, we recently described an assay that quantifies an episomal DNA by-product of the T-cell receptor (TCR) rearrangement process.2 These TCR rearrangement excision circles (TRECs) contain the signal joint sequences from the TCRAD locus δRec to ψJα recombination event, which is common to approximately 70% of thymocytes destined to become mature TCRαβ T cells. TRECs are stable and do not replicate with cellular proliferation,2 13; therefore, the TREC content in a peripheral cell population is proportional to the frequency of recent thymic emigrants (RTEs). This frequency, however, is affected not only by changes in thymic output but also by the proliferative history of the cells. Because RTEs have TRECs at levels that would not have been affected by peripheral expansion and cellular replication, quantification of TREC levels in peripheral blood mononuclear cells (PBMCs) represents a sensitive measurement of thymopoietic capacity.
Although thymic function declines with age, substantial output is maintained into late adulthood.2,3 Furthermore, the adult thymus can contribute to immune reconstitution in individuals following antiretroviral therapy,2,14,15 and following myeloablative chemotherapy and autologous hematopoietic stem cell transplantation.16 T cells generated de novo from thymopoiesis have a broad TCR repertoire and are theoretically more capable of responding to neoantigens effectively.16,17 In contrast, peripheral expansion of existing T-cell pools may lead to limited T-cell repertoires and antigen responsiveness.1 17-22 Therefore, in patients with human immunodeficiency virus (HIV) infection or in those who have received chemotherapy, the ability of the thymus to generate naı̈ve T cells with a broad TCR repertoire should allow for recovery of T cell–mediated immunity that is qualitatively better than if the recovery were only through expansion of pre-existing naı̈ve and memory T cells.
Interleukin-7 (IL-7), which was originally reported as a pre-B cell growth factor,23 is produced by stromal cells in the thymus and bone marrow and appears to play a role at multiple stages of T- and B-lymphocyte development.24,25 In mice, the IL-7 receptor (IL-7R) is first expressed in lymphoid lineage-restricted progenitors in bone marrow26 and later can be detected in various tissues including thymus.27 IL-7R is composed of the IL-7Rα27 chain and the common cytokine receptor γ chain,28 the latter of which is the indispensable subunit receptor for several lymphoid-related cytokines such as IL-2, IL-4, IL-9, and IL-15.29 Several studies have shown the essential role of this cytokine in the survival and normal differentiation of thymocytes into mature naı̈ve T cells in mice. It has been suggested that IL-7 is a cofactor for V(D)J rearrangement of the TcRb gene locus30 and that it is also required for TcRb D-J rearrangement.31 In mice with targeted deletions of the IL-7 gene (IL-7−/−), there was a 20-fold reduction in the number of pro–T cells,32 yet the percentage of TcRαβ thymocytes was relatively normal,25,32 whereas the number of TcRγδ thymocytes was substantially reduced.32In mice with deletions of the IL-7R gene (IL-7Rα−/−and γc−/−), the number of TcRαβ thymocytes was markedly decreased and TcRγδ thymocytes were completely absent.33,34 On the other hand, in humans, it has been reported that mutations that prevent the expression of IL-7Rα chain and γc result in severe combined immunodeficiency (SCID).35,36 Thus, it appears that IL-7 is necessary for the survival and proliferation of early thymic progenitor cells and production of TcRγδ T cells, although it may not be unconditionally required for rearrangement of TcRa andb genes in mice. However, the regulatory mechanism of TCRαβ or γδ rearrangement in human thymus and the roles of IL-7 in thymopoiesis are as yet not fully understood. Importantly, it has been recently shown that IL-7 levels are increased during T lymphopenia in HIV infection, strongly suggesting that IL-7 may play a major role in both peripheral and central T-cell homeostasis.37 38
We evaluated the effects of exogenous IL-7 on human thymopoiesis in vitro using human thymic organ culture (TOC) and in vivo with NOD/LtSz-scid mice implanted with human thymus and liver (NOD-SCID-hu). The addition of IL-7 to TOC increased thymocyte proliferation and TREC levels and decreased apoptosis in thymuses obtained from fetuses and infants. In vivo, exogenous IL-7 enhanced TREC levels in thymic grafts in NOD-SCID-hu mice. Our results indicate that IL-7 results in increased TCR rearrangement in human thymus, both in vitro and in vivo, and suggest that immune reconstitution in humans could be augmented through stimulating thymus-dependent T-cell generation with exogenous IL-7.
Materials and methods
Thymus and liver
Human fetal thymus and liver tissue at 18 to 22 weeks of gestation were obtained from Advanced Bioscience Resources (Alameda, CA) and were processed within 24 hours of harvest. Newborn, infant, and adult thymuses were obtained from patients undergoing corrective cardiac surgery or adult thymectomy (Children's Medical Center or Parkland Memorial Hospital, Dallas, TX) and were processed within 6 hours of harvest.
TOC
Thymus tissue was dissected into pieces of about 2 mm3 so that every piece was likely to contain cortex and have similar cellularity. In adult samples, only the lobes that, on visual inspection, contained cell-dense areas were selected and used for TOC. Each thymic fragment was cultured on polyethylene terephthalate track-etched trans-well membranes (Becton Dickinson, San Jose, CA) suspended in 12-well plates in UltraCULTURE fetal calf serum (FCS)–free medium (Biowittaker, Walkersville, MD) supplemented with 50 U/mL penicillin and 50 mg/mL streptomycin in the presence or absence of recombinant human IL-7 (Peproteck, Rocky Hill, NJ). The cultures were maintained in a 5% CO2 incubator at 37°C.
Treatment of NOD-SCID-hu mice with IL-7
The NOD/LtSz-scid mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were implanted with pieces (∼2 mm3 each) of human fetal thymus and liver under the kidney capsule as previously described39 40 and maintained under specific pathogen-free conditions. Twenty-eight days after implantation, the size of the grafts was checked and the mice were accordingly divided into 4 groups with similarly sized thymic grafts. Four days later, 5 mice from each group were injected intraperitoneally twice daily with 100 ng each of IL-7 or phosphate-buffered saline. This was continued daily for 10 days. On days 7 and 21 after the last injection, IL-7–treated and control mice were autopsied and the graft, blood, spleen, and mesenteric lymph nodes were recovered for evaluation of TREC levels and phenotypic analysis.
Flow cytometry
Thymocytes were stained with combinations of the following directly labeled antihuman mouse monoclonal antibodies and isotype-matched controls: CD1a (HI149), CD3 (SK6), CD4 (SK3), CD8 (SK1) (Becton Dickinson); CDw127 (IL-7Rα) (R34.34) (Beckman Coulter, Brea, CA); CD3 (UCHT1), Ki67 (B56), BrdU (3D4), annexin V, CD45RA (HI00), (BD Pharmingen, San Diego, CA); and BCL-2 (124) (DAKO, Glostrup, Denmark). After staining, the cells were fixed in 1% paraformaldehyde and 4-color flow cytometry was performed using a FACSCalibur (Becton Dickinson) flow cytometer. For Ki67 and BCL-2, the cells were first stained for surface CD3, CD4, and CD8 and then fixed and permeabilized using the Cytofix/Cytoperm kit (BD Pharmingen) prior to staining of these intracellular proteins. Bromodeoxyuridine (BrdU) staining was carried out after staining for surface antigens using the BrdU Flow Kit (BD Pharmingen) according to the manufacturer's instructions. The data were analyzed using CellQuest software (Becton Dickinson).
Sorting of thymocytes
After mechanical disruption of thymus fragments, the cells were labeled with CD3 MicroBeads (Miltenyi Biotech, Auburn, CA) and separated into CD3+ and CD3− populations using separation columns (Miltenyi Biotech) according to the manufacturer's instructions. Thymic subsets were sorted by FACStar cell sorter (Becton Dickinson) after being stained for CD1a, CD3, CD4, and CD8.
Quantitative polymerase chain reaction of TRECs
The sorted cells were lysed in 100 μg/mL proteinase K (Roche Diagnostics, Indianapolis, IN) for 3 hours at 56°C and then 20 minutes at 95°C. TREC levels were measured by real-time quantitative polymerase chain reaction (PCR) using the 5′-nuclease (TaqMan) assay in an AB17700 system (Perkin-Elmer, Norwalk, CT). As previously described,16 each 25-μL reaction contained 5 μL cell lysate, and the final concentration of each component was as follows: 1.0 times reaction buffer, 20 U/mL platinum taq polymerase (Gibco BRL, Grand Island, NY), 3.5 mM MgCl2, 0.2 mM dNTPs, 500 nM each primer, 150 nM probe, and Blue-636 reference (Megabases, Chicago, IL). The sense and antisense primers were 5′-cacatccctttcaaccatgct-3′ and 5′-cctaaaccctgcagctggc-3′, respectively, and the probe was FAM-acacctctggtttttgtaaaggtgcccact-TAMRA (MegaBases). PCR conditions were 95°C for 5 minutes followed by 40 cycles of 95°C for 30 seconds and 60°C for 1 minute. A standard curve was plotted and TREC values for samples were calculated using ABI7700 software. Samples were analyzed in duplicate. TRECs are present at 0, 1, or 2 copies per diploid cell and there is no pseudogene sequence.
Results
TREC levels in FACS-sorted thymocyte subsets in the fresh human thymus
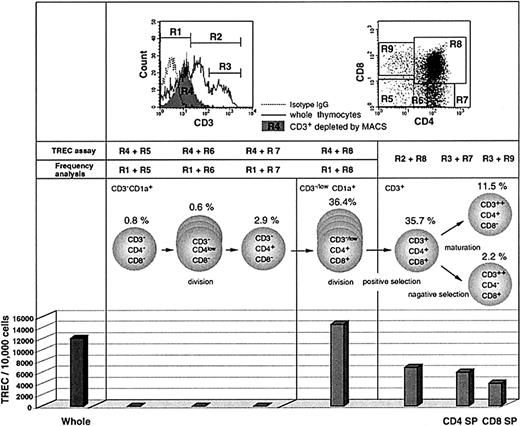
To investigate the timing of TREC generation and dilution during thymic development in vivo, we measured TREC levels in sorted thymocyte subsets from a newborn infant. The cells were separated by magnetic cell sorting (MACS) and flow cytometry based on surface phenotype. Commitment of progenitor cells to the T-cell lineage occurs at or around the transition of CD1a−CD34+ thymocytes into CD1a+CD34+ thymocytes.41,42Cells were sorted by flow cytometry for CD1a+/++CD3− and CD3+ subsets so as to exclude the CD1a−CD3− subset, which contains precursors for natural killer (NK) cells and thymic dendritic cells.43-45 Quantitative PCR demonstrated that the TREC level in unfractionated thymocytes was 12 194/10 000 cells (Figure1). No TRECs were detected at the CD3−CD4−CD8− triple negative (TN), CD3−CD4lowCD8−, or CD3−CD4+CD8− stages of thymocyte development. However, the highest level of TRECs was detected within CD1a+ cells that are CD3−CD4+CD8+ double positive (CD3− DP; 14 692/10 000 cells), confirming previous experiments that address the timing of TCRD gene rearrangement.46 This indicates that the excision leading to TRECs that commits a cell to the TCRαβ lineage occurs concomitant with the expansion of CD3− DP thymocytes. If we assume that TCRD deletion occurs in both alleles,47 then a TREC level of 14 692/10 000 cells would indicate than an average of 75% of cells have undergone rearrangement of both alleles to generate TREC. However, this value may vary depending on the possible TCR deletion on only one allele in part of the cells.48 Because cellular divisions after TREC generation will dilute TRECs, these results indicate that the initiation of cell expansion precedes TREC generation in CD3− DP cells. High levels of TREC were still detected in the CD3+ DP subset, which had undergone expansion (6900/10 000 cells), and also in the more mature CD4+ and CD8+ single positive (SP) subsets (6064 and 4099/10 000 cells, respectively). The decrease in TRECs in mature cells is likely to represent dilution secondary to cellular proliferation that occurs during the processes of positive and negative selection. There was no evidence to suggest that TREC generation still occurs after the CD3+ stage.
TREC levels in FACS-purified thymocyte subsets from the human thymus.
TREC levels were measured in FACS-sorted cells from a fresh thymus of a newborn infant. For CD3− cells, the thymocytes were first depleted of CD3+ cells by MACS and then sorted by FACS for the subsets based on CD4 and CD8 expression (dot plot, upper right) to more precisely determine the timing of peak TREC production. Cells were also sorted for CD1a+/++ cells so as to exclude the CD1a−CD3− subset, which may contain precursors for NK cells and thymic dendritic cells. The sorted cells were subjected to TREC assay. The CD3+-depleted fraction (shown as R4 in the histogram, upper left) contained cells expressing CD3 at the intermediate level (referred to as CD3low) as well as CD3− cells. On the other hand, for measuring the frequency of each subpopulation in CD3− and CD3−/low populations, we analyzed whole thymocytes from the same thymus sample using gate R1. For CD3+ subsets, the whole thymocytes were analyzed for frequency measurement using gates R2 or R3 and then sorted for the TREC assay. Therefore, it should be noted that the actual frequency of CD3−/lowCD4+CD8+ and CD3+CD4+CD8+ cells should be slightly higher and lower, respectively, than the values shown here (36.4% and 35.7%, respectively). The TREC values are expressed as copies per 10 000 cells. These data represent 3 different experiments.
TREC levels in FACS-purified thymocyte subsets from the human thymus.
TREC levels were measured in FACS-sorted cells from a fresh thymus of a newborn infant. For CD3− cells, the thymocytes were first depleted of CD3+ cells by MACS and then sorted by FACS for the subsets based on CD4 and CD8 expression (dot plot, upper right) to more precisely determine the timing of peak TREC production. Cells were also sorted for CD1a+/++ cells so as to exclude the CD1a−CD3− subset, which may contain precursors for NK cells and thymic dendritic cells. The sorted cells were subjected to TREC assay. The CD3+-depleted fraction (shown as R4 in the histogram, upper left) contained cells expressing CD3 at the intermediate level (referred to as CD3low) as well as CD3− cells. On the other hand, for measuring the frequency of each subpopulation in CD3− and CD3−/low populations, we analyzed whole thymocytes from the same thymus sample using gate R1. For CD3+ subsets, the whole thymocytes were analyzed for frequency measurement using gates R2 or R3 and then sorted for the TREC assay. Therefore, it should be noted that the actual frequency of CD3−/lowCD4+CD8+ and CD3+CD4+CD8+ cells should be slightly higher and lower, respectively, than the values shown here (36.4% and 35.7%, respectively). The TREC values are expressed as copies per 10 000 cells. These data represent 3 different experiments.
IL-7Rα expression in thymocyte subsets
As a basis for studying the role of IL-7 in TREC generation in human thymus, IL-7Rα expression was examined by flow cytometry on thymocytes from a fetus (22 weeks' gestation), a 2-month-old infant, and a 14-year-old youth (Figure 2). IL-7Rα expression was highest in immature CD3−CD4lowCD8− cells (89.0%-95.5%), just before TREC generation (Figures 1 and 2). Because cells with this phenotype are thought to be initiating the process of TCRαβ or γδ lineage commitment and are known to undergo proliferation,49 these results suggest the involvement of IL-7Rα in regulating the proliferation of thymocytes and their commitment to TCRαβ or γδ lineage. Although IL-7Rα expression tended to decrease after the CD3−CD4lowCD8− stage, it was still fairly high (52.2%-85.9%) in CD3− DP cells regardless of the age of the thymus. IL-7Rα expression was lowest in CD3+ DP cells (31.4%-57.7%) and then increased again in CD3+ SP cells (43.6%-69.2%).
IL-7Rα (CD127) expression in thymuses from subjects of varying ages.
Freshly isolated thymocytes from a 22-week gestation fetus, a 2-month-old infant, and a 14-year-old youngster were analyzed by flow cytometry for expression of IL-7Rα (CD127) on multiple cell types.
IL-7Rα (CD127) expression in thymuses from subjects of varying ages.
Freshly isolated thymocytes from a 22-week gestation fetus, a 2-month-old infant, and a 14-year-old youngster were analyzed by flow cytometry for expression of IL-7Rα (CD127) on multiple cell types.
The effects of IL-7 on proliferation and apoptosis of thymocytes
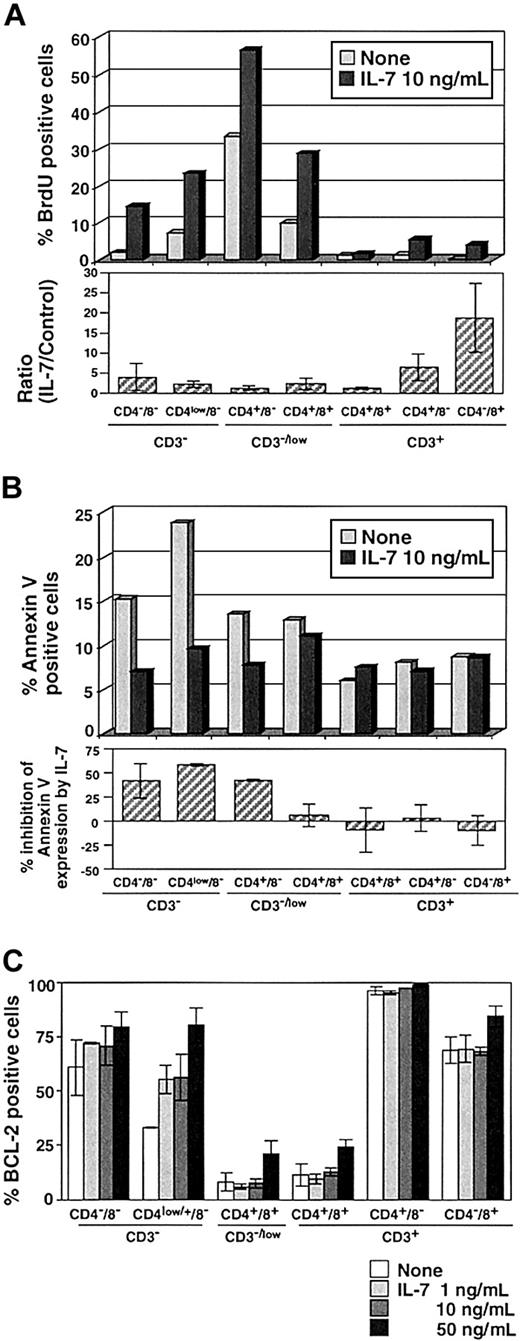
Several studies have suggested that IL-7 is a physiologic survival factor for early lymphoid progenitor cells.24 50-52 The evaluation of TREC levels in each thymic subpopulation will be affected by proliferation and apoptosis of thymocytes. To evaluate the in vitro effects of exogenous IL-7 on TREC generation in our TOC system, we first assessed the effect of IL-7 on thymocyte proliferation by measuring the incorporation of BrdU. The upper panel of Figure3A represents several experiments using different thymuses. The rate of BrdU incorporation was highest in CD3−/lowCD4+CD8− cells and low in mature CD3+ cells (Figure 3A). We observed a marked increase in BrdU uptake in most thymocyte populations with the administration of 10 ng/mL IL-7 (Figure 3A). Increased BrdU uptake with IL-7 was observed consistently in 5 different TOCs (Figure 3A, lower panel), not only in immature cells (4.0 ± 3.3-fold and 2.3 ± 0.8-fold in TN and CD3−CD4lowCD8−, respectively) but also in more mature CD3+CD4+ SP (6.6 ± 3.3-fold) and CD3+CD8+ SP cells (18.8 ± 8.6-fold). This effect was dependent on IL-7 dose (data not shown).
Proliferation and apoptosis of thymocytes in response to IL-7.
(A) The incorporation of BrdU was measured in thymocytes in the presence (10 ng/mL) or absence of IL-7 on day 4 of TOC. Upper panel shows representative results from 5 different experiments. Ratio of IL-7 to control is shown in the lower panel. (B) The inhibition effect of IL-7 on apoptosis of thymocytes was evaluated by flow cytometry by the expression of annexin V after 4 days of TOC. Upper panel shows the representative result from 5 different experiments. Lower panel shows the percent inhibition of annexin V expression by IL-7 to control TOC. BCL-2 expression was evaluated in TOC with 1, 10, and 50 ng/mL IL-7. Data are expressed as mean value ± SD in triplicate.
Proliferation and apoptosis of thymocytes in response to IL-7.
(A) The incorporation of BrdU was measured in thymocytes in the presence (10 ng/mL) or absence of IL-7 on day 4 of TOC. Upper panel shows representative results from 5 different experiments. Ratio of IL-7 to control is shown in the lower panel. (B) The inhibition effect of IL-7 on apoptosis of thymocytes was evaluated by flow cytometry by the expression of annexin V after 4 days of TOC. Upper panel shows the representative result from 5 different experiments. Lower panel shows the percent inhibition of annexin V expression by IL-7 to control TOC. BCL-2 expression was evaluated in TOC with 1, 10, and 50 ng/mL IL-7. Data are expressed as mean value ± SD in triplicate.
We next assessed the effects of IL-7 on apoptosis in TOCs by measuring expression of annexin V after 4 days of TOC in the presence or absence of IL-7. IL-7 significantly lowered annexin V expression by about 50% in CD3−CD8− immature thymocytes (Figure 3B, upper and lower panels), which were pre–TREC-generating cells (Figure1). We then examined BCL-2, a well-established protein, which is involved in the suppression of apoptosis. It has been reported that BCL-2 expression during T-cell differentiation is multiphasic; it is high in CD3− immature thymocytes, down-regulated in CD4+CD8+ thymocytes to facilitate selection process, and up-regulated in mature CD3+ SP thymocytes thus enhancing prolonged survival.53 As expected, IL-7 enhanced BCL-2 expression in CD3− immature thymocytes in a dose-dependent way (Figure 3C). These results were consistent with previous studies in mice demonstrating that short-term culture with IL-7 of immature thymocytes from IL-7−/− mice caused up-regulation of BCL-2 and increased cell survival.51Thus, in human thymus, IL-7 led to an increase in proliferation and decrease in apoptosis of immature CD3− to CD3low cells, which correlated with the increased expression of BCL-2.
The in vitro effects of IL-7 on TREC generation in TOCs
The effects of IL-7 on TREC generation were examined in TOCs. After culturing newborn thymus in the presence (10 and 50 ng/mL) or absence of IL-7 for 4 days, thymocytes were separated into CD3− and CD3+ fractions by MACS (Figure4A). The majority of CD3lowcells were contained in the CD3− fraction (Figure 4A), whereas most of CD3+ DP cells were collected into the CD3+ fraction (data not shown). Figure 4B shows the TREC frequency in TOCs. IL-7 increased TREC levels in both CD3−and CD3+ subsets in a dose-dependent way. Although there was a difference between 10 ng/mL IL-7–treated and untreated TOC, the only statistically significant difference existed between the 50 ng/mL and untreated TOCs (P < .09 and P < .001 in CD3− and CD3+ subsets, respectively).
The in vitro effects of IL-7 on TREC generation in TOCs.
Thymocytes from a newborn thymus were separated by MACS for CD3− and CD3+ subsets before or after 4 days of TOC in the absence or presence of IL-7 (10 and 50 ng/mL) and TREC levels were measured. (A) The representative data of CD3 expression is shown in CD3− (thin line) and CD3+ (bold line) fractions sorted by selection column from preculture and day 4 of TOC in the absence or presence of IL-7 (10 ng/mL). (B) TREC levels in the 2 fractions were measured. Three separate experiments were performed. Each experiment was performed in quadruplicate with all 3 concentrations of IL-7. A representative result is shown with error bars pertaining to the experiment.
The in vitro effects of IL-7 on TREC generation in TOCs.
Thymocytes from a newborn thymus were separated by MACS for CD3− and CD3+ subsets before or after 4 days of TOC in the absence or presence of IL-7 (10 and 50 ng/mL) and TREC levels were measured. (A) The representative data of CD3 expression is shown in CD3− (thin line) and CD3+ (bold line) fractions sorted by selection column from preculture and day 4 of TOC in the absence or presence of IL-7 (10 ng/mL). (B) TREC levels in the 2 fractions were measured. Three separate experiments were performed. Each experiment was performed in quadruplicate with all 3 concentrations of IL-7. A representative result is shown with error bars pertaining to the experiment.
If IL-7 were to be used as a therapeutic agent to improve immune reconstitution, its effect would have to be maintained in postnatal thymuses. The effects of IL-7 on TREC levels were further evaluated in TOCs using thymuses from subjects of differing ages. Fetal (18, 19, and 22 weeks' gestation), newborn (2 and 15 day, and 3, 4, 7, and 11 month), and infant (3 and 5.6 year) thymuses were placed in TOCs in the presence of IL-7, and TRECs were measured (Table1). In all TOCs, TREC levels increased in the presence of IL-7 (1.3 ± 0.1-fold for whole cells, 2.0 ± 0.8-fold for CD3− fractions, and 1.5 ± 0.2-fold for CD3+ fractions).
The effect of IL-7 on TREC levels in TOCs using thymuses of differing ages
| TOC no. . | Age of thymus . | TREC/10,000 cells . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 4 or Day 5* . | ||||||||||
| Whole . | CD3− . | CD3+ . | ||||||||
| Control . | IL-7 . | Ratio† . | Control . | IL-7 . | Ratio† . | Control . | IL-7 . | Ratio† . | ||
| 1 | Fetal 18 wk-1 | 4 738 | 6 074 | 1.3 | 4 120 | 13 561 | 3.3 | ND | ND | ND |
| 2 | 18 wk-2 | ND | ND | ND | 4 047 | 11 318 | 2.8 | ND | ND | ND |
| 3 | 19 wk | ND | ND | ND | 3 970 | 4 883 | 1.2 | 6 618 | 8 347 | 1.3 |
| 4 | 22 wk | ND | ND | ND | 1 703 | 3 885 | 2.3 | 3 310 | 5 321 | 1.6 |
| 5 | Newborn 2 d | 10 639 | 15 265 | 1.4 | 7 733 | 13 462 | 1.7 | 7 808 | 12 697 | 1.6 |
| 6 | 15 d | 9 790 | 12 333 | 1.3 | 2 080 | 5 044 | 2.4 | 7 507 | 9 751 | 1.3 |
| 7 | 3 mo | 4 682 | 6 943 | 1.5 | ND | ND | ND | ND | ND | ND |
| 8 | 4 mo | 5 175 | 6 756 | 1.3 | 2 616 | 3 232 | 1.2 | ND | ND | ND |
| 9 | 7 mo | 6 071 | 7 503 | 1.2 | 2 389 | 5 585 | 2.3 | 9 700 | 14 979 | 1.5 |
| 10 | 11 mo | 5 820 | 7 884 | 1.4 | 4 195 | 4 387 | 1.0 | 5 768 | 8 416 | 1.5 |
| 11 | Infant 3 y | 8 590 | 12 183 | 1.4 | ND | ND | ND | ND | ND | ND |
| 12 | 5.6 y | 8 297 | 10 200 | 1.2 | ND | ND | ND | ND | ND | ND |
| Mean ± SD | 1.3 ± 0.1 | 2.0 ± 0.8 | 1.5 ± 0.2 | |||||||
| TOC no. . | Age of thymus . | TREC/10,000 cells . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 4 or Day 5* . | ||||||||||
| Whole . | CD3− . | CD3+ . | ||||||||
| Control . | IL-7 . | Ratio† . | Control . | IL-7 . | Ratio† . | Control . | IL-7 . | Ratio† . | ||
| 1 | Fetal 18 wk-1 | 4 738 | 6 074 | 1.3 | 4 120 | 13 561 | 3.3 | ND | ND | ND |
| 2 | 18 wk-2 | ND | ND | ND | 4 047 | 11 318 | 2.8 | ND | ND | ND |
| 3 | 19 wk | ND | ND | ND | 3 970 | 4 883 | 1.2 | 6 618 | 8 347 | 1.3 |
| 4 | 22 wk | ND | ND | ND | 1 703 | 3 885 | 2.3 | 3 310 | 5 321 | 1.6 |
| 5 | Newborn 2 d | 10 639 | 15 265 | 1.4 | 7 733 | 13 462 | 1.7 | 7 808 | 12 697 | 1.6 |
| 6 | 15 d | 9 790 | 12 333 | 1.3 | 2 080 | 5 044 | 2.4 | 7 507 | 9 751 | 1.3 |
| 7 | 3 mo | 4 682 | 6 943 | 1.5 | ND | ND | ND | ND | ND | ND |
| 8 | 4 mo | 5 175 | 6 756 | 1.3 | 2 616 | 3 232 | 1.2 | ND | ND | ND |
| 9 | 7 mo | 6 071 | 7 503 | 1.2 | 2 389 | 5 585 | 2.3 | 9 700 | 14 979 | 1.5 |
| 10 | 11 mo | 5 820 | 7 884 | 1.4 | 4 195 | 4 387 | 1.0 | 5 768 | 8 416 | 1.5 |
| 11 | Infant 3 y | 8 590 | 12 183 | 1.4 | ND | ND | ND | ND | ND | ND |
| 12 | 5.6 y | 8 297 | 10 200 | 1.2 | ND | ND | ND | ND | ND | ND |
| Mean ± SD | 1.3 ± 0.1 | 2.0 ± 0.8 | 1.5 ± 0.2 | |||||||
ND indicates not done.
TOC no. 3 and 11 were cultured for 5 days and others were cultured for 4 days.
IL-7(+)/IL-7(−).
The in vivo effects of IL-7 on TREC generation in NOD-SCID-hu mice
Several studies have demonstrated the in vivo effects of IL-7 on mouse thymopoiesis.54 55 In the present study we used NOD-SCID-hu chimeric mice to measure the in vivo effects of IL-7 on human thymopoiesis. Twenty-eight days after engraftment of human fetal thymus and liver under the mouse kidney capsule, the growth of thymic grafts was readily apparent; typically, a 2-mm3 fragment grew up to a diameter of 5 to 7 mm. These grafts contained comparable levels of TRECs per thymocytes to those of native human thymuses (data not shown), indicating the generation of new thymocytes in the grafts. More importantly, we were able to detect human CD45+ cells in the peripheral blood and spleen. Most of these human cells were positive for CD3 (data not shown) and represented 1.0% and 1.3% of the total lymphocytes in PBMCs and spleen, respectively (Figure5A, upper panel). These CD3+cells comprised mature CD4+ and CD8+ SP cells (Figure 5A, middle panel), the majority of which were CD45RA+, suggesting that they were naı̈ve T cells (Figure 5A, bottom panel). The frequency of human CD3+CD45RA+CD4+ or CD3+CD45RA+CD8+ T cells correlated positively with the size of the graft (data not shown).
The in vivo effects of IL-7 on TREC generation in NOD-SCID-hu mice.
Four weeks after implantation of human fetal thymus and liver, NOD-SCID-hu mice were treated with 100 ng IL-7 twice daily for 10 days. Seven (control, n = 3; IL-7, n = 4) and 21 days (control, n = 5; IL-7, n = 7) after the last injection, the grafts, spleen, and PBMCs were recovered from the mice for phenotypic analysis and TRECs. The upper panels of panel A indicate percentage of human CD3+cells and the panels in the middle row of panel A indicate human CD4+ and CD8+ expression on the CD3+ population of PBMC (left) and spleen (right). The bottom figures of panel A show CD45RA+ cells on CD3+CD4+ or CD3+CD8+populations of PBMCs (left) and spleen (right). The TREC levels of grafts and the graft weights in control and IL-7–treated mice are shown in panels B and C, respectively. The percentages of Ki67+ cells in each subpopulation of grafts are shown in panel D.
The in vivo effects of IL-7 on TREC generation in NOD-SCID-hu mice.
Four weeks after implantation of human fetal thymus and liver, NOD-SCID-hu mice were treated with 100 ng IL-7 twice daily for 10 days. Seven (control, n = 3; IL-7, n = 4) and 21 days (control, n = 5; IL-7, n = 7) after the last injection, the grafts, spleen, and PBMCs were recovered from the mice for phenotypic analysis and TRECs. The upper panels of panel A indicate percentage of human CD3+cells and the panels in the middle row of panel A indicate human CD4+ and CD8+ expression on the CD3+ population of PBMC (left) and spleen (right). The bottom figures of panel A show CD45RA+ cells on CD3+CD4+ or CD3+CD8+populations of PBMCs (left) and spleen (right). The TREC levels of grafts and the graft weights in control and IL-7–treated mice are shown in panels B and C, respectively. The percentages of Ki67+ cells in each subpopulation of grafts are shown in panel D.
We treated NOD-SCID-hu mice with 100 ng IL-7 (n = 11) or saline (n = 8) twice daily for 10 days beginning 4 days after checking the size of the grafts. TREC levels in thymocytes from the grafts were significantly higher in the IL-7–treated group (14 276 ± 1290 and 14 344 ± 2449 for day 7 and day 21, respectively) than the control group (8135 ± 1070 and 9877 ± 1869 for day 7 and day 21, respectively) at day 7 (P < .002) and day 21 (P < .007) after the last administration of IL-7 (Figure5B). Although the average graft weight (Figure 5C) was higher in IL-7–treated mice after 7 days, the difference between the 2 groups was not statistically significant either after 7 or 21 days. Moreover, there was no change by IL-7 treatment in the percentage of Ki67+ cells in any of the subsets except for CD3+ DP and CD3+ CD8 SP at day 7 (Figure 5D). Also, there was no significant increase either in the percentage of human CD3+ cells or TREC levels in the peripheral blood after IL-7 treatment (data not shown).
Discussion
It is well established that IL-7 exerts potent effects on T-cell progenitors and is required for T-cell development. It is critical for homeostatic proliferation and survival of not only thymocytes but also naı̈ve T cells in the peripheral blood and lymph nodes.56 In the present study we used the quantitative TREC assay to assess more definitively the effects of exogenous IL-7 on TCR rearrangement in thymocytes using both in vitro TOCs and thymic engraftments in NOD-SCID mice.
To understand the timing of TREC generation and its dilution during thymic development, we quantified TREC level in each thymic subpopulation. We found the highest level of TREC in the CD1a+CD3− DP stage. Recently, TCRβ selection in the human thymus was reported to be initiated at the transition of CD3−CD4+CD8− into the CD4+CD8α+β−stage,57 whereas TCRA gene expression has been known to be restricted to CD3+ DP and later stages of T-cell development. Together with these, our data suggest thatTCRB gene rearrangement precedes and induces the initiation of TREC generation, followed by TCRArearrangement. TREC generation may represent a decisive step in the αβ versus γδ lineage commitment of differentiating thymocytes because this is an intermediate rearrangement between TCRDand TCRA gene rearrangement, which excises most of theTCRD locus.58 The only apparent reason for this rearrangement is to delete the TCRD locus to prepare the allele for subsequent TCRA gene rearrangement. Thus, our finding that IL-7 increases TREC levels of thymocytes most likely indicates that IL-7 eventually leads to the enhancement of induction ofTCRA and TCRB gene rearrangement.
Although IL-7 increased TREC in our TOC system, we cannot definitively prove that this is due to the promotion of TREC generation in the individual thymocytes because TREC levels are also affected by the ability of IL-7 to regulate apoptosis and proliferation of cells at the pre–TREC-generating stage.49,50,51 59 Indeed, BrdU, annexin V, and BCL-2 staining in our TOC system clearly demonstrated that exogenous IL-7 can enhance the proliferation and prevent apoptosis of immature CD3−/low subsets (Figure 3) which, according to our results (Figure 1), precede TREC generation. It is quite possible, therefore, that IL-7 indirectly increases the TREC frequency in the thymus by promoting the proliferation and survival of thymocytes pre-TREC generation.
On the other hand, our in vivo experiments suggested another pathway of IL-7 effects. Exogenous IL-7 was administered to NOD-SCID-hu mice, where the growth of human thymic grafts appeared to be fairly active. At 7 and 21 days after the last administration, TREC levels in thymocytes from the grafts were significantly higher in the IL-7–treated group than the control group (Figure 5B). However, the graft weights and also Ki67 expression in any of the subsets did not show a significant change by IL-7 treatment (Figure 5C and 5D, respectively). Considering that IL-7 can exhibit distinct effects on T cells at different concentrations,60 one possible interpretation of our data would be that the endogenous human IL-7 in these grafts was sufficient to maintain the normal development of thymocytes, whereas the addition of exogenous IL-7 further promoted TCR gene rearrangement and therefore TREC generation, but was not enough to promote further proliferation of immature cells. In this experiment we did not measure the rate of apoptosis in the thymic grafts. However, it is unlikely that this increase in TREC by IL-7 was caused solely by lengthening the survival of immature cells because the percentage of annexin V+ cells in fresh human thymus is typically as low as 0.5% to 4.0% in each subpopulation (Yukari Okamoto unpublished data, May 1999). Therefore, the logical interpretation is that IL-7 can directly stimulate TREC generation. Based on transgenic mouse studies, it has been suggested that there is a regulatory sequence near the human δRec gene segment, which is recognized by putative regulatory proteins that induce δRec rearrangement.61 Moreover, transcription of the T-early α (TEA) element is assumed to be responsible for opening theTCRAJ locus to the V(D)J recombinase complex.62 63 Further identification of regulatory mechanism of the δRec-ψJα recombination event will eventually allow a determination of whether IL-7 has a direct effect on intracellular processes that lead to enhanced TREC production.
In our NOD-SCID-hu mouse system, we observed no significant increase for the percentage of human CD3+ cells in the peripheral blood as a result of IL-7 treatment. It has been reported that mouse NK cells affect the efficiency of engraftment of human cells in this model.64 Although NOD/LtSz-scid mice have relatively low NK cell activity,65 it is possible that IL-7 caused an enhancement of this.66 67 Be that as it may, our data clearly show that IL-7 increased TCR gene rearrangement and TREC generation, which would suggest that thymopoietic function was augmented in vivo.
Complete recovery of broad T-cell immunity after insults such as HIV infection or chemotherapy will require the generation of new naı̈ve T cells from the thymus.2,16,17,21 The administration of thymopoietic cytokines may be able to improve immune reconstitution by augmenting thymic function in these situations. IL-7 is a major factor for thymopoiesis, and systemic administration of recombinant IL-7 to murine bone marrow transplant recipients has been shown to normalize thymopoiesis and improve immune function after bone marrow transplantation.21,68,69 Furthermore, endogenous IL-7 may play a major role in the homeostatic response to T lymphopenia.37 Our data suggest that IL-7 can enhance the function of fetal and infant thymuses, although in therapeutic practice it may be necessary to antagonize the effects of thymic atrophic factors.70 The decrease in thymocyte apoptosis mediated by high concentrations of exogenous IL-7 in CD3+ DP population may affect negative selection. Furthermore, it should also be considered that high concentrations of exogenous IL-7 may break unresponsiveness of residual autoreactive T cells in healthy individuals by stimulating the anergic T cells that suppress the autoreactive cells71 and may cause autoimmune disease. Nevertheless, our results provide a basis for understanding some of the regulatory mechanisms of thymocyte development in the human thymus and encourage the further evaluation of cytokine-based immune reconstitution strategies, through the stimulation of thymus-dependent T-cell generation.
We thank Brenna Hill, Joseph Beckham, and Angie Mobley for technical help; Dr Michael Betts and Dr Takeshi Kurata for helpful advice; and Dr Steven Leonard for procurement of postnatal thymuses.
Supported by a grant from Japanese Foundation of AIDS Prevention (to Y.O.), and by a Leukemia and Lymphoma Society of America translational research grant 6540-00 and American Foundation for AIDS Research (AmFAR) grant 02680-28-RGV (to D.C.D.). The opinions expressed are those of the author (R.D.M.) and not necessarily those of the US Food and Drug Administration.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard A. Koup, Immunology Laboratory, National Institutes of Health, Vaccine Research Center, Rm 3502, 40 Convent Dr, Bethesda, MD 20892; e-mail: rkoup@mail.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal