Abstract
Most plasmas from patients with inhibitors contain antibodies that are reactive with the C2 domain of factor VIII. Previously, we have shown that the variable heavy chain (VH) regions of antibodies to the C2 domain are encoded by the closely related germline gene segments DP-10, DP-14, and DP-88, which all belong to the VH1 gene family. Here, we report on the isolation and characterization of additional anti-C2 antibodies that are derived from VH gene segments DP-88 and DP-5. Competition experiments using murine monoclonal antibodies CLB-CAg 117 and ESH4 demonstrated that antibodies derived from DP-5 and DP-88 bound to different sites within the C2 domain. Epitope mapping studies using a series of factor VIII/factor V hybrids revealed that residues 2223 to 2332 of factor VIII are required for binding of the DP-10–, DP-14–, and DP-88–encoded antibodies. In contrast, binding of the DP-5–encoded antibodies required residues in both the amino- and carboxy-terminus of the C2 domain. Inspection of the reactivity of the antibodies with a series of human/porcine hybrids yielded similar data. Binding of antibodies derived from germline gene segments DP-10, DP-14, and DP-88 was unaffected by replacement of residues 2181 to 2243 of human factor VIII for the porcine sequence, whereas binding of the DP-5–encoded antibodies was abrogated by this replacement. Together these data indicate that antibodies assembled from VH gene segments DP-5 and the closely related germline gene segments DP-10, DP-14, and DP-88 target 2 distinct antigenic sites in the C2 domain of factor VIII.
Introduction
Hemophilia A is an X-linked bleeding disorder that is characterized by the absence or dysfunction of blood coagulation factor VIII. Current treatment of hemophilia A consists of infusion of therapeutic amounts of factor VIII that can evoke an immune response in some patients. The presence of neutralizing antibodies to factor VIII, commonly termed factor VIII inhibitors, presents a serious complication of hemophilia care.1 The biochemical properties of factor VIII inhibitors have been extensively studied with emphasis on the epitope specificity and mode of action of these antibodies. Binding sites for inhibitors have been identified within the A2, A3, and C2 domains of factor VIII.2-6 In general, heterogeneous mixtures of anti–factor VIII antibodies are present in plasma from patients with factor VIII inhibitors, and in more than 80% of inhibitor plasmas antibodies directed against the C2 domain are present.7 Epitope mapping of anti-C2 domain antibodies revealed the presence of an inhibitor binding site comprising amino acid residues Val2248-Ser2312 of the C2 domain.3 A second inhibitor epitope in the C2 domain has been attributed to region Glu2181-Val2243.6 Antibodies reactive with the C2 domain prevent factor VIII from binding to phospholipid surfaces and von Willebrand factor.8,9 Both findings are in agreement with the presence of binding sites for phospholipids and von Willebrand factor in the C2 domain.10,11 An additional mechanism of factor VIII inhibition has been described for less common human antibodies directed to the C2 domain.12 These antibodies did not block the binding of factor VIII to von Willebrand factor but reduced the release of activated factor VIII from von Willebrand factor. Although both the factor VIII inhibitory mechanism and epitope specificity of C2 inhibitors are well understood, knowledge about the primary structure of these antibodies is limited.
Recently, anti-C2 antibodies have been studied at the clonal level using phage display technology.13 The variable heavy chain (VH) domains of these antibodies were encoded by VH germline gene segments DP-10, DP-14, and DP-88 derived from the VH1 gene family. Furthermore, these VHdomains contain hypervariable complementarity determining region 3 (CDR3) loops that were relatively large (20-23 residues) compared with the average CDR3 length of 14 residues.14,15 Another human anti-C2 antibody has been isolated from a hemophilia A patient with an inhibitor using classical Epstein-Barr virus immortalization.16 The VH domain of this antibody was encoded by germline gene segment DP-5, which is also derived from the VH1 gene family. This antibody inhibited factor VIII activity by preventing binding of factor VIII to von Willebrand factor and phospholipid surfaces. Interestingly, the anti-C2 antibodies expressed as single-chain variable domain antibody fragments (scFv) composed of DP-10–, DP-14–, and DP-88–encoded VHdomains did not inhibit factor VIII activity.13 In the present study, additional antibodies were isolated from the immunoglobulin repertoire of a mild hemophilia A patient with anti-C2 antibodies. The isolated antibodies were encoded by heavy chain germline gene segments DP-5 and DP-88. Epitope mapping studies revealed that antibodies encoded by these 2 classes of VH gene segments bind to distinct antigenic sites within the C2 domain.
Materials and methods
Materials
DNA-modifying enzymes were purchased from Life Technologies (Breda, The Netherlands) and New England Biolabs (Beverly, MA). Immunotubes and microtiter plates were purchased from Nunc (Life Technologies). Plasma-derived factor VIII light chain was purified as described.17 Monoclonal antibodies CLB-CAg 12 and 117 have been characterized previously4,17; monoclonal antibody ESH418 was purchased from American Diagnostica (Greenwich, CT).
Factor VIII assays
Phage display library construction, selection, and characterization of selected clones
In this study, plasma and peripheral blood mononuclear cells were used from a mild hemophilia A patient with an Arg2163His mutation who developed a factor VIII inhibitor.22 The patient's IgG4-specific VH gene repertoire was amplified and combined with a variable light chain (VL) gene repertoire of nonimmune origin in pHEN-1-VLrep and displayed as scFv on the surface of filamentous phage.13 Phages from the library were selected on factor VIII light chain immobilized via antibody CLB-CAg 12–coated microtiter wells as described previously.13After 3 rounds of selection for binding to the factor VIII light chain, the factor VIII domain specificity of phages obtained from single infected colonies was determined. Phages were tested for reactivity with factor VIII light chain immobilized to CLB-CAg 12 as described previously.13 Phages corresponding to clones reactive with factor VIII light chain were selected for further study. VHand VL genes were sequenced on an Applied Biosystems 377XL automated DNA sequencer (Foster City, CA) using the BigDye Terminator sequencing kit. Nucleotide sequences were aligned to their most homologous germline sequences as present in the V-BASE sequence directory.23 The selected clones were subsequently tested for reactivity with the C2 domain of factor VIII in the following manner. Monoclonal antibodies ESH4 or CLB-CAg 117 were immobilized on microtiter wells overnight at 4°C. Subsequently, wells were incubated for 1.5 hours with recombinant C2 domain (3.5 nM in 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 2% [wt/vol] human serum albumin; and 0.1% [vol/vol] Tween 20). Wells were washed 3 times with 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; and 0.1% Tween 20. Recombinant phages derived of single infected clones were then added in 150 mM NaCl; 50 mM Tris-HCl, pH 7.4; 3% (wt/vol) human serum albumin; and 0.5% (vol/vol) Tween 20 and incubated for 2 hours at room temperature. Wells were washed 3 times with 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; and 0.1% Tween 20. Bound phages were detected following a 2-hour incubation at room temperature with peroxidase-labeled anti-M13 antibody (Pharmacia-LKB, Woerden, The Netherlands) diluted 2000-fold in 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 2% (wt/vol) human serum albumin; and 0.05% (vol/vol) Tween 20.
Production and purification of scFv was performed as described previously.13 Purified scFv were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and protein concentrations were measured spectrophotometrically at 280 nm. Reactivity of scFv with recombinant factor VIII and factor V hybrid molecules was evaluated by immunoprecipitation analysis. Construction and expression of hybrid human factor VIII molecules containing porcine C2 domain substitutions and hybrid human factor V molecules containing factor VIII C2 domain substitutions have been described previously.6,24,25 Immunoprecipitation of metabolically labeled factor VIII fragments by scFv/Ni-NTA agarose (Qiagen, Hilden, Germany) complexes was performed as described previously.13
Surface plasmon resonance
The kinetics of the interaction between scFv and factor VIII light chain were determined by surface plasmon resonance using a BIAcore 2000 biosensor system (Biacore, Uppsala, Sweden). Factor VIII light chain was covalently coupled to the dextran surface of an activated CM5 sensor chip at a density of 88.5 fm/mm2 using the amine-coupling kit according to the manufacturer's instructions (Biacore). As a control, one of the 4 channels on a chip was activated and blocked in the absence of protein. Binding to coated channels was corrected for binding to noncoated channels (< 5% of binding to coated channels). Binding (association) of scFv was assessed in 100 mM NaCl, 100 mM imidazole, and 50 mM sodium phosphate, pH 7.5, at 25°C for 2 minutes at a flow rate of 20 μL/min. Dissociation was allowed for 2 minutes in the same buffer flow. Association (kon) and dissociation (koff) rate constants were calculated from data sets using the BIAevaluation 3.1 software (Biacore). Binding data were corrected for bulk refractive index changes and fitted according to a one-site model. Equilibrium dissociation constants (Kd) were calculated from the measured values ofkoff and kon.
Results
Isolation of anti–factor VIII antibodies using phage display
The domain specificity of antibodies present in the plasma of an inhibitor patient with mild hemophilia A was determined by immunoprecipitation analysis. The patient's antibodies reacted with metabolically labeled factor VIII light chain and the C2 domain. Approximately 80% of the inhibitory activity originated from anti-C2 antibodies as was determined by neutralization assays (data not shown). These data indicate that the C2 domain is the major target for factor VIII inhibitors present in plasma of this patient. To further examine the patient's anti-C2 domain antibodies, we used phage display to isolate factor VIII–reactive antibodies from the patient's immunoglobulin repertoire. Therefore, a phage display library derived from the patient's IgG4-specific VH gene repertoire and a VL gene repertoire of nonimmune source was constructed. The library, which consisted of 1.9 × 107 clones, was subsequently selected for binding to plasma-derived factor VIII light chain immobilized via antibody CLB-CAg 12. After 3 rounds of selection, phages derived from 19 of 20 clones were found to react with factor VIII light chain. Nucleotide sequences of the VH and VL domains of the 19 clones were determined and aligned to their most homologous germline genes in the V-BASE directory.23 The VH domains of 5 clones were encoded by germline gene segment DP-5. Germline gene segment DP-88 could be identified as the most homologous gene segment encoding the VH domains of the other 14 clones. Both gene segments belong to the VH1 gene family. In total, 3 unique VH domains encoded by germline gene segment DP-5 were identified (Figure 1). The VHdomains of WR1 and WR17 are highly homologous, varying in only 2 amino acid residues. At the nucleotide level, 4 substitutions were observed, which makes it unlikely that the observed differences resulted from polymerase chain reaction artifacts. The VH domains of WR1/17 and WR16 were generated by VDJ recombination using JH gene segments JH4b and JH3b, respectively. Rearrangement of particular D gene segments could not be determined as a consequence of limited sequence homology with known D genes. CDR3 sequences of WR1/17 and WR16 consisted of 11 and 9 amino acids, respectively.
Deduced protein sequences of scFv that bind to the C2 domain.
Sequence numbering is according to Kabat et al.26Sequences are available from GenBank under accession numbers AY050689(VHWR1), AY050690 (VHWR10), AY050691 (VLWR10), AY050692 (WR11),AY050693 (VLWR11), AY050694 (VHWR13), AY050695 (VHWR14), AY050696(VHWR15), AY050697 (VLWR15), AY050698 (VHWR16), AY050699 (VLWR16),AY050700 (VHWR17), AY050701 (VLWR17), AY050702 (VHWR19), AY050703(VLWR1), AY050704 (VHWR2), AY050705 (VHWR20), AY050706 (VHWR3),AY050707 (VLWR3), AY050708 (VHWR7), AY050709 (VHWR8), AY050710(VLWR10), AY050711 (VHWR9), and AY050712 (VLWR9). FR indicates framework region; dashes, sequence identity to germline. Amino acid substitutions most likely introduced by the use of VH gene family–specific oligonucleotide primers during amplification of VH repertoires are indicated in lowercase.
Deduced protein sequences of scFv that bind to the C2 domain.
Sequence numbering is according to Kabat et al.26Sequences are available from GenBank under accession numbers AY050689(VHWR1), AY050690 (VHWR10), AY050691 (VLWR10), AY050692 (WR11),AY050693 (VLWR11), AY050694 (VHWR13), AY050695 (VHWR14), AY050696(VHWR15), AY050697 (VLWR15), AY050698 (VHWR16), AY050699 (VLWR16),AY050700 (VHWR17), AY050701 (VLWR17), AY050702 (VHWR19), AY050703(VLWR1), AY050704 (VHWR2), AY050705 (VHWR20), AY050706 (VHWR3),AY050707 (VLWR3), AY050708 (VHWR7), AY050709 (VHWR8), AY050710(VLWR10), AY050711 (VHWR9), and AY050712 (VLWR9). FR indicates framework region; dashes, sequence identity to germline. Amino acid substitutions most likely introduced by the use of VH gene family–specific oligonucleotide primers during amplification of VH repertoires are indicated in lowercase.
Of 14 VH domains encoded by the DP-88 germline gene segment, 8 unique VH domains were identified (Figure 1). All VH domains were heavily hypermutated compared with the DP-88 germline. Seven of 8 VH domains shared homologous patterns of somatic hypermutation, suggesting that these VHdomains share an identical B-cell precursor. It should be noted that amino acid replacements in WR3, WR7, WR11, and WR15 were caused by single nucleotide substitutions. Consequently, it cannot be excluded that the nucleotide substitutions in these VH domains were introduced during amplification of the patient's VH gene repertoire. All VH domains, except that of WR10, have a similar CDR3, which is partially encoded by the largest known JH gene segment, JH6b. The VHdomain of WR10 has a different pattern of somatic mutation compared with the other DP-88–encoded VH domains (Figure 1). Furthermore, the CDR3 of WR10, which comprises 20 amino acid residues, has been assembled using gene segments D3-10 and JH3b. Different VL domains encoded by germline gene segments of V and V gene families were paired with single VH domains derived from DP-5 and DP-88 germline gene segments (Table1).
Most homologous germline gene segments used by human antibodies directed against the C2 domain
| Clone . | VH domain germline . | VL domain . | Reference . | |
|---|---|---|---|---|
| Germline . | Family . | |||
| WR1 | DP-5 (1-24) | DPK8 (L8) | VI | This study |
| WR17 | DPL5 (V1-19) | V1 | This study | |
| WR16 | DPK3 (L11) | VI | This study | |
| BO2C11 | DPK22 (A27) | VIII | 16 | |
| YK3-3-38 | — | — | 28 | |
| YK3-3-40 | — | — | 28 | |
| YK3-3-50 | — | — | 28 | |
| EL-14 | DP-10 (1-69) | DPK5 (L5) | VI | 13 |
| EL-5 | DP-14 (1-18) | L12a | VI | 13 |
| EL-16 | DPK8 (L8) | VI | 13 | |
| EL-25 | DPK7 (L15) | VI | 13 | |
| EL-9 | DP-88 (1-e) | DPK8 (L8) | VI | 13 |
| WR10 | DPL11 (V1-4) | V2 | This study | |
| WR2/7/13 | ND | ND | This study | |
| WR3 | DPK3 (L11) | VI | This study | |
| WR8 | DPK24 (B3) | VIV | This study | |
| WR9 | V1-2 (V1-2) | V2 | This study | |
| WR11 | DPL11 (V1-4) | V2 | This study | |
| WR15 | DPL16 (V2-13) | V3 | This study | |
| Clone . | VH domain germline . | VL domain . | Reference . | |
|---|---|---|---|---|
| Germline . | Family . | |||
| WR1 | DP-5 (1-24) | DPK8 (L8) | VI | This study |
| WR17 | DPL5 (V1-19) | V1 | This study | |
| WR16 | DPK3 (L11) | VI | This study | |
| BO2C11 | DPK22 (A27) | VIII | 16 | |
| YK3-3-38 | — | — | 28 | |
| YK3-3-40 | — | — | 28 | |
| YK3-3-50 | — | — | 28 | |
| EL-14 | DP-10 (1-69) | DPK5 (L5) | VI | 13 |
| EL-5 | DP-14 (1-18) | L12a | VI | 13 |
| EL-16 | DPK8 (L8) | VI | 13 | |
| EL-25 | DPK7 (L15) | VI | 13 | |
| EL-9 | DP-88 (1-e) | DPK8 (L8) | VI | 13 |
| WR10 | DPL11 (V1-4) | V2 | This study | |
| WR2/7/13 | ND | ND | This study | |
| WR3 | DPK3 (L11) | VI | This study | |
| WR8 | DPK24 (B3) | VIV | This study | |
| WR9 | V1-2 (V1-2) | V2 | This study | |
| WR11 | DPL11 (V1-4) | V2 | This study | |
| WR15 | DPL16 (V2-13) | V3 | This study | |
VH and VL germline gene use and nomenclature according to V-BASE.23 All observed VH germline genes belong to the VH1 gene family.
ND indicates not determined.
Epitope specificity of scFv reactive with the C2 domain
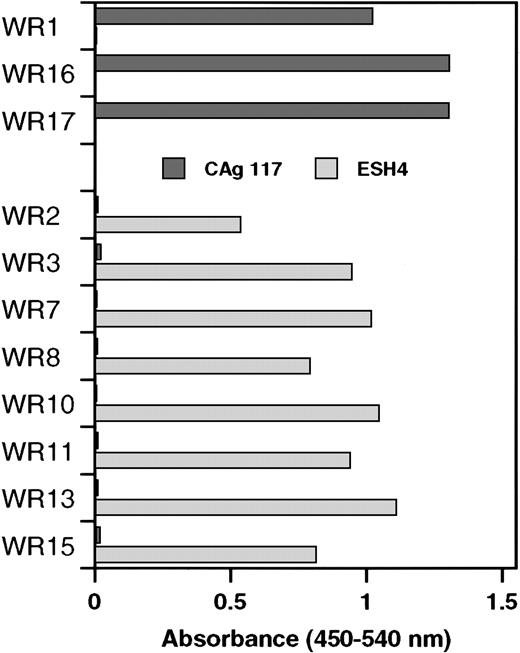
Similar to clones WR2, WR3, WR7, WR8, WR10, WR11, WR13, and WR15, the VH domain of the previously described scFv EL-9 is also derived from germline gene DP-88 (Table 1). Competition experiments have shown that the binding site for EL-9 in the C2 domain overlapped with that of monoclonal antibody CLB-CAg 117.13 Therefore, phages derived from the newly isolated clones were tested as to whether they also competed with CLB-CAg 117 for binding to the C2 domain. Phages bearing a VH domain derived from germline gene segment DP-88 did not bind to the C2 domain that was immobilized via CLB-CAg 117 (Figure 2). Interestingly, phages corresponding to clones consisting of a DP-5–encoded VH domain bound readily to the C2 domain that was immobilized via CLB-CAg 117. We also tested reactivity of phages with C2 domain immobilized via ESH4. Previously, we have shown that ESH4 and CLB-CAg 117 bind nonoverlapping epitopes in the C2 domain of factor VIII.4 Phages derived from clones using germline gene segment DP-5 did not bind to ESH4-immobilized C2 domain. In contrast, the C2 domain immobilized via ESH4 exclusively allowed for binding of phages derived from clones consisting of a DP-88–encoded VH domain (Figure 2). These data suggest that DP-5 and DP-88 germline gene segments generate antibodies with distinct binding sites in the C2 domain of factor VIII.
Specificity of phages isolated by selection on plasma-derived factor VIII light chain for the C2 domain.
Binding of phages to recombinant C2 domain that was immobilized via monoclonal antibodies CLB-CAg 117 or ESH4 was determined by enzyme-linked immunosorbent assay. The results were corrected for binding to both antibodies in the absence of C2 domain. Clones were divided into 2 groups, DP-5 and DP88, respectively, based on the variable heavy gene segment that encodes the VHdomain.
Specificity of phages isolated by selection on plasma-derived factor VIII light chain for the C2 domain.
Binding of phages to recombinant C2 domain that was immobilized via monoclonal antibodies CLB-CAg 117 or ESH4 was determined by enzyme-linked immunosorbent assay. The results were corrected for binding to both antibodies in the absence of C2 domain. Clones were divided into 2 groups, DP-5 and DP88, respectively, based on the variable heavy gene segment that encodes the VHdomain.
Previously, we have shown that scFv EL-5, EL-9, and EL-14 did not inhibit factor VIII activity.13 The capacity of scFv isolated in this study to inhibit factor VIII activity was assessed in a Bethesda assay. None of the scFv significantly inhibited factor VIII activity (titer < 15 Bethesda units [BU]/mg scFv). Subsequently, we determined the affinity of the scFv for the factor VIII light chain using surface plasmon resonance. All scFv readily associated with factor VIII light chain, and for all but 3 scFv the rate of dissociation was extremely slow (Table2). The relative high dissociation rate observed for EL-5, EL-9, and WR16 results in a reduced affinity for factor VIII light chain when compared with the other scFv (Table2).
Binding kinetics of isolated scFvKd, kon, andkoff were determined by surface plasmon resonance as described in “Materials and methods”
| scFv . | Kon(M−1s−1) . | Koff(s−1) . | Kd (nM) . |
|---|---|---|---|
| EL5 | 1.6 × 104 | 3.6 × 10−3 | 226 |
| EL9 | 3.1 × 104 | 1.3 × 10−3 | 42 |
| EL14 | 3.7 × 104 | 4.2 × 10−5 | 1.1 |
| WR1 | 2.7 × 104 | 3.9 × 10−4 | 14 |
| WR8 | 1.2 × 104 | 5.3 × 10−5 | 4.2 |
| WR10 | 4.1 × 104 | 4.9 × 10−4 | 12 |
| WR11 | 8.8 × 103 | 4.2 × 10−5 | 4.8 |
| WR13 | 3.0 × 105 | 6.4 × 10−4 | 2.1 |
| WR16 | 1.6 × 104 | 1.9 × 10−3 | 115 |
| scFv . | Kon(M−1s−1) . | Koff(s−1) . | Kd (nM) . |
|---|---|---|---|
| EL5 | 1.6 × 104 | 3.6 × 10−3 | 226 |
| EL9 | 3.1 × 104 | 1.3 × 10−3 | 42 |
| EL14 | 3.7 × 104 | 4.2 × 10−5 | 1.1 |
| WR1 | 2.7 × 104 | 3.9 × 10−4 | 14 |
| WR8 | 1.2 × 104 | 5.3 × 10−5 | 4.2 |
| WR10 | 4.1 × 104 | 4.9 × 10−4 | 12 |
| WR11 | 8.8 × 103 | 4.2 × 10−5 | 4.8 |
| WR13 | 3.0 × 105 | 6.4 × 10−4 | 2.1 |
| WR16 | 1.6 × 104 | 1.9 × 10−3 | 115 |
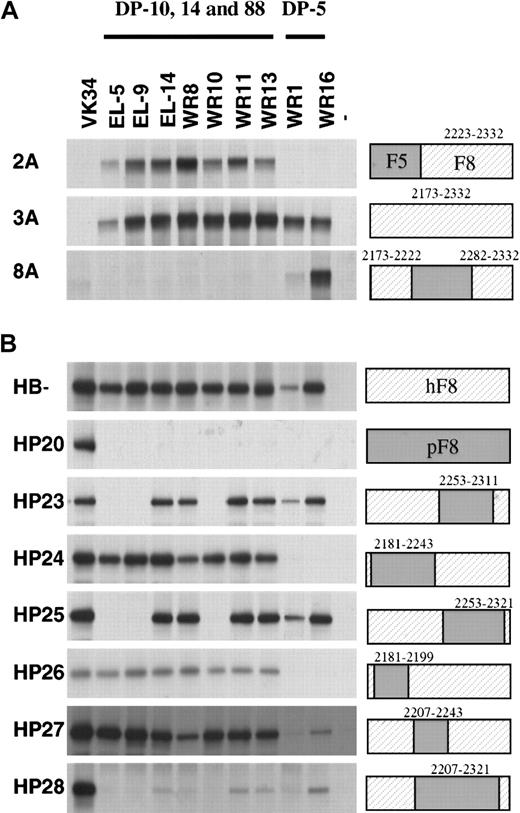
To further explore the epitope specificity of the isolated scFv, their reactivity with labeled hybrid factor VIII/factor V molecules was evaluated by immunoprecipitation analysis. Three previously isolated scFv EL-5, EL-9, and EL-14 (encoded by VH germline gene segments DP-14, DP-88, and DP-10) were also included in this analysis.13 Factor VIII/factor V hybrids in which (part of) the C2 domain of factor V was replaced by factor VIII did not react with scFv VK-34 that is directed to the A2 domain of factor VIII (Figure 3A, lane 1).27All scFv tested reacted with the factor VIII/factor V hybrid in which the C2 domain of factor V was replaced by that of factor VIII (Figure 3A). The scFv composed of VH domains derived from germline gene segments DP-10, DP-14, and DP-88 (scFv EL-5, EL-9, EL-14, WR8, WR10, WR11, and WR13) reacted with the factor V hybrid 2A, which indicates that the epitope for these scFv is located within residues 2223 to 2332 of factor VIII. This group of scFv did not react with factor VIII/V hybrids in which residues 2173 to 2222 or 2282 to 2332 of factor VIII were present. Binding of scFv containing VHdomains encoded by germline gene segment DP-5 solely react with a hybrid that contained residues 2173 to 2222 and 2282 to 2332 of factor VIII (Figure 3A, lane 8). Apparently, residues contained in both these regions contribute to the epitope of this class of scFv (Figure3A, lane 8). Taken together, these results suggest that DP-5–encoded scFv and DP-10–, DP-14–, and DP-88–encoded scFv bind to distinct antigenic sites within the C2 domain.
Reactivity of isolated scFv with factor V/factorVIII and human/porcine factor VIII hybrids.
Binding of scFv to recombinant factor V/VIII (A) and porcine/human factor VIII (B) hybrids was assessed by immunoprecipitation. (Lane 1) positive control (34, anti-A2 scFv VK34); (lanes 2-10) 5, 9, 14, 8, 10, 11, 13, 1, and 16: scFv corresponding to clones EL-5, EL-9, EL-14, WR8, WR10, WR11, WR13, WR1, and WR16; (lane 11) negative (−) control. On the right, the fragments used are indicated. Sequences corresponding to human factor VIII are depicted as hatched boxes. At the top of the figure, the 2 classes of VH gene segments that encode the different clones are given. Clone EL-5, EL-9, EL-14, WR8, WR10, WR11, and WR13 are derived from VH gene segments DP-10, DP-14, and DP-88, whereas clones WR1 and WR16 are derived from VH gene segment DP-5.
Reactivity of isolated scFv with factor V/factorVIII and human/porcine factor VIII hybrids.
Binding of scFv to recombinant factor V/VIII (A) and porcine/human factor VIII (B) hybrids was assessed by immunoprecipitation. (Lane 1) positive control (34, anti-A2 scFv VK34); (lanes 2-10) 5, 9, 14, 8, 10, 11, 13, 1, and 16: scFv corresponding to clones EL-5, EL-9, EL-14, WR8, WR10, WR11, WR13, WR1, and WR16; (lane 11) negative (−) control. On the right, the fragments used are indicated. Sequences corresponding to human factor VIII are depicted as hatched boxes. At the top of the figure, the 2 classes of VH gene segments that encode the different clones are given. Clone EL-5, EL-9, EL-14, WR8, WR10, WR11, and WR13 are derived from VH gene segments DP-10, DP-14, and DP-88, whereas clones WR1 and WR16 are derived from VH gene segment DP-5.
We also evaluated the reactivity of the scFv with a series of human-porcine hybrids that have been described previously.6 All scFv reacted with B domain–deleted factor VIII, whereas no reactivity was observed with hybrid HP20 in which the human C2 domain was replaced by the corresponding part of porcine factor VIII (Figure 3B). Based on the lack of reactivity with HP28, residues within amino acid sequence 2207 to 2321 are likely to contribute to the epitopes of scFv derived from heavy chain germline gene segments DP-10, DP-14, and DP-88 (Figure 3B). Interestingly, both hybrids HP23 and HP25 are not recognized by scFv EL-5, EL-9, and WR10, suggesting that residues 2253 to 2311 contain amino acids crucial for binding of these scFv. The pattern observed for scFv derived of germline gene segment DP-5 is clearly different. Hybrids HP24 and HP26 are not recognized by WR1 and WR16, suggesting that residues located between 2181 and 2199 are crucial for binding of these scFv to the C2 domain of factor VIII. Overall, the data suggest that the amino-terminal portion of the C2 domain is involved in binding of DP-5–encoded scFv. Binding of scFv derived from heavy chain segments DP-10, DP-14, and DP-88 requires the presence of residues contained within the central and/or carboxy-terminal part of the C2 domain.
Discussion
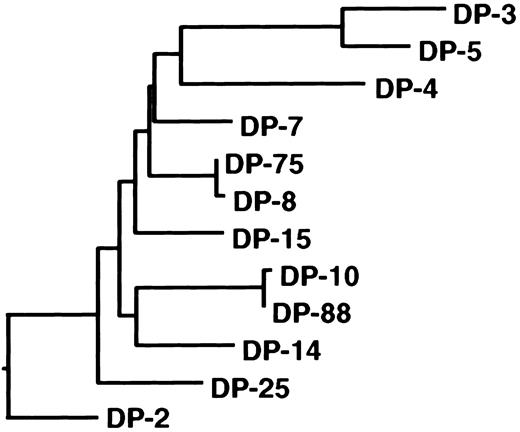
Antibodies directed to the C2 domain of factor VIII are frequently found in plasma of hemophilia A patients with an inhibitor.7 In this study, we defined the primary structure of human C2 domain–specific antibodies. Two classes of anti-C2 antibodies directed to distinct epitopes were isolated from the immunoglobulin repertoire of a mild hemophilia A patient with an inhibitor. The isolated antibodies consisted of VH domains encoded by germline gene segments DP-5 and DP-88 from the VH1 gene family. Previously, we reported on the isolation of a panel of anti-C2 domain antibodies encoded by germline gene segments DP-10, DP-14, and DP-88.13 The nonmutated germline genes DP-10 and DP-88 are highly homologous and vary by only 1 nucleotide. The DP-14 and DP-88 genes are more distantly related and vary by 29 nucleotides, which lead to 17 amino acid substitutions. In 2 recent studies, anti-C2 antibodies derived from VH gene segment DP-5 were isolated (Table 1).16,28 The nonmutated germline gene DP-5 differs substantially from DP-10, DP-14, and DP-88 (46-47 nucleotides, which results in 21-22 amino acid substitutions), although all these genes belong to the VH1 gene family. Phylogenetic analysis of germline gene segments of the VH1 family confirms the close relation between germline genes DP-10, DP-14, and DP-88 (Figure 4). In contrast, DP-5 appears to be present in a different branch of the phylogenetic tree of the VH1 family. Interestingly, the VH domains encoded by DP-10, DP-14, and DP-88 all contain a large CDR3 consisting of 18 to 23 residues, whereas the CDR3 length of DP-5–derived VH domains ranges between 8 to 11 amino acids.13 16 It cannot be excluded that antibody fragments selected by phage display are not fully representative of the complete spectrum of antibodies present in a patient's plasma. Additional anti-C2 antibodies with different characteristics than reported in this study may exist. The common features of human anti-C2 antibodies described in this and other studies, however, suggest that these antibodies are derived from a limited proportion of the human immunoglobulin repertoire.
Phylogeny of the VH1 family germline gene family.
Dendrogram of all human VH1 family germline genes shows relatedness of DP-10, DP-88, and DP-14. The nucleotide sequences were aligned using the CLUSTAL alignment of the DNAstar (Madison, WI) MegALIGN application. The evolutionary distance between the different gene segments correlates with the length of the closed lines.
Phylogeny of the VH1 family germline gene family.
Dendrogram of all human VH1 family germline genes shows relatedness of DP-10, DP-88, and DP-14. The nucleotide sequences were aligned using the CLUSTAL alignment of the DNAstar (Madison, WI) MegALIGN application. The evolutionary distance between the different gene segments correlates with the length of the closed lines.
Both epitope mapping studies and competition experiments suggest that scFv derived from DP-10, DP-14, DP-88, and DP-5 germline genes bind to distinct antigenic sites within the C2 domain of factor VIII. Based on the results with human/porcine hybrids, it is likely that residues 2181 to 2243 contribute to binding of DP-5–encoded antibodies. This observation is consistent with the identification of a major epitope for factor VIII inhibitors in this part of factor VIII.6,29 Based on their reactivity with factor VIII/V hybrid 8A, it is likely that residues contained within the carboxy-terminus of the C2 domain also contribute to binding of DP-5–encoded scFv (Figure 3). Recently, the 3-dimensional structure of DP-5–encoded human monoclonal antibody BO2C11 in complex with the C2 domain has been determined.30 A number of amino acid residues within 2181 to 2243 have been implicated in binding of BO2C11 to the C2 domain. However, residues His2315 and Gln2316 present at the carboxy-terminus of the C2 domain also have been shown to interact with BO2C11. From the 3-dimensional structure it appears that the interactive surface of BO2C11 is acidic, whereas the antigenic surface of the C2 epitope contains a number of basic residues. In contrast to other germlines, the CDR1 and CDR2 of DP-5 harbor 5 negatively charged Glu (E) and Asp (D) amino acid residues (Figure 1). These residues contribute to the net negative charge of VH domains encoded by this particular germline gene segment. The average calculated isoelectric point (pI) of nonmutated germline VH segments is 8.74 ± 1.06. In a previous study, the average pI of rearranged VH domains of 39 randomly picked clones was 9.24 ± 0.8.31 Conversely, germline DP-5, being one of the 3 VH segments with a net negative charge, has a predicted pI of 4.84. It can be anticipated that surface Ig molecules on immature B cells carrying a DP-5–encoded VH domains may more readily interact with a complementary positively charged site in the C2 domain. This may result in selective amplification of these B-cell clones and explain the presence of the DP-5 gene segment in a subset of human antibodies directed against the C2 domain.
Human anti-C2 antibodies encoded by VH gene segments DP-10, DP-14, and DP-88 compete with binding of CLB-CAg 117 but not ESH4 for binding to the C2 domain. In contrast, DP-5–encoded clones compete with binding of ESH4 to the C2 domain. This observation suggests that DP-10–, DP-14–, and DP-88–encoded clones bind to an antigenic site within the C2 domain that does not overlap with the epitope of the DP-5–encoded scFv. Epitope mapping studies support this notion and clearly indicate that the C2 domain contains at least 2 antigenic sites. DP-10–, DP-14–, and DP-88–encoded scFv bind to HP24, whereas DP-5–encoded scFv do not bind to this hybrid molecule. These data are in agreement with the results obtained for factor V/VIII hybrid 2A and suggest that residues contained within 2243 to 2332 contribute to binding of DP-10–, DP-14–, and DP-88–encoded scFv to the C2 domain of factor VIII. More detailed information on the epitope specificity of this group of scFv can be derived from the results obtained with hybrids HP23 and HP25. ScFv EL-5, EL-9, and WR10 most likely bind to an epitope contained within residues 2253 to 2311 because these scFv do not react with human porcine hybrid HP23 (Figure 3). Interestingly, the related scFv EL-14, WR8, WR11, and WR13 do bind to hybrid HP23. Only when the replaced region was extended from residues 2207 to 2321 was reduced binding of these scFv observed (hybrid HP28; Figure 3). The results obtained with hybrid HP23 and HP25 suggest that within amino acid sequence 2207 to 2321, different amino acid residues may contribute to binding of human antibodies to the C2 domain of factor VIII. Inspection of the primary sequence of DP-10–, DP-14–, and DP-88–derived scFv does not reveal characteristics that may explain different reactivity of the scFv with hybrids HP23 and HP25. Because the overall structure of the heavy chain of the scFv is likely to be similar, the observed differences may reflect a modulating role of the CDR3 region on the epitope specificity. Alternatively, amino acid changes introduced by somatic hypermutation may result in the formation of additional contacts between antibodies and the C2 domain. At present we cannot discriminate between these 2 possibilities. Future studies aim at defining amino acid residues within 2207 to 2321 that contribute to binding of DP-10–, DP-14–, and DP-88–encoded human antibodies to the C2 domain of factor VIII.
In a previous study we have shown that scFv encoded by germline gene segments DP-10, DP-14, and DP-88 do not inhibit factor VIII in a Bethesda assay.13 Here, we have isolated additional scFv encoded by germline gene segment DP-88 (Table 1). In accordance with previous data, these scFv do not inhibit factor VIII activity. The lack of inhibition may be explained by the epitope of this subset of human antibodies, which may not overlap with a functional site within the C2 domain. Alternatively, the affinity of the scFv may be too low to compete for binding of factor VIII to phospholipids. In this study we also show that DP-5–encoded scFv do not inhibit factor VIII activity. At first sight these findings do not correspond with the properties of human monoclonal BO2C11, a DP-5–encoded antibody that does inhibit factor VIII with a specific activity of 7000 BU/mg.16 However, the affinity of BO2C11 for factor VIII is 1000- to 10 000-fold higher than observed for the DP-5–encoded scFv WR1 and WR16 (0.014 nM vs 14 and 115 nM). Apparently, high-affinity binding is required for human antibodies to inhibit the interaction of factor VIII with phospholipid membranes. Therefore, we favor the view that the inability of the isolated scFv to inhibit factor VIII is primarily due to their reduced affinity for the C2 domain compared with that of complete antibody molecules.
Our data show the presence of 2 distinct antigenic sites within the C2 domain of factor VIII that are recognized by 2 classes of human antibodies. The first class of antibodies is encoded by VHgene segments DP-10, DP-14, and DP-88, whereas the second class of antibodies is encoded by VH gene segment DP-5. As argued previously, the preferential use of VH gene segment DP-5 for the assembly of anti-C2 antibodies may be explained by its overall negative charge, which can complement positively charged residues in the C2 domain. We do not have an explanation for the presence of DP-10, DP-14, and DP-88 gene segments in human antibodies that target the C2 domain. A common property of this class of human antibodies is their exceptionally large CDR3, which ranges from 20 to 23 amino acids. Selection of DP-10, DP-14, and DP-88 for the assembly of anti-C2 antibodies can be related to their ability to incorporate a large CDR3 segment. In addition, other common structural elements shared between these closely related VH gene segments may explain their presence in anti-C2 antibodies. Collectively, our data suggest that 2 classes of antibodies recognize 2 distinct sites in the C2 domain of factor VIII. Identification and subsequent modification of the interactive surfaces in the C2 domain that reacts with these 2 classes of scFv can potentially reduce both the antigenicity and immunogenicity of this portion of factor VIII.
The authors thank Dr J. A. van Mourik and Dr A. B. Meijer for critical evaluation of the manuscript.
Supported by The Netherlands Organization for Scientific Research (NWO) (grant 902-26-204), a travel grant from the Haemophilia Foundation, National Institutes of Health grant R01-HL46215, and a March of Dimes research grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan Voorberg, Dept of Plasma Proteins, CLB, Plesmanlaan 125, 1066 CX Amsterdam; e-mail: j_voorberg@clb.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal