Abstract
Telomere length must be tightly regulated in highly proliferative tissues, such as the lymphohematopoietic system. Under steady-state conditions, the levels and functionality of hematopoietic-committed or multipotent progenitors were not affected in late-generation telomerase-deficient mice (mTerc−/−) with critically short telomeres. Evaluation of self-renewal potential of mTerc−/− day-12 spleen colony-forming units demonstrated no alteration as compared with wildtype progenitors. However, the replating ability of mTerc−/− granulocyte-macrophage CFUs (CFU-GMs) was greatly reduced as compared with wildtype CFU-GMs, indicating a diminished capacity of late-generation mTerc−/− committed progenitors when forced to proliferate. Long-term bone marrow cultures of mTerc−/−bone marrow (BM) cells show a reduction in proliferative capacity; this defect can be mainly attributed to the hematopoietic, not to the stromal, mTerc−/− cells. In serial and competitive transplantations, mTerc−/− BM stem cells show reduced long-term repopulating capacity, concomitant with an increase in genetic instability compared with wildtype cells. Nevertheless, in competitive transplantations late-generation mTerc−/− precursors can occasionally overcome this proliferative impairment and reconstitute irradiated recipients. In summary, our results demonstrate that late-generation mTerc−/− BM cells with short telomeres, although exhibiting reduced proliferation ability and reduced long-term repopulating capacity, can still reconstitute myeloablated animals maintaining stem cell function.
Introduction
Eukaryotic chromosomes are capped by a special structure, the telomere, that in all vertebrates consists of tandem repeats of the DNA sequence TTAGGG and of associated proteins. Telomeres guarantee chromosome integrity by preventing illegitimate recombination, degradation, and end fusions.1,2 Telomere shortening occurs in each replication cycle and is proposed to mediate replicative senescence in human cells in culture, as well as the aging process.3,4 Telomere maintenance involves a ribonucleoprotein with reverse-transcriptase activity, called telomerase.5,6 Telomerase is active during human embryonic development and is downregulated immediately after birth.7,8 In adults, most normal somatic cells lack detectable telomerase activity, whereas cells from germline tissues and most tumors express high levels of telomerase activity.9Telomerase activity is also detected in normal human somatic tissues containing cells with self-renewal capacity, such as those of the lymphohematopoietic system10 and the skin epithelium.11
Hematopoiesis requires self-renewal of stem cells, as well as proliferation and differentiation of the committed progenitors. This process demands an extraordinary replicative capacity in certain cell types, especially those of the immune system. Telomeres in blood cells from bone marrow (BM) transplant recipients are shorter than those in cells from the BM donor,12,13 suggesting that the additional cell divisions in the stem cell compartment required for BM regeneration result in a measurable decline in telomere length. Analysis of human BM cells showed that, in vitro, telomerase activity is repressed in quiescent stem cells, expressed at low levels in cycling stem cells, and up-regulated following cytokine stimulation.10,14,15 Moreover, cytokine-induced differentiation of CD34+ cells results in a decrease in telomerase activity.16 In murine fetal liver and adult BM, results based on single-cell analysis17 showed that the majority of long-term reconstituting BM hematopoietic stem cells (HSCs) and transiently self-renewing multipotent progenitors exhibit telomerase activity.
Mice genetically deficient for the mouse telomerase RNA (mTerc) gene lack telomerase activity and show telomere shortening at a rate of 4 to 5 kilobases (kb) per mouse generation.7,18 This shortening is accompanied by an increase in the number of chromosome ends with no detectable telomeres and in the frequency of chromosome fusions.18,19 The mTerc−/− mice have been studied on 2 different genetic backgrounds, the original mixed C57BL6/129Sv (B6/Sv) and pure C57BL6 (B6) backgrounds. The mTerc−/− mice survive 3 to 4 generations on the B6 background20 and up to 6 generations on the B6/Sv background.19 Late-generation mTerc−/− mice are infertile, show reduced viability, and exhibit defects in highly proliferative tissues, such as the hematopoietic system and the gut.20,21 In particular, late-generation mTerc−/− B6 mice show splenic atrophy, abnormal hematology, an impaired B- and T-cell reaction to mitogen stimulation, and a defective germinal center reaction following antigen immunization.22 Whereas the committed progenitor compartment of early-generation mTerc−/− mice shows no alterations on the basis of colony-forming unit (CFU) assays, a statistically significant decrease in the total number of colonies in late-generation mTerc−/− mice has been reported,19 suggesting that the long-term renewal of HSCs is compromised following telomere loss. Although telomerase is sufficient for telomere maintenance, alternative telomerase-independent mechanisms for telomere lengthening have been postulated on the basis of mechanisms found in yeast.23-25 In situations demanding high proliferation, telomerase-independent telomere-elongation mechanisms may operate in the hematopoietic organs of late-generation mTerc−/− mice. Specifically, telomeres appear to be elongated in late-generation mTerc−/− mice during B-lymphocyte clonal expansion in spleen germinal centers.22 Here we study the effects of telomerase deficiency and telomere shortening in the murine HSC compartment. We evaluated the self-renewal potential and competitive long-term repopulating ability of late-generation mTerc−/− HSCs compared with wildtype controls. Our results indicate that under steady-state conditions telomerase deficiency does not impair stem-cell function.
Materials and methods
Mice
Different-generation mTerc−/− mice and the corresponding wildtype controls were analyzed on 2 different genetic backgrounds, a 90% pure C57BL6 background20 and the original mixed background (60% C57BL6, 37.5% 129Sv, 2.5% SJL).18 Generation 3 (G3) mTerc−/−C57BL6 and G6 mTerc−/− C57BL6/129Sv mice were used as late-generation animals; in some experiments, G3 mTerc−/−C57BL6/129Sv mice were used as early-generation animals.
Flow fluorescence in situ hybridization, quantitative fluorescence in situ hybridization, and telomeric restriction fragment analysis
Fresh BM samples were obtained by flushing the femora of wildtype and mTerc−/− littermates with sterile phosphate-buffered saline. BM was dispersed immediately in RPMI medium plus 10% fetal calf serum with the use of a 24-gauge needle. Flow fluorescence in situ hybridization (flow-FISH) was performed as described.26 To normalize the flow-FISH protocol, 2 murine leukemia cell lines of known telomere lengths, L5178Y-L and L5178Y,27 were used as internal controls for each experiment; telomere length is expressed as telomere fluorescence units (1 telomere fluorescence unit equals 1 kb of telomeric repeats). BM cells (3 × 105) were hybridized with the telomeric peptide nucleic acid (PNA)–fluorescein isothiocyanate probe (PE Biosystems, Framingham, MA), and the total telomere fluorescence of at least 5000 single cells with a G0-G1 DNA content was analyzed by means of a Coulter EPICS-XL flow cytometer (Beckman Coulter, Fullerton, CA) with the System 2 proprietary software package.
First, 5 × 106 fresh BM cells from wildtype and G3 mTerc−/− animals were cultured for 72 hours in Myelocult 5300 medium (StemCell Technologies, Vancouver, BC, Canada) supplemented with 15% Wehi 3B cell-conditioned media as a source of interleukin (IL)–3. Metaphase cells were prepared by means of standard protocols. After dropping cells onto wet slides, we hybridized metaphases with the telomeric PNA-Cy3 probe (PE Biosystems) as described previously.28 Quantitative-FISH (Q-FISH) analysis was performed as described,29 and analysis of telomere fluorescence was carried out by means of the TFL telo program kindly provided by Peter Lansdorp (Terry Fox, British Columbia Cancer Center, Vancouver, Canada). Telomeric restriction fragment (TRF) analysis was carried out as described.18 Y-chromosome painting experiments were carried out on cells with a mouse painting probe (Cambio, Cambridge, United Kingdom) according to the manufacturer′s instructions.
Spectral karyotyping analysis
Spectral karyotyping analysis (SKY) was performed on BM cells with SkyPaint M10 probes (Applied Spectral Imaging, Migdal Ha'Emek, Israel) as described.30
Long-term bone marrow cultures
Primary stroma were obtained by flushing the BM cells from one tibia and one femur from wildtype and mTerc−/− mice directly into a 25-cm2 flask with 10 mL Myelocult M5300 medium, supplemented with 10−6 M hydrocortisone and cultured at 32°C. Weekly exchange of half of the medium was performed for up to 5 weeks, and total cell number and granulocyte-macrophage CFUs (CFU-GMs) were evaluated every week. At termination of culture, flasks were trypsinized to detach the stromal cell layer, and adherent cells were allowed to readhere to the culture plastic for 1 hour at 37°C; total hematopoietic cells and CFU-GMs were also analyzed in the stromal layer. In seeding experiments, wildtype and G3 mTerc−/− B6 long-term BM cultures (LTBMCs) were established as described above and 21 days later were irradiated with a dose of 17 Gy; 3 days after irradiation, cultures were washed and seeded with 7 × 105 wildtype or G3 mTerc−/− B6 Lin− BM cells in 10 mL Myelocult M5300. Every 5 days, half of the medium was changed, and total cells and CFU-GMs in suspension were evaluated.
Clonogenic assays
CFU-GMs were analyzed (105 cells per milliliter) in MethoCult M3530 medium (StemCell Technologies). Erythroid burst-forming units (BFU-Es) were analyzed in MethoCult M3230 (StemCell Technologies) supplemented with 6 U/mL erythropoietin (StemCell Technologies), 10 ng/mL murine IL-3 (Biosource International, Camarillo, CA), and 50 ng/mL murine stem cell factor (Biosource International). The pre-B CFUs were analyzed in MethoCult M3630 (StemCell Technologies). Cells were added in 300 μL and mixed thoroughly, and duplicates of 1 mL were dispensed into 35-mm plates (Falcon, Plymouth, United Kingdom). Cultures were incubated at 37°C, and colonies were scored at day 7 for CFU-GMs and at day 12 for BFU-Es. Megakaryocyte-CFUs (CFU-Mks) were analyzed in serum-free cultures as described31; cultures were incubated at 37°C for 7 days; individual colonies were stained for acetylcholinesterase activity32 and counted. For high proliferative potential colony-forming cell (HPP-CFC) evaluation, CFU-GM culture dishes were incubated for 14 days; colonies larger than 0.5 mm in diameter consisting of tightly packed cells were scored as HPP-CFCs. In replating experiments, day-7 CFU-GM colonies harvested from methylcellulose cultures were resuspended in 300 μL Iscoves modified Dulbecco medium (Gibco, Rockville, MD) and replated in secondary methylcellulose cultures established as above.
Assay of day-12 spleen CFUs
Exogenous day-12 spleen CFUs (CFU-S12) were assayed as described previously.33 Briefly, groups of ten 3- to 4-month-old C57BL6 mice were irradiated with a split dose of 10.5 Gy (2 doses of 5.25 Gy spaced 4 hours apart); an appropriate number of BM cells were injected into the recipients via the lateral tail vein to obtain about 8 to 10 colonies per spleen. At 12 days after transplantation, recipients were killed; their spleens were removed and fixed in Telleyeniczky solution (44% ethanol, 31% acetic acid, and 2.3% formaldehyde); and the number of macroscopic spleen colonies was scored.
CFU-S12 self-renewal capacity
The self-renewal capacity of the CFU-S12 population was determined by measuring the mean number of CFU-S12contained in primary spleen colonies. Groups of 15 irradiated mice (2 doses of 5.25 Gy, 4 hours apart) were inoculated with appropriate hematopoietic cell dilutions to generate between 8 and 10 colonies per spleen. At 12 days later, 10 spleens per group were excised and used for colony counting; the remaining 5 spleens were removed and the cells dispersed through a nylon mesh in Hanks balanced salt solution. The cell suspension was diluted, and appropriate aliquots were injected into groups of 15 irradiated recipients to generate a countable number of spleen colonies 12 days after transplantation.
Long-term bone marrow repopulation assays
Female mice were conditioned as described in the CFU-S12 assays; the irradiation protocol was optimized to minimize endogenous reconstitution.34 These assays were performed essentially as described,35 with the use of BM cells from wildtype and G3 mTerc−/− B6 mice to generate the chimeric grafts. Groups of 10 irradiated recipients received transplants of 5 × 105 male wildtype or G3 mTerc−/− B6 BM cells obtained from a pool of cells from 3 animals. Recipients were killed 150 days after transplantation, and BM cells were pooled and transplanted into secondary irradiated female recipients. At 60 days after transplantation, secondary recipients were killed, and BM cells were pooled and transplanted into tertiary irradiated female recipients, which were analyzed 60 days after transplantation.
For competitive transplantations, groups of 10 female irradiated recipients received transplants of chimeric BM that contained different proportions of female wildtype BM cells and male mTerc−/−BM cells, as follows: 2 × 105 wildtype cells plus 2 × 105 mTerc−/− cells; 2 × 105 wildtype cells plus 2 × 106mTerc−/− cells. The mTerc−/− BM cell suspension was a pool of cells from 4 different G3 B6 mTerc−/− mice. Recipients were killed at 90, 145, and 200 days after transplantation to determine the competitive repopulating ability of the test populations.
Dot blot analysis
The extent of reconstitution from mTerc−/− cells in recipient mice was analyzed by evaluating the engraftment in BM and spleen of cells bearing the neomycin resistance gene(neor), which replaced the entire mTerc gene in the knockout mice.18 Organs were removed and DNA was extracted as described.36 Dot blot analyses were performed as reported previously.34 Membranes were probed with an EcoRI/SalI fragment (1.2 kilobases [kb]) from the pTZ18Neo plasmid (kind gift of J.C. Segovia; CIEMAT, Madrid, Spain). Different proportions of mTerc−/−/wildtype spleen DNA were mixed and used as a neor internal standard. Hybridization with a fragment of the glyceraldehyde 3-phosphate dehyrogenase (GAPDH) monocopy gene was carried out to confirm correct DNA loading in the dot blot membranes.
Results
Long-term mTerc−/− BM cultures
To examine the process of HSC self-renewal and differentiation in mice lacking telomerase activity and with short telomeres, we used LTBMCs of wildtype, G3, and G6 mTerc−/− B6/Sv mice to mimic the BM microenvironment. At 5 weeks after initiation of culture, the number of hematopoietic cells released from the stroma to the culture medium greatly diminished in G6 mTerc−/− B6/Sv LTBMCs, compared with wildtype controls. The average cell numbers for wildtype, G3, and G6 mTerc−/− cultures were, respectively, 6.4 ± 2.7 × 106; 2.5 ± 1.7 × 106; and 0.65 ± 0.35 × 106 cells (Figure1A). At this time, the CFU-GM content of cells in suspension was also examined in these cultures, and again a clear reduction in colony number was observed in G6 mTerc−/− cultures compared with wildtype controls. The mean CFU-GM values for wildtype, G3, and G6 mTerc−/−B6/Sv cultures were 311 ± 109, 122 ± 86, and 39 ± 37 CFU-GMs, respectively (Figure 1B). CFU-GM content was also reduced (90%) in the hematopoietic cells attached to the stroma of G6 mTerc−/−B6/Sv LTBMC; the mean CFU-GM content for wildtype, G3, and G6 mTerc−/− B6/Sv cultures after 5 weeks of culture was 9379 ± 1890, 3887 ± 2736, and 914 ± 622 CFU-GMs, respectively (Figure 1C).
Hematopoietic cell production, CFU-GM production, and telomere length of hematopoietic cells in wildtype, G3, and G6 mTerc−/− LTBMCs from different generations and genetic backgrounds.
Cultures were established from G3 and G6 mTerc−/− B6/Sv mice (panels A-C) or G3 mTerc−/− B6 mice (panel D) and were maintained in Myelocult M5300 medium plus 10−6 M hydrocortisone at 32°C, with weekly exchange of half of the medium. (A) (B) Total cells (panel A) and total CFU-GMs (panel B) in suspension per culture flask, were evaluated at weekly intervals. (C) At 5 weeks after initiation of the culture, the stromal layer of LTBMC was detached, and total CFU-GMs in stroma were evaluated. (D) Telomere fluorescence of total BM cells at the time of establishing LTBMC (day 0) was evaluated by flow-FISH (black bars); at the end of the cultures (day 35), telomere length of hematopoietic cells in suspension cells (gray bars) and hematopoietic cells in stroma (white bars) was also evaluated by gating the population of intermediate forward light scatter and low right-angle light scatter, characteristic of lymphoblastoid hematopoietic cells. Results are expressed as the mean ± SD of 3 different experiments *P < .01; mTerc−/− versus wildtype animals, for panels A-C; day 35 versus day 0 telomere length, for panel D.
Hematopoietic cell production, CFU-GM production, and telomere length of hematopoietic cells in wildtype, G3, and G6 mTerc−/− LTBMCs from different generations and genetic backgrounds.
Cultures were established from G3 and G6 mTerc−/− B6/Sv mice (panels A-C) or G3 mTerc−/− B6 mice (panel D) and were maintained in Myelocult M5300 medium plus 10−6 M hydrocortisone at 32°C, with weekly exchange of half of the medium. (A) (B) Total cells (panel A) and total CFU-GMs (panel B) in suspension per culture flask, were evaluated at weekly intervals. (C) At 5 weeks after initiation of the culture, the stromal layer of LTBMC was detached, and total CFU-GMs in stroma were evaluated. (D) Telomere fluorescence of total BM cells at the time of establishing LTBMC (day 0) was evaluated by flow-FISH (black bars); at the end of the cultures (day 35), telomere length of hematopoietic cells in suspension cells (gray bars) and hematopoietic cells in stroma (white bars) was also evaluated by gating the population of intermediate forward light scatter and low right-angle light scatter, characteristic of lymphoblastoid hematopoietic cells. Results are expressed as the mean ± SD of 3 different experiments *P < .01; mTerc−/− versus wildtype animals, for panels A-C; day 35 versus day 0 telomere length, for panel D.
LTBMCs were also established with BM from wildtype and G3 mTerc−/− B6 animals, again revealing growth impairment in the G3 mTerc−/− B6 cultures in production of both total hematopoietic cells and of CFU-GMs (data not shown). Telomere length of hematopoietic cells, both in adherent and in nonadherent cell populations, was estimated by flow-FISH at the endpoint of culture (day 35) by gating the lymphoblastoid population and was compared with the mean telomere fluorescence of total BM cells at the time of establishing LTBMCs. For this telomere analysis, LTBMCs established on the pure B6 background were used because of their stronger phenotype and lower heterogeneity.20 At day 0, telomeres were shorter in G3 mTerc−/− cells than in wildtype cells (Figure 1D). For wildtype and G3 mTerc−/− B6 LTBMCs, telomere length of hematopoietic cells in stroma after 35 days of culture was similar to that of total BM cells at initiation of culture (Figure 1D), in accordance with the fact that these cells are slowly proliferating hematopoietic progenitors.37 In contrast, average telomere fluorescence of hematopoietic cells in suspension was reduced to 67% ± 6% of the initial telomere fluorescence values for wildtype and 76% ± 4% of the initial telomere fluorescence values for G3 mTerc−/− B6 LTBMCs (Figure 1D). This loss of telomeric signal, equivalent to 7.1 and 5.2 kb for wildtype and G3 mTerc−/− B6 cells, respectively, is probably a consequence of the accumulated cell divisions undergone by the hematopoietic cells released from the stromal layer.
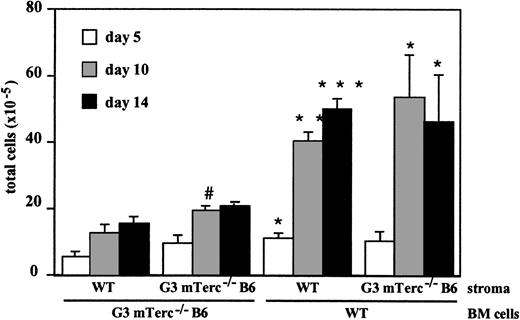
To elucidate whether the defective hematopoietic proliferation in mTerc−/− LTBMC can be attributed to a defect in hematopoietic progenitors or to a defective stromal support, we analyzed cultures of G3 mTerc−/− B6 Lin−hematopoietic cells seeded on irradiated wildtype stromal layers and wildtype Lin− hematopoietic cells seeded on G3 mTerc−/− B6 irradiated stromal layers. The Lin− cells used in the seeding experiments were shown to be free of fibroblast-CFU activity; moreover, the irradiated stroma were not able to produce hematopoietic cells (data not shown). The proliferation of mTerc−/− hematopoietic cells on irradiated stroma was significantly reduced as compared with wildtype cells, irrespective of the genotype of the irradiated stroma. Correspondingly, G3 mTerc−/− B6 irradiated stroma was able to support the proliferation of wildtype hematopoietic cells similarly to wildtype irradiated stroma (Figure2). No differences in CFU-GM concentration were observed (data not shown).
Kinetics of wildtype and G3 mTerc−/− B6 Lin− BM cell proliferation on irradiated wildtype and G3 mTerc−/− B6 stromal layers.
Wildtype and G3 mTerc−/− B6 LTBMCs were established and maintained as described in Figure 1. After 21 days, cultures were iradiated with a dose of 17 Gy; 3 days after irradiation, cultures were seeded with 7 × 105 wildtype or G3 mTerc−/− B6 Lin− BM cells. CFU-GM content of the seeded cells was 178 and 177 CFU-GMs per 104 cells for wildtype and G3 mTerc−/− B6 Lin− BM cells, respectively. Every 5 days, half of the medium was changed, and total cells and total CFU-GMs in suspension were evaluated. Results are expressed as the mean ± SD of 4 different experiments. *P < .01, **P < 001, ***P < .0001; for mTerc−/− BM cells versus WT BM cells seeded on the same type of stroma.#P < .01; for WT stroma versus mTerc−/− stroma seeded with the same type of BM cells.
Kinetics of wildtype and G3 mTerc−/− B6 Lin− BM cell proliferation on irradiated wildtype and G3 mTerc−/− B6 stromal layers.
Wildtype and G3 mTerc−/− B6 LTBMCs were established and maintained as described in Figure 1. After 21 days, cultures were iradiated with a dose of 17 Gy; 3 days after irradiation, cultures were seeded with 7 × 105 wildtype or G3 mTerc−/− B6 Lin− BM cells. CFU-GM content of the seeded cells was 178 and 177 CFU-GMs per 104 cells for wildtype and G3 mTerc−/− B6 Lin− BM cells, respectively. Every 5 days, half of the medium was changed, and total cells and total CFU-GMs in suspension were evaluated. Results are expressed as the mean ± SD of 4 different experiments. *P < .01, **P < 001, ***P < .0001; for mTerc−/− BM cells versus WT BM cells seeded on the same type of stroma.#P < .01; for WT stroma versus mTerc−/− stroma seeded with the same type of BM cells.
Analysis of multipotent and committed hematopoietic progenitors in mTerc−/− BM cells
BM cell suspensions derived from wildtype and late-generation mTerc−/− mice were compared for their content in hematopoietic-committed progenitors (CFU-GMs, BFU-Es, pre-B CFUs, CFU-Mks, and HPP-CFCs), and for the most primitive clonogenic progenitor, CFU-S12. The mTerc−/− mice of the 2 previously described backgrounds, B6 and B6/Sv, were used. When BM cells from wildtype, G3, and G6 mTerc−/− B6/Sv mice were assayed for CFU-GM content, no differences were observed (Figure3A). Similarly, no significant differences in number, colony size, or composition were observed between wildtype and G3 mTerc−/− B6 mice (Figure 3A). Although telomere length in BM cells from late-generation mTerc−/− mice was significantly shorter than in wildtype animals,19 20 no severe hematopoietic imbalance could be detected in physiological conditions.
Analysis of multipotent and committed hematopoietic progenitors in mTerc−/− BM cells from different generations and genetic backgrounds.
(A) BM cells were analyzed for CFU-GM, pre-B CFU, CFU-Mk, HPP-CFC, and CFU-S12 content as described in “Materials and methods.” (B) The replating ability of CFU-S12 and CFU-GMs was analyzed. Individual CFU-S12 spleen colonies generated in irradiated mice were dissected, dispersed, and injected into secondary irradiated recipients and assayed for secondary CFU-S12; CFU-GM colonies were isolated, replated in methylcellulose cultures, and assayed for secondary CFU-GMs. Results are shown independently; the statistical significance of differences between mean values (■) was determined by means of the 2-tailed Student t test.
Analysis of multipotent and committed hematopoietic progenitors in mTerc−/− BM cells from different generations and genetic backgrounds.
(A) BM cells were analyzed for CFU-GM, pre-B CFU, CFU-Mk, HPP-CFC, and CFU-S12 content as described in “Materials and methods.” (B) The replating ability of CFU-S12 and CFU-GMs was analyzed. Individual CFU-S12 spleen colonies generated in irradiated mice were dissected, dispersed, and injected into secondary irradiated recipients and assayed for secondary CFU-S12; CFU-GM colonies were isolated, replated in methylcellulose cultures, and assayed for secondary CFU-GMs. Results are shown independently; the statistical significance of differences between mean values (■) was determined by means of the 2-tailed Student t test.
The replating potential of cells from primary CFU-GMs of wildtype and G3 mTerc−/− B6 mice was analyzed in secondary methylcellulose cultures. Interestingly, the clonogenic ability of primary colonies was significantly lower in G3 mTerc−/−B6 CFU-GM cells compared with the corresponding wildtype cells (21.3 ± 8.9 and 4.0 ± 3.3 secondary CFU-GMs/primary CFU-GM, for wildtype and mTerc−/− cells, respectively) (Figure 3B). To determine whether the proliferative disadvantage of late-generation mTerc−/− versus wildtype cells could influence the self-renewal potential of HSCs in late-generation G3 mTerc−/− B6 animals, CFU-S12 were assayed; individual CFU-S12 colonies were dissected, dispersed, and injected into irradiated secondary recipients, and the number of secondary CFU-S12 was scored. The number of secondary CFU-S12 per primary CFU-S12 is a direct measure of the self-renewal ability of the multipotent hematopoietic precursor that originated the primary CFU-S12 colony. An increased (but not statistically significant) number of secondary CFU-S12 was observed in mice inoculated with G3 mTerc−/− B6 cells compared with wildtype mice (12.9 ± 6.4 and 21.4 ± 16.6 CFU-S12/primary CFU-S12 for wildtype and mTerc−/− mice, respectively) (Figure 3B).
Long-term repopulating ability of mTerc−/− BM cells
To assay in vivo the role of telomerase in the maintenance of the hematopoietic function, the long-term repopulating ability of G3 mTerc−/− B6 BM cells was analyzed by serial and competitive transplantation experiments. First, telomere length of wildtype and G3 mTerc−/− B6 BM cells that were used as inocula for the transplants was evaluated by 3 methods: TRF analysis on pulse-field gel electrophoresis, flow-FISH analysis, and Q-FISH analysis on metaphase spreads. As previously described for mTerc−/− B6/Sv mice,18 38 telomeres of the G3 mTerc−/− B6 cells showed a reduced telomeric signal by the 3 different methods. TRF analysis separates high–molecular weight DNA fragments, which consist of telomeric DNA and a small portion of subtelomeric DNA. TRF size range was 60 to 20 kb for wildtype BM cells and 70 to 6 kb with a smear of low–molecular weight telomeres for G3 mTerc−/− B6 BM cells (Figure4A). Further characterization of telomere fluorescence by flow-FISH showed a 40% reduction in the telomeric fluorescence intensity in G3 mTerc−/− B6 cells compared with wildtype controls (Figure 4B), in accordance with TRF results. Finally, the telomere size distribution of metaphase chromosomes analyzed by Q-FISH indicated a shift in the length distribution toward shorter telomeres, with a small proportion of undetectable telomeres in G3 mTerc−/− B6 cells (Figure 4C). Measurement of telomeres by all 3 techniques indicated that G3 mTerc−/− B6 BM cells show shorter telomeres than wildtype controls. Cytogenetic inspection of the primary BM metaphases did not show a significant increase in cytogenetic aberrations in the 25 metaphases analyzed.
Characterization of telomeres of BM cells from wildtype and G3 mTerc−/− B6 animals by telomere restriction fragment, Q-FISH, and flow-FISH analysis.
(A) Telomere length estimation by telomere restriction fragments in pools of primary BM cells from 3 wildtype and 3 G3 mTerc−/− B6 animals used for transplants into irradiated hosts. The telomere restriction fragments were separated by pulse-field gel electrophoresis, blotted, and hybridized to a P32-labeled TTAGGG probe. Note the presence of low–molecular weigth TRFs in the G3 mTerc−/− cells, which correspond to telomeres of 20 kb to fewer than 6 kb. (B) The same cells were hybridized with a flourescent PNA probe to measure telomere length by flow-FISH. Results are expressed as the mean ± SD of 3 different animals. (C) Telomere length distribution of primary and transplanted BM metaphase cells as determined by Q-FISH with the use of a telomeric PNA-Cy3 probe. The G3 mTerc−/− cells showed a lower telomere flourescence and a change in size distribution toward shorter telomeres. Ten metaphases obtained from a pool of BM cells from 3 different animals were analyzed. A telomere fluorescent unit (TFU) corresponds to 1 kb telomeric DNA.
Characterization of telomeres of BM cells from wildtype and G3 mTerc−/− B6 animals by telomere restriction fragment, Q-FISH, and flow-FISH analysis.
(A) Telomere length estimation by telomere restriction fragments in pools of primary BM cells from 3 wildtype and 3 G3 mTerc−/− B6 animals used for transplants into irradiated hosts. The telomere restriction fragments were separated by pulse-field gel electrophoresis, blotted, and hybridized to a P32-labeled TTAGGG probe. Note the presence of low–molecular weigth TRFs in the G3 mTerc−/− cells, which correspond to telomeres of 20 kb to fewer than 6 kb. (B) The same cells were hybridized with a flourescent PNA probe to measure telomere length by flow-FISH. Results are expressed as the mean ± SD of 3 different animals. (C) Telomere length distribution of primary and transplanted BM metaphase cells as determined by Q-FISH with the use of a telomeric PNA-Cy3 probe. The G3 mTerc−/− cells showed a lower telomere flourescence and a change in size distribution toward shorter telomeres. Ten metaphases obtained from a pool of BM cells from 3 different animals were analyzed. A telomere fluorescent unit (TFU) corresponds to 1 kb telomeric DNA.
Serial BM transplantations were carried out with irradiated female recipients; 5 × 105 total BM cells (containing approximately 50 HSCs) obtained from a pool of 4 BM samples from wildtype or G3 mTerc−/− B6 male donor mice were used as inoculum. The extent of exogenous reconstitution from male cells was analyzed 5 months after transplantation by Y-chromosome painting of total BM cells of the recipient animals. Unexpectedly, although G3 mTerc−/− B6 BM cells showed a significant reduction in telomere length (Figure 5A), their contribution to the reconstitution of the lymphohematopoietic system after BM transplantation was remarkable (45%) when compared with the corresponding wildtype BM cells (84%) (Figure 5A). Similar results were previously obtained when BM transplantations were carried out with the use of G6 mTerc−/− B6/Sv BM cells (data not shown). Additionally, the telomere fluorescence of Y-chromosome–positive nuclei from wildtype and G3 mTerc−/− B6 inocula, in comparison with primary transplanted BM cells, was analyzed. As seen in Figure 5B, a significant reduction (38%) in the telomere fluorescence of the transplanted wildtype cells versus the primary wildtype inoculum was observed. On the contrary, the transplanted G3 mTerc−/− B6 BM cells showed a slight reduction (9%) in the telomeric signal, but it was not significant (P > .05).
Evaluation of exogenous reconstitution of transplanted irradiated hosts by wildtype and G3 B6 mTerc−/− bone marrow cells.
(A) Quantification of Y chromosome–positive cells in the BM of irradiated female hosts after primary, secondary, and tertiary transplants with wildtype and G3 mTerc−/− B6 male BM cells. Quantification was performed by chromosome painting with a mouse whole-chromosome painting probe directly labeled with Cy3. A pool of BM cells from 4 different animals was used; the number of cells analyzed is represented above each bar. Grey bars indicate the expected exogenous reconstitution, taking into account the reduction in the percentage of Y+ cells inoculated in each sequential transplant. (B) Quantification of telomere fluorescence by Q-FISH in male nuclei of wildtype and G3 mTerc−/− B6 BM cells used as inoculum for transplants and 5 months after transplantation of those cells into irradiated female recipients. The telomere fluorescence was analyzed in nuclei that were positive for the Y-chromosome painting probes in a second sequential hybridization. The number of nuclei analyzed is represented above each bar. Results are expressed as the mean ± SE. *P < .01 for inoculum versus primary transplant.
Evaluation of exogenous reconstitution of transplanted irradiated hosts by wildtype and G3 B6 mTerc−/− bone marrow cells.
(A) Quantification of Y chromosome–positive cells in the BM of irradiated female hosts after primary, secondary, and tertiary transplants with wildtype and G3 mTerc−/− B6 male BM cells. Quantification was performed by chromosome painting with a mouse whole-chromosome painting probe directly labeled with Cy3. A pool of BM cells from 4 different animals was used; the number of cells analyzed is represented above each bar. Grey bars indicate the expected exogenous reconstitution, taking into account the reduction in the percentage of Y+ cells inoculated in each sequential transplant. (B) Quantification of telomere fluorescence by Q-FISH in male nuclei of wildtype and G3 mTerc−/− B6 BM cells used as inoculum for transplants and 5 months after transplantation of those cells into irradiated female recipients. The telomere fluorescence was analyzed in nuclei that were positive for the Y-chromosome painting probes in a second sequential hybridization. The number of nuclei analyzed is represented above each bar. Results are expressed as the mean ± SE. *P < .01 for inoculum versus primary transplant.
A pool of 5 × 105 BM cells from 4 primary recipients were inoculated into secondary irradiated female recipients, analyzed 2 months after transplantation, and retransplanted into tertiary irradiated female recipients following an identical procedure; BM cells from recipients of the secondary transplants were also analyzed 2 months after transplantation. A decrease in the percentage of Y-chromosome–positive cells was observed with succesive transplantations. This decrease cannot be explained solely by the fact that each sequential BM-transplanted cohort is receiving fewer male cells, because of the endogenous reconstitution that is taking place in primary and secondary recipients. Both wildtype and G3 mTerc−/− B6 cells show a decreased BM-reconstituting ability, with this effect being observed earlier in mTerc−/− cells than in wildtype cells (Figure 5A).
Spectral karyotyping analysis was performed in 25 to 27 donor-derived metaphases from transplanted wildtype and G3 mTerc−/− B6 BM cells. Transplanted male mTerc−/− cells showed an increased chromosomal instability (Table1); interestingly, 8 out of 9 translocations were clonal robertsonian translocations between chromosomes 5 and 6 (Figure 6). Despite the increased number of translocations in mTerc−/− G3 BM transplanted cells, no indications of any preleukemic phenotype were observed in hemograms of these mice (data not shown).
Chromosomal instability of wildtype and G3 in Terc−/− C57BL6 bone marrow cells 5 months after primary transplantation
| Transplanted cells . | Metaphases analyzed, no. . | Translocations found . | Cells with translocations, % . |
|---|---|---|---|
| Wildtype (XY) | 25 | t(16;?), in 1 metaphase | 4 |
| G3 mTerc−/− B6 (XY) | 27 | t(5;6), in 8 metaphases | 36* |
| t(7;13), in 1 metaphase |
| Transplanted cells . | Metaphases analyzed, no. . | Translocations found . | Cells with translocations, % . |
|---|---|---|---|
| Wildtype (XY) | 25 | t(16;?), in 1 metaphase | 4 |
| G3 mTerc−/− B6 (XY) | 27 | t(5;6), in 8 metaphases | 36* |
| t(7;13), in 1 metaphase |
mTerc indicates telomerase-deficient mice; B6 indicates C57BL6.
P < .01.
Increase in cytogenetic aberrations in Y+ primary transplanted G3 mTerc−/− BM cells.
Spectral karyotyping was performed on metaphases of transplanted wildtype and G3 mTerc−/− B6 BM male cells after 5 months of transplantation into female myeloablated hosts. (A) Image of a typical mTerc−/− metaphase after SKY analysis showing a robertsonian translocation between chromosomes 5 and 6 (classified colors). (B) Corresponding reverse 4′6-diamidino-2-phenylindole·2HCl (DAPI) image of the same metaphase. (C) Complete karyotype of the same metaphase showing the chromosomes by spectral, DAPI, and classified images, respectively. (D) Examples of the clonal t(5;6) translocation in G3 mTerc−/− B6 BM cells.
Increase in cytogenetic aberrations in Y+ primary transplanted G3 mTerc−/− BM cells.
Spectral karyotyping was performed on metaphases of transplanted wildtype and G3 mTerc−/− B6 BM male cells after 5 months of transplantation into female myeloablated hosts. (A) Image of a typical mTerc−/− metaphase after SKY analysis showing a robertsonian translocation between chromosomes 5 and 6 (classified colors). (B) Corresponding reverse 4′6-diamidino-2-phenylindole·2HCl (DAPI) image of the same metaphase. (C) Complete karyotype of the same metaphase showing the chromosomes by spectral, DAPI, and classified images, respectively. (D) Examples of the clonal t(5;6) translocation in G3 mTerc−/− B6 BM cells.
To further study the effect of telomerase deficiency and short telomeres on the long-term repopulating stem cell (LTRSC) compartment, competitive long-term repopulating assays were performed. Lethally irradiated mice were reconstituted with various ratios of BM cells from wildtype and G3 mTerc−/− B6 mice. The extent of lymphohematopoietic reconstitution by G3 mTerc−/− B6 cells in BM and spleen of transplanted mice was analyzed at 90, 145, and 200 days after transplantation by genomic hybridization with aneor-specific probe. In mice transplanted with 1:1 (G3 mTerc−/−-to-wildtype) ratio, reconstitution after 90 days was almost exclusively by wildtype cells (16% ± 12% and 28% ± 6% BM and spleen reconstitution, respectively, by G3 mTerc−/− cells) (Figure 7), indicating a competitive disadvantage in G3 mTerc−/− HSC proliferation. When the ratio was increased to 10:1, BM reconstitution by G3 mTerc−/− cells increased to 38% ± 21%, with 1 of 4 lethally irradiated recipients partially reconstituted (69% reconstitution) by G3 mTerc−/− B6 HSCs (Figure 7). Analysis 145 days after transplantation showed a reduced contribution of G3 mTerc−/− B6 cells to the BM reconstitution of myeloablated animals transplanted with 1:1 ratio (38% ± 21% and 41% ± 28% reconstitution in BM and spleen, respectively) (Figure7). In mice transplanted with the highest mTerc−/−-to-wildtype cells ratio, 10:1, the contribution of G3 mTerc−/− B6 cells to hematopoietic reconstitution was more evident, even at 200 days after transplantation (42% ± 20% and 51% ± 15% mTerc−/−reconstitution in BM and spleen, respectively). In 1 of 5 animals, reconstitution was almost fully accomplished by G3 mTerc−/− cells (Figure 7).
Competitive long-term repopulating ability of G3 mTerc−/− B6 BM cells.
Groups of 10 irradiated recipient mice were inoculated with different proportions (1:1 and 10:1) of G3 mTerc−/− B6 versus wild type BM cells; 2 × 105 wildtype cells were inoculated in all animals, and the number of mTerc−/−cells was 2 × 105 and 2 × 106 in the 2 groups, respectively. The wild type and mTerc−/− BM cell suspensions were a pool of cells from 4 different animals. Recipients were killed at 90 (all groups), 145 (1:1 group), and 200 days (10:1 group) after transplantation, and the competitive repopulating ability of the test populations was evaluated by hybridization of BM and spleen (Sp) DNA with a neor-specific probe (which has replaced the mTerc gene in the mTerc−/− mice). Signal intensity was analyzed by densitometer by means of the ImageQuant program (Amersham Biosciences, Sunnyvale, CA). A titration of mixtures of different proportions of mTerc−/−-to-wild type splenic DNA is shown in the bottom of the Figure; this was used to quantitate the densitometric analysis of the autoradiography. Hybridization with a fragment of the GAPDH monocopy gene was carried out to correct DNA loading in the dot blot membranes; the corresponding control for GAPDH hybridization is shown in the bottom panel. Each sample in the dot blot represents a transplanted individual. Data are represented as the percentage of reconstitution by G3 mTerc−/− B6 BM cells.
Competitive long-term repopulating ability of G3 mTerc−/− B6 BM cells.
Groups of 10 irradiated recipient mice were inoculated with different proportions (1:1 and 10:1) of G3 mTerc−/− B6 versus wild type BM cells; 2 × 105 wildtype cells were inoculated in all animals, and the number of mTerc−/−cells was 2 × 105 and 2 × 106 in the 2 groups, respectively. The wild type and mTerc−/− BM cell suspensions were a pool of cells from 4 different animals. Recipients were killed at 90 (all groups), 145 (1:1 group), and 200 days (10:1 group) after transplantation, and the competitive repopulating ability of the test populations was evaluated by hybridization of BM and spleen (Sp) DNA with a neor-specific probe (which has replaced the mTerc gene in the mTerc−/− mice). Signal intensity was analyzed by densitometer by means of the ImageQuant program (Amersham Biosciences, Sunnyvale, CA). A titration of mixtures of different proportions of mTerc−/−-to-wild type splenic DNA is shown in the bottom of the Figure; this was used to quantitate the densitometric analysis of the autoradiography. Hybridization with a fragment of the GAPDH monocopy gene was carried out to correct DNA loading in the dot blot membranes; the corresponding control for GAPDH hybridization is shown in the bottom panel. Each sample in the dot blot represents a transplanted individual. Data are represented as the percentage of reconstitution by G3 mTerc−/− B6 BM cells.
These results suggest that G3 mTerc−/− B6 BM cells have a proliferative disadvantage in competitive transplantations with wildtype BM cells. We also show that G3 mTerc−/− B6 HSCs can occasionally reconstitute myeloablated animals and overcome their proliferative disadvantage; this becomes more likely when the ratio of transplanted wildtype cells is reduced, thus subjecting G3 mTerc−/− HSCs to strong proliferative stress.
Discussion
Highly proliferative tissues, and those specific cell populations subjected to demanding proliferative stimuli, require telomerase activity to perform their physiological functions without compromising cell viability through exhaustion of telomeres.18 HSC cells are a scarce and heterogeneous population lodged in adult BM that is responsible for functional maintenance of the lymphohematopoietic system.39 HSCs are thought to be quasiquiescent under steady-state conditions.40 The capacity of HSCs to modulate telomerase activity after proliferation or differentiation stimuli may be critical in completing the cell-renewal process that maintains blood cell turnover throughout the lifespan of an individual.10,41 In human BM cells, low telomerase activity levels were demonstrated in multipotent HSCs (CD34+CD38−), with significant upregulation of enzyme activity in the presence of proliferation-inducing cytokines to reach the levels found in committed progenitors (CD34+CD38+). CD34−cells appeared to have telomerase levels similar to those found in stem cells.10,14-16,41 In contrast, single-cell analysis of telomerase activity in murine Lin−Sca-1+ cells suggested that the frequency of telomerase-expressing multipotent progenitors appears to be closely related to the frequency of cells with self-renewal potential.17 The studies reported here evaluated the consequences of genetic telomerase deficiency in the murine hematopoietic system, focusing specifically on the HSC compartment.
In mTerc−/− mice, telomere length declines at a rate ranging from 3 to 5 kb per mouse generation, depending on the genetic background.18-20 No abnormalities in blood cell counts have been reported for G6 mTerc−/− B6/Sv mice,19 although reduced lymphocyte numbers and increased neutrophil numbers have been observed in G3 mTerc−/− mice on a C57BL6 background,20 a genetic background with a shorter telomere length. Analysis of hematopoietic progenitors from mTerc−/− B6/Sv mice showed that BM cells from G1 and G3 mTerc−/− mice are comparable to wildtype BM cells in their ability to generate CFU-GMs, granulocyte-erythrocyte-monocyte-megakaryocyte CFUs and HPP-CFCs, whereas a statistically significant decrease in the total number of colonies obtained in late-generation G6 B6/Sv mice has been reported.19 In contrast, we observed no significant differences in CFU-GM content in BM samples from G3 and G6 mTerc−/− mice on the same mixed genetic background. This discrepancy may be due to the greater heterogeneity in telomere length and the higher frequency of very long telomeres found in BM cells of the mixed background.19 Similarly, CFU-GM levels were not altered in late-generation G3 mTerc−/− B6 mice, confirming the same result in a different genetic background. A detailed analysis of other committed progenitors (BFU-Es, pre-B CFUs, CFU-Mks, and HPP-CFCs) and multipotent progenitors (CFU-S12) showed no alteration in late-generation G3 mTerc−/− B6 mice. These results indicate that the reduction in telomere length observed in late-generation mTerc−/− mice (G6 for B6/Sv and G3-G4 for B6 backgrounds) does not influence the steady-state levels of different hematopoietic progenitors, concurring with normal blood cell homeostasis in late-generation mTerc−/− mice.21 The replating ability of G3 mTerc−/− B6 CFU-GMs was greatly reduced as compared with wildtype CFU-GM, while that of CFU-S12 was not altered; this may indicate a diminished proliferative capacity of late-generation mTerc−/− BM progenitors when they are forced to proliferate. This effect is observed only in committed progenitors (CFU-GMs), not in pluripotent progenitors (CFU-S12), probably because CFU-GMs have gone through more cell divisions and may have exhausted their proliferative capacity earlier.
Proliferative disadvantage has been observed when late-generation mTerc−/− LTBMCs on the 2 genetic backgrounds were established and analyzed. In LTBMCs, HSCs proliferate and differentiate under conditions mimicking the BM microenvironment. Total BM cell and CFU-GM production in late-generation mTerc−/− LTBMCs was reduced as compared with similar wildtype cultures on both genetic backgrounds. The proliferative disadvantage was fully evident in all LTBMCs from late-generation mTerc−/− mice analyzed, but not in all early-generation mTerc−/− cultures. This heterogeneity of response may be attributed to existing variation in the average telomere length among different same-generation animals.20 A similarly impaired proliferative capacity has also been observed in late-generation mTerc−/−splenocytes on the 2 different genetic backgrounds.19 22That the proliferative disadvantage of G3 mTerc−/− B6 cells observed in expansion cultures and LTBMCs does not correlate with any alteration in the phenotype of the expanded cells indicated that all cell lineages represented in the cultures must be equally affected, thereby reflecting an alteration in the multipotent progenitor compartment. The proliferative disadvantage of mTerc−/−cells in LTBMCs can be attributed mainly to the hematopoietic, not to the stromal, compartment, as evidenced by seeding experiments on irradiated stroma.
Transplantation of a limiting number of HSCs exerts a high proliferative demand on the stem cells that have to repopulate irradiated recipients. After 2 rounds of transplantation, the telomere length has been shown to decrease by 7 kb; moreover, the extent of reduction in telomere length was found to be dependent on the initial dose of transplanted HSCs. At 4 months after transplantation, mice reconstituted with limiting numbers of HSCs30 had significantly shorter telomeres than mice reconstituted with 3000 HSCs.42 In our experimental conditions for BM transplantation, the percentage of donor cells in the BM of the recipients was 84%; similar transplantations using late-generation mTerc−/− BM cells showed their repopulating potential to be much lower (45%) compared with wildtype cells. Secondary and tertiary transplantations indicated a progressive decrease in the repopulating ability of both wildtype and mTerc−/− BM cells, an effect that cannot be attributed exclusively to the underlying endogeneous reconstitution. This may reflect that telomere length has reached a critical length after the first transplantation and is therefore hindered in competing with the endogeneous reconstitution in subsequent transplantations. Although the reconstitution capacity of mTerc−/− BM cells decreases earlier than in wildtype cells, it is surprising that mTerc−/− BM cells are still able to reconstitute the irradiated hosts. This finding, together with the fact that a significant telomere shortening is not observed in G3 mTerc−/− B6 BM cells after transplantation, could reflect the existence of alternative mechanisms of telomere maintenance20 25 or the clonal selection of BM repopulating cells with longer telomeres. In this regard, SKY analysis indicated that 35% of cells in the BM of mice reconstituted with G3 mTerc−/− B6 BM cells are clonal because of the same chromosomal translocation. These results indicate that late-generation mTerc−/− HSCs have a disadvantage when forced to reconstitute the hematopoietic compartment, but their self-renewal potential is not severely affected. The forced proliferation of mTerc−/− BM cells in primary transplantations results in increased end-to-end fusions and therefore increased genetic instability owing to telomere dysfunction.
Competitive BM repopulation assay is currently the experimental procedure that most closely defines HSC function.43,44Analysis of the ability of mTerc−/− LTRSCs to compete with wildtype LTRSCs (ratio, 1:1) confirmed the previously described proliferative advantage for wildtype BM cells. This concurs with the disadvantage of G3 mTerc−/− B6 BM cells observed in our in vitro assays. When the G3 mTerc−/−-versus-wildtype cell ratio was increased to 10:1 in the graft, mTerc−/−LTRSCs repopulate BM and spleen for periods longer than 6 months. Accordingly, when the self-renewal ability of multipotent CFU-S12 progenitors was analyzed, no alterations were observed in G3 mTerc−/− B6 cells, reflecting that telomerase deficiency does not alter short-term function of the HSC compartment. These results lead to conclusions that differ with the report indicating that telomerase activity in murine hematopoietic cells is associated with self-renewal potential.17Interestingly, an increased chromosomal instability in transplanted G3 mTerc−/− B6 BM cells was demonstrated in comparison with transplanted wildtype BM cells, although no evidence of a preleukemic phenotype could be revealed.
In summary, our results demonstrate that telomerase deficiency does not impair HSC function under steady-state conditions; nonetheless, in situations that demand high proliferative capacity, such as transplantations of limiting numbers of HSCs, late-generation mTerc−/− BM cells exhibit a proliferative disadvantage that is occasionally associated with a moderate increase in chromosomal instability. This conclusion is in agreement with the diminished capacity of late-generation mTerc−/− mice to respond to stresses known to challenge the proliferative reserve of the hematopoietic system, such as myeloablation with 5-fluorouracil.21
We thank Elisa Santos and Rosa Serrano for mouse care and genotyping; Juan C. Cigudosa for technical help with SKY; Juan Martı́n-Caballero, M. Carmen Moreno, Irene López, and Asunción Garcı́a for their assistance; and Fermı́n Goytisolo, Hans Riese, and Cathy Mark for critical reading of the manuscript.
Supported by Swiss Bridge Award 2000; by grants PM97-0133 from the Ministerio de Educación y Cultura (MEC), 08.1/0030/98 from the Comunidad Autonoma de Madrid (CAM), EURATOM/991/0201 and FIGH-CT-1999/00002 to M.A.B.; and by grants 07/057/96 and 08.6/0021/1997 from CAM and SAF98-0008-CO4-O3 from the Plan Nacional de Salud y Farmacia, CICYT, to A.B. Authors E.S., R.E., and L.M.R. are supported by predoctoral fellowships from the CAM and MEC, respectively. The Department of Immunology and Oncology was founded and is supported by the Spanish Research Council (CSIC) and by Pharmacia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miguel Aracil, Department of Immunology and Oncology, Centro Nacional de Biotecnologı́a–CSIC, Campus Cantoblanco, E-28049 Madrid, Spain; e-mail: maracil@cnb.uam.es.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal