Abstract
We hypothesized that incremental improvements in the cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) chemotherapy regimen through optimization of drug selection, schedule, and pharmacokinetics would improve outcome in patients with large B-cell lymphomas. A prospective multi-institutional study of administration of etoposide, vincristine, and doxorubicin for 96 hours with bolus doses of cyclophosphamide and oral prednisone (EPOCH therapy) was done in 50 patients with previously untreated large B-cell lymphomas. The doses of etoposide, doxorubicin, and cyclophosphamide were adjusted 20% each cycle to achieve a nadir absolute neutrophil count below 0.5 × 109/L. The median age of the patients was 46 years (range, 20-88 years); 24% were older than 60 years; and 44% were at high-intermediate or high risk according to International Prognostic Index (IPI) criteria. There was a complete response in 92% of patients, and at the median follow-up time of 62 months, the progression-free survival (PFS) and overall survival (OS) rates were 70% and 73%, respectively. Neither IPI risk factors nor the index itself was associated with response, PFS, or OS. Doses were escalated in 58% of cycles, and toxicity levels were tolerable. Significant inverse correlations were observed between dose intensity and age for all adjusted agents, and drug clearance of doxorubicin and free etoposide was also inversely correlated with age (r = −0.54 andP2 = .08 and r = −0.45 andP2 = .034, respectively). Free-etoposide clearance increased significantly during successive cycles (P2 = .015). Lymphomas with proliferation of at least 80% had somewhat lower progression and those expressing bcl-2 had significantly higher progression (P2 = .04). Expression of bcl-2 may discriminate the recently described activated B-like from germinal-center B-like large-cell lymphomas and provide important pathobiologic and prognostic information. Dose-adjusted EPOCH may produce more cell kill than CHOP-based regimens. Dynamic dose adjustment may overcome inadequate drug concentrations, particularly in younger patients, and compensate for increased drug clearance over time.
Introduction
Since the development of cyclophosphamide-hydroxydaunomycin-vincristine-prednisone (CHOP) chemotherapy 25 years ago, many efforts have been made to improve up-front treatment of aggressive lymphomas.1,2 Early strategies were empiric and focused on the addition of new cytotoxic agents to the CHOP regimen, whereas later approaches were based on prevailing hypotheses of treatment design, such as those pertaining to dose intensity (DI).1,2 These efforts led to the development of regimens that were more complex and often more toxic, but none were found in randomized trials to be more effective than CHOP for treating aggressive lymphomas.3 Unfortunately, with standard CHOP therapy, only one third of patients remain free of progressive disease at 5 years3 (R. Fisher, personal communication, March 2000).
Ten years ago, we began development of a new treatment regimen by assessing the components of drug selection, schedule, and dose in the CHOP regimen. Etoposide was selected for inclusion with the CHOP drugs because of its single-agent activity and synergy.4 In vitro studies by us showed that tumor cells had relatively less resistance with prolonged low-concentration exposure to the natural-product–derived agents vincristine and doxorubicin than with brief higher-concentration exposure, and clinical reports also suggested that the effectiveness of vincristine and etoposide was schedule dependent.5 Thus, we designed a regimen including etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) and incorporated our findings on schedule dependency by administering the natural-product agents as a continuous intravenous infusion during 96 hours. EPOCH was initially evaluated in 131 patients with relapsed or refractory lymphomas and showed a high (74%) overall response rate and tolerable toxicity.6 Pharmacokinetic analyses of etoposide and doxorubicin showed significant interpatient variations in steady-state plasma concentrations and suggested the need for individual-patient dose adjustment.7 Such variations are a particular concern with continuous-infusion schedules because of the need to exceed threshold concentrations, and this led to incorporation of a dose-adjustment strategy based on hematopoietic nadir.8
We here report long-term outcomes in 50 patients with previously untreated large B-cell lymphomas who received dose-adjusted EPOCH chemotherapy. We describe the effect of a novel dose-adjustment strategy on toxicity and DI, as well as the effects of age and drug metabolism on drug doses. We found high progression-free survival (PFS) and overall survival (OS) rates and observed no relation between validated International Prognostic Index (IPI) risk factors and outcome or between the index itself and outcome. Our results suggest that optimization of treatment components may increase the possibility of curing large B-cell lymphomas.
Patients and methods
Patients and staging
Between May 1993 and September 1999, 50 patients with newly diagnosed large B-cell lymphomas were entered into a phase II multi-institutional study of dose-adjusted EPOCH chemotherapy for aggressive lymphomas at the National Cancer Institute (NCI), Bethesda, MD; Massachusetts General Hospital, Boston, MA; and the Frederick Cancer Center, Frederick, MD. Patients were all previously untreated and had the histologic subtypes of diffuse large B-cell, primary mediastinal (thymic) large B-cell, and follicular large B-cell (grade 3) lymphomas, confirmed by central path review (by E.S.J. or S.P.).9 No patient had evidence of an underlying or pre-existing indolent histologic feature. Eligibility requirements included disease stages II to IV and stage I bulky thymic (mediastinal mass > 5 cm) lymphomas, a nonreactive test for human immunodeficiency virus (HIV), negative pregnancy test in women, written informed consent, serum creatinine level below 133 μM/L (1.5 mg/dL), serum bilirubin level below 43 μM/L (2.5 mg/dL), absolute neutrophil count (ANC) of at least 1 × 109/L, and platelet count of at least 100 × 109/L unless due to organ involvement by tumor.
Initial evaluation included history taking and physical examination, standard blood tests including assessment of lactic acid dehydrogenase (LDH) level and HIV antibody, whole-body computed tomography, and bilateral bone marrow biopsies. Staging complied with the recommendations of the Cotswolds meeting, and response criteria complied with the guidelines of the International Workshop to Standardize Response Criteria.10 11 Sites of disease were restaged after cycle 4 and every 2 cycles thereafter. Patients received 2 cycles beyond complete remission or establishment of stable disease for a minimum of 6 cycles and a maximum of 8 cycles.
Chemotherapy and dose adjustments
Dose-adjusted EPOCH was administered as shown in Tables 1 and2. All patients received granulocyte colony-stimulating factor (G-CSF; Neupogen; Amgen, Thousand Oaks, CA) beginning on day 6 and continued until the ANC was more than 5 × 109/L above the nadir level. To prevent Pneumocystis carinii pneumonia, patients received sulfamethoxazole-trimethoprim twice daily for 3 days per week. Cycles were begun every 21 days, providing that the ANC was at least 1 × 109/L and the platelet count was at least 100 × 109/L. If the values were below these levels, counts were checked daily until recovery and G-CSF was administered as indicated. Patients with or at risk of central nervous system disease received standard intrathecal chemotherapy, and one patient received radiation to a brain mass.12 13 No other patients received radiation to any site.
EPOCH starting dose level (level 1)
| Drug . | Dose . | Route . | Treatment days . |
|---|---|---|---|
| Infused agents* | |||
| Etoposide | 50 mg/m2/day | CIV | 1, 2, 3, 4 (96 hours) |
| Doxorubicin | 10 mg/m2/day | CIV | 1, 2, 3, 4 (96 hours) |
| Vincristine† | 0.4 mg/m2/day | CIV | 1, 2, 3, 4 (96 hours) |
| Bolus agents | |||
| Cyclophosphamide | 750 mg/m2/day | IV | 5 |
| Prednisone | 60 mg/m2/bid | Oral | 1, 2, 3, 4, 5 |
| G-CSF | 5 μg/kg/day | SC | 6 to ANC > 5 × 109/L past nadir |
| Next cycle‡ | Day 21 |
| Drug . | Dose . | Route . | Treatment days . |
|---|---|---|---|
| Infused agents* | |||
| Etoposide | 50 mg/m2/day | CIV | 1, 2, 3, 4 (96 hours) |
| Doxorubicin | 10 mg/m2/day | CIV | 1, 2, 3, 4 (96 hours) |
| Vincristine† | 0.4 mg/m2/day | CIV | 1, 2, 3, 4 (96 hours) |
| Bolus agents | |||
| Cyclophosphamide | 750 mg/m2/day | IV | 5 |
| Prednisone | 60 mg/m2/bid | Oral | 1, 2, 3, 4, 5 |
| G-CSF | 5 μg/kg/day | SC | 6 to ANC > 5 × 109/L past nadir |
| Next cycle‡ | Day 21 |
Etoposide, doxorubicin, and vincristine could be mixed in the same solution.
The vincristine dose was never routinely capped.
Began on day 21 if the ANC was at least 1 × 109/L and the platelet count was at least 100 × 109/L.
EPOCH dose-adjustment paradigm
| Nadir measurements6-150 . | Dose-adjustment6-151 . |
|---|---|
| If Nadir ANC at least 0.5 × 109/L | 20% increase in etoposide, doxorubicin, and cyclophosphamide above last cycle |
| If Nadir ANC less than 0.5 × 109/L on 1 or 2 measurements | Same dose(s) as last cycle |
| If Nadir ANC less than 0.5 × 109/L on at least 3 measurements | 20% decrease in etoposide, doxorubicin, and cyclophosphamide below last cycle |
| Or | |
| If Nadir platelet count less than 25 × 109/L on 1 measurement | 20% decrease in etoposide, doxorubicin, and cyclophosphamide below last cycle |
| Nadir measurements6-150 . | Dose-adjustment6-151 . |
|---|---|
| If Nadir ANC at least 0.5 × 109/L | 20% increase in etoposide, doxorubicin, and cyclophosphamide above last cycle |
| If Nadir ANC less than 0.5 × 109/L on 1 or 2 measurements | Same dose(s) as last cycle |
| If Nadir ANC less than 0.5 × 109/L on at least 3 measurements | 20% decrease in etoposide, doxorubicin, and cyclophosphamide below last cycle |
| Or | |
| If Nadir platelet count less than 25 × 109/L on 1 measurement | 20% decrease in etoposide, doxorubicin, and cyclophosphamide below last cycle |
Measurements of ANC and platelet nadir are based ontwice weekly CBC only.
Dose adjustments above starting dose level(level 1) apply to etoposide, doxorubicin and cyclophosphamide. Dose adjustments below starting dose level (level 1) apply to cyclophosphamide only.
Dose adjustments are a fundamental component of the EPOCH regimen. Adjustments above the starting-dose level always applied to etoposide, doxorubicin, and cyclophosphamide; and adjustments below the starting-dose level applied only to cyclophosphamide. All drug doses were based on true body weight. The adjustment paradigm was based on the ANC nadir in the previous cycle and was designed to achieve a shallow period of neutropenia (Table 2). If a platelet nadir value below 25 × 109/L was observed, doses were reduced only 20%, regardless of the ANC nadir. The adjustment paradigm was based specifically on twice-weekly complete blood counts obtained 3 days apart, such as Monday and Thursday or Tuesday and Friday, and was not intended to identify every neutropenic event. For example, if the lowest ANC was 0.5 × 109/L, doses were escalated irrespective of whether the physician thought the patient had a lower nadir on a day on which an ANC was not done. Deviations from the adjustment paradigm were made only rarely in the event of a critical illness during the previous cycle and not for uncomplicated infections.
Vincristine was administered at a fixed dose of 1.6 mg/m2of body-surface area (0.4 mg/m2 per day), and the dose was never capped. The vincristine dose was reduced 25% if grade 2 motor neuropathy developed, 50% for grade 3 motor neuropathy, and 50% for grade 3 sensory neuropathy. Common side effects such as moderate constipation and sensory neuropathy were managed aggressively, without routine reductions in the dose of vincristine. Severe constipation was more frequent during the first cycle and decreased substantially during subsequent cycles. The dose of vincristine was increased up to a full dose if the toxic effect for which it had been reduced lessened.
Infusions were administered through a central venous access. Most patients had a temporary percutaneous intravenous central catheter placed each cycle, although permanent catheters or Port-a-Caths were also used. Patients usually received their 96-hour infusions as outpatients by using a single portable infusion pump. A solution containing vincristine, doxorubicin, and etoposide could be mixed in a 0.9% normal saline (NS) injection at the respective drug concentrations of 1, 25, and 125 μg/mL; 1.4, 35, and 175 μg/mL; or 2, 50, and 250 μg/mL. The solution was stable for at least 48 hours at room temperature if protected from light.14 A 24-hour supply of vincristine, doxorubicin, and etoposide mixed in 500 mL 0.9% NS was prepared daily for delivery through the portable infusion pump. After standard hydration, cyclophosphamide (diluted in 100 mL NS) was administered during 15 minutes on the fifth day after completion of the 96-hour infusion.
Drug-resistance markers
Immunohistochemical studies were conducted on paraffin-embedded, formalin-fixed tissue sections as described previously.15Briefly, sections were stained with monoclonal antibodies that included D07 antibody against wild-type and mutant p53 (Dako, Carpinteria, CA), MIB-1 antibody against the homologous nuclear proliferating antigen to assess tumor proliferation (Dako), and an antibody against bcl-2 protein (Dako clone 124). All immunohistochemical analyses were done in the Laboratory of Pathology, National Cancer Institute.
After deparaffinization, the slides were subjected to an antigen-retrieval procedure (placement in 10 mM citrate buffer [pH 6.0] in a pressure cooker and exposure to microwaves for 40 minutes at 700 W), and immunohistochemical studies were then done by using an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's instructions. Sections were scored as described previously.15 Samples were considered positive for p53 if more than 10% of the tumor cells had nuclear staining. A control slide was run with the tumor samples to confirm adequacy of staining. For bcl-2, the staining intensity was compared with that in control T cells present in the tumor samples. If the control cells did not stain, the sample was considered unsatisfactory. Staining for bcl-2 was scored as follows: 0, tumor cells were negative, but interspersed reactive T cells were positive; 1, tumor cells stained more weakly than T cells; 2, tumor cells stained the same as T cells; and 3, tumor cells stained more strongly than T cells. Tumor cells were considered positive for bcl-2 if they were scored 1 or higher. MIB-1 staining was scored as a mean percentile for 200 tumor cells averaged over 3 high-power (×40) fields, or in cases with a minute amount of tissue, as a mean percentile of all neoplastic cells and was reported as 0% to 100%.
Pharmacokinetics of etoposide
Duplicate blood samples for measurement of total and free (not protein bound) etoposide were drawn into heparin-coated tubes before the start of the etoposide infusion and at hours 22 and 94 of each 96-hour infusion. Plasma was separated by centrifugation and stored frozen at −70°C until analysis. Etoposide concentrations were measured by using a modification of a previously described reverse-phase high-performance liquid chromatography (HPLC) method.16 Duplicate plasma samples (0.1 mL) for determination of total drug were extracted into 1.0 mL chloroform twice. The organic phase was collected after centrifugation, evaporated to dryness by using nitrogen, and reconstituted in mobile phase. Chromatography was done on a Waters 2690 HPLC system (Waters, Milford, MA). Twenty microliters of the reconstituted sample was injected into a Zorbax 4.6 × 12.5-mm, 5-μm phenyl guard column and then a 4.6 × 250-mm, 5-μm phenyl analytical column (Agilent Technologies, Palo Alto, CA). The mobile phase consisted of water, methanol, acetonitrile, and glacial acetic acid (45.5:25:25:0.5) with 1.81 g/L tetramethylammonium hydroxide at a flow rate of 1.0 mL/minute. Etoposide was detected and quantified at 550 mV with a Coulochem II electrochemical detector equipped with an analytic cell (model 5011; ESA, Chelmsford, MA). The signal data were collected and integrated by using Millennium software (Waters). Ultrafiltrates for the measurement of non–protein-bound etoposide were prepared by using Microcon 30 centrifugal filtration units (Amicon, Beverly, MA). Fifty microliters of nonextracted ultrafiltrate was injected, detected, and quantified as described above. The steady-state concentrations of total and free etoposide were the means of the 22- and 94-hour concentrations. Total and free-etoposide clearance was derived by dividing the drug-infusion rate by the steady-state concentration.
Statistical analysis
Survival time and time to disease progression in months were calculated from the day of enrollment in the study until death, relapse, progression, or last follow-up, as appropriate. The probability of OS and PFS was calculated by using the Kaplan-Meier method, and the significance of the difference between pairs of Kaplan-Meier curves was determined by using the Mantel-Haenszel procedure.17,18 The Cox proportional hazards model was used to identify factors that were jointly significant in the association with OS or PFS.19 The factors considered for inclusion in univariate and Cox analyses were patient age, patient sex, disease stage, Eastern Cooperative Oncology Group (ECOG) performance status, number of extranodal sites, LDH, IPI score, and marker values. Initially, in the univariate actuarial analyses, continuous variables such as age and MIB-1 value were divided into quartiles. To account for the pooling of groups after the initial examination of results,P values for the univariate analysis were adjusted by multiplying by a factor equal to the number of implicit evaluations made that led to the comparison reported. For example, suppose age was initially examined in 4 groups and was then reduced to 2 groups that had distinctly different outcomes. Because there could have been 3 possible formations for 2 groups consisting of patients in 1 to 3 adjacent quartiles, the adjusted P value is reported as the unadjusted P value multiplied by 3.
Only factors that were associated with at least a trend toward significance in the univariate analysis (unadjusted Pvalue < .20) were evaluated in the Cox model. Because the trends in OS and PFS were not necessarily linear over the 4 quartiles, the data for the Cox models were dichotomized at the cut point that yielded the most significant result in the univariate analysis. As a result, the Cox model P values may be biased, because the cut points were selected on the basis of an examination of the same data that went into the model. In view of the limited number of patients in this study, the Cox models, the thresholds used in variables evaluated in the Cox models, and the resulting P values should be interpreted as hypothesis generating rather than definitive. The thresholds and P values reported are thus intended to be suggestive of effect rather than absolute.
The resulting model variables (bi) were converted to relative risks by computing exp(bi), where exp(a) equals 2.7183. The 95% confidence interval for the relative risk was computed as [exp(biL), exp(biH)], where biL equals bi − 1.96 [estimated standard error (bi)] and biH equals bi plus 1.96 [estimated standard error (bi)].20 The relative risk was the risk associated with dying or having disease progression while being in a higher-risk category compared with the risk while being in the lowest risk category. For example, a risk of 2.0 indicates twice the risk of dying during any interval for a patient with the trait associated with elevated risk compared with a patient without the trait. Using the procedure described by Simon and Altman,21 we used a likelihood ratio test to assess the importance of new variables (specifically, marker values) after adjusting for standard prognostic factors such as number of extranodal sites. All P values are 2-tailed and denoted as P2.
Results
Characteristics of patients
All 50 enrolled patients with large B-cell lymphoma were included in the analysis of survival outcomes and toxicity, and 49 patients were assessed for response; one patient had no measurable disease after surgery. The median age of the patients was 46 years, and 24% were older than 60 (Table 3). Aside from older age, other poor prognostic factors were ECOG performance status of 2 or more in 10% of patients, advanced disease stage in 74%, and elevated LDH in 70%. Assessment using the IPI found high-intermediate-risk or high-risk scores in 44% of patients and, after adjustment for age, high-intermediate-risk or high-risk scores in 52%.22Diffuse large B-cell lymphoma that was not otherwise specified (NOS) was the predominant histologic finding (64% of patients) and was followed by thymic large B-cell lymphoma (28%) and follicular large B-cell lymphoma (8%).
Patient characteristics and outcome
| Characteristic . | No. (%)* . | % PFS at 62 mo† . | P2‡ . | % OS at 62 mo† . | P2‡ . |
|---|---|---|---|---|---|
| Total patients | 50 (100) | 70 | 73 | ||
| Sex | |||||
| Male | 25 (50) | 71 | .92 | 75 | .83 |
| Female | 25 (50) | 68 | 71 | ||
| Median age, y (range) | 46 (20-88) | ||||
| Younger than or equal to 60 y | 38 (76) | 68 | .85 | 78 | .14 |
| Older than 60 y | 12 (24) | 73 | 58 | ||
| Performance status | |||||
| ECOG 0 to 1 | 45 (90) | 68 | .59 | 70 | .19 |
| ECOG at least 2 | 5 (10) | 80 | 100 | ||
| Disease stage | |||||
| I/II | 6/7 (26) | 85 | .47 | 85 | .39 |
| III/IV | 9/28 (74) | 64 | 69 | ||
| LDH level | |||||
| Normal | 15 (30) | 80 | .40 | 86 | .18 |
| Above normal | 35 (70) | 65 | 68 | ||
| Extranodal sites | |||||
| 0 to 1 | 33 (66) | 78 | .07 | 81 | .09 |
| At least 2 | 17 (34) | 53 | 58 | ||
| IPI score | |||||
| Low (0-1) | 19 (38) | 79 | .52 | 84 | 2-153 |
| Low intermediate (2) | 9 (18) | 78 | 100 | ||
| High intermediate (3) | 16 (32) | 54 | 42 | ||
| High (4-5) | 6 (12) | 67 | 83 | ||
| Age-adjusted IPI score | |||||
| Low (0) | 4 (8) | 100 | .50 | 100 | .10 |
| Low intermediate (1) | 20 (40) | 75 | 80 | ||
| High intermediate (2) | 21 (42) | 56 | 55 | ||
| High (3) | 5 (10) | 80 | 100 | ||
| Bcl-2 status (n = 29) | |||||
| Negative | 17 (59) | 82 | .04 | 82 | .37 |
| Positive | 12 (41) | 50 | 67 | ||
| MIB-1 status (n = 33) | |||||
| At least 80% | 19 (58) | 74 | .24 | 79 | .35 |
| Less than 80% | 14 (42) | 62 | 64 | ||
| P53 status (n = 33) | |||||
| Negative | 25 (76) | 72 | .17 | 80 | .04 |
| Positive | 8 (24) | 60 | 50 |
| Characteristic . | No. (%)* . | % PFS at 62 mo† . | P2‡ . | % OS at 62 mo† . | P2‡ . |
|---|---|---|---|---|---|
| Total patients | 50 (100) | 70 | 73 | ||
| Sex | |||||
| Male | 25 (50) | 71 | .92 | 75 | .83 |
| Female | 25 (50) | 68 | 71 | ||
| Median age, y (range) | 46 (20-88) | ||||
| Younger than or equal to 60 y | 38 (76) | 68 | .85 | 78 | .14 |
| Older than 60 y | 12 (24) | 73 | 58 | ||
| Performance status | |||||
| ECOG 0 to 1 | 45 (90) | 68 | .59 | 70 | .19 |
| ECOG at least 2 | 5 (10) | 80 | 100 | ||
| Disease stage | |||||
| I/II | 6/7 (26) | 85 | .47 | 85 | .39 |
| III/IV | 9/28 (74) | 64 | 69 | ||
| LDH level | |||||
| Normal | 15 (30) | 80 | .40 | 86 | .18 |
| Above normal | 35 (70) | 65 | 68 | ||
| Extranodal sites | |||||
| 0 to 1 | 33 (66) | 78 | .07 | 81 | .09 |
| At least 2 | 17 (34) | 53 | 58 | ||
| IPI score | |||||
| Low (0-1) | 19 (38) | 79 | .52 | 84 | 2-153 |
| Low intermediate (2) | 9 (18) | 78 | 100 | ||
| High intermediate (3) | 16 (32) | 54 | 42 | ||
| High (4-5) | 6 (12) | 67 | 83 | ||
| Age-adjusted IPI score | |||||
| Low (0) | 4 (8) | 100 | .50 | 100 | .10 |
| Low intermediate (1) | 20 (40) | 75 | 80 | ||
| High intermediate (2) | 21 (42) | 56 | 55 | ||
| High (3) | 5 (10) | 80 | 100 | ||
| Bcl-2 status (n = 29) | |||||
| Negative | 17 (59) | 82 | .04 | 82 | .37 |
| Positive | 12 (41) | 50 | 67 | ||
| MIB-1 status (n = 33) | |||||
| At least 80% | 19 (58) | 74 | .24 | 79 | .35 |
| Less than 80% | 14 (42) | 62 | 64 | ||
| P53 status (n = 33) | |||||
| Negative | 25 (76) | 72 | .17 | 80 | .04 |
| Positive | 8 (24) | 60 | 50 |
PFS indicates progression-free survival; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; LDH, lactic acid dehydrogenase; and IPI, International Prognostic Index.
Values are numbers (%) unless otherwise indicated.
Kaplan-Meier estimates at median follow-up time of 62 months.
P2 derived from log-rank test of Kaplan-Meier curves.
The inverse order of the curves within the low-risk and high-risk categories indicates that the significant difference observed (P2 = .009) does not accurately reflect the expected association of the IPI with survival.
Treatment outcome
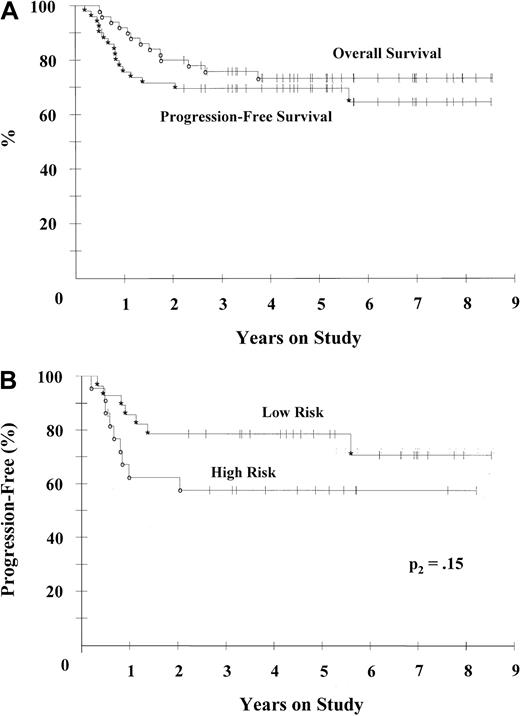
Among the 49 patients assessed, 45 (92%) had a complete response (CR) and 4 (8%) had a partial response (PR) to EPOCH therapy; thus, the overall response rate was 100% (Table4). Most patients (78%) had a CR or establishment of stable disease within 4 cycles of treatment and received a total of 6 cycles; 22% received 8 cycles. Among patients who had a CR, 26% subsequently had relapses, all but one of which occurred in the first 20 months of follow-up. The 4 patients with a PR had disease progression within a median time of 4.5 months (range, 2-6 months) after the beginning of EPOCH therapy. The median OS and PFS points have not been reached, but at the median follow-up time of 62 months, the PFS probability was 70% and the OS probability was 73% (Figure 1A). There was no significant difference in PFS and OS at 62 months between the 2 largest subgroups of patients, that is, those with large B-cell lymphoma NOS and those with thymic large B-cell lymphoma: PFS rates were 68% and 64% and OS rates were 68% and 79%, respectively.
Response to EPOCH chemotherapy
| Category . | Total no. . | CR, no. (%; 95% CI) . | PR, no. (%) . | RR, no. (%) . |
|---|---|---|---|---|
| All patients3-150 | 49 | 45 (92; 80-98) | 4 (8) | 49 (100) |
| IPI score | ||||
| Low risk (0-2) | 27 | 26 (96; 81-100) | 1 (4) | 27 (100) |
| High risk (3-5) | 22 | 19 (86; 65-97) | 3 (14) | 22 (100) |
| Category . | Total no. . | CR, no. (%; 95% CI) . | PR, no. (%) . | RR, no. (%) . |
|---|---|---|---|---|
| All patients3-150 | 49 | 45 (92; 80-98) | 4 (8) | 49 (100) |
| IPI score | ||||
| Low risk (0-2) | 27 | 26 (96; 81-100) | 1 (4) | 27 (100) |
| High risk (3-5) | 22 | 19 (86; 65-97) | 3 (14) | 22 (100) |
CR indicates complete response; CI, confidence interval; PR, partial response; RR, overall response rate; and IPI, International Prognostic Index.
One patient had no measurable disease after tumor resection.
Kaplan-Meier plots for PFS and OS.
(A) For all patients in the study, PFS (*) and OS (○) were 70% and 73%, respectively, at the median follow-up time of 62 months. (B) PFS for all patients divided into modified low (0-2) (*) and high (3-5) (○). The rates according to IPI risk group were 79% and 58%, respectively, at 62 months (P2 = .15 for all patients).
Kaplan-Meier plots for PFS and OS.
(A) For all patients in the study, PFS (*) and OS (○) were 70% and 73%, respectively, at the median follow-up time of 62 months. (B) PFS for all patients divided into modified low (0-2) (*) and high (3-5) (○). The rates according to IPI risk group were 79% and 58%, respectively, at 62 months (P2 = .15 for all patients).
Prognostic factors
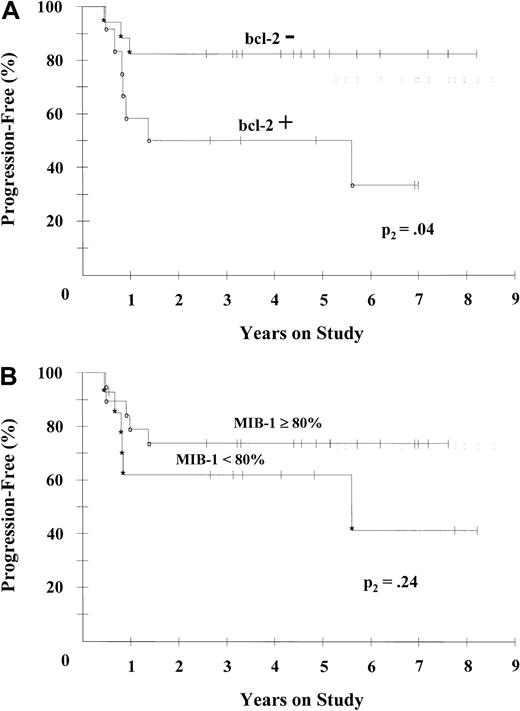
There was no significant association between any of the validated IPI risk factors or the non–age-adjusted or age-adjusted index itself and any survival end point (Table 3). Because of the similarity of the 2 low IPI PFS curves (IPI 0/1 and 2) and the 2 high IPI risk curves (IPI 3 and 4/5), they were combined to form a modified IPI to increase the power of the comparison, and the results were correlated with outcome. No significant association with PFS was observed (P2 = .15; Figure 1B), although there was a significant association with OS (P2 = .007) in the univariate analyses. To investigate whether known mechanisms of drug resistance were predictors of outcome, tumor tissue was analyzed for expression of the bcl-2 and p53 proteins, which are associated with apoptosis, and for tumor proliferation (MIB-1), which is associated with drug sensitivity.15,23,24 Notably, overexpression of bcl-2 was the only factor associated with treatment failure: PFS rates at 62 months were 82% in patients without bcl-2 and 50% in patients with bcl-2 expression (Figure 2A). Also of interest, and in contrast to findings with CHOP-based regimens, patients with high tumor proliferation rates (> 80%) were somewhat less likely to have disease progression than patients with lower rates (P2 = .24; Figure 2B), and they had similar survival rates (Table 3).25,26 Overexpression of p53, which we previously showed to be a surrogate (with 98% specificity and 79% sensitivity) for gene mutation in B-cell lymphomas, was associated with a moderately higher probability of disease progression (P2 = .17) and a significantly lower probability of OS (P2 = .04; Table 3).15
Analysis of PFS in patients with bcl-2 and tumor proliferation (MIB-1) markers.
(A) PFS rates in 29 patients divided into bcl-2–negative (*) and bcl-2–positive groups (○) were 82% and 50%, respectively, at 62 months (P2 = .036 overall). (B) PFS rates in 33 patients divided into MIB-1 of 80% or greater (○) and less than 80% (*) were 74% and 62%, respectively, at 62 months (P2 = .24 overall).
Analysis of PFS in patients with bcl-2 and tumor proliferation (MIB-1) markers.
(A) PFS rates in 29 patients divided into bcl-2–negative (*) and bcl-2–positive groups (○) were 82% and 50%, respectively, at 62 months (P2 = .036 overall). (B) PFS rates in 33 patients divided into MIB-1 of 80% or greater (○) and less than 80% (*) were 74% and 62%, respectively, at 62 months (P2 = .24 overall).
A Cox proportional hazards analysis was done to determine whether any prognostic factors were independently associated with outcome (Table5). We found no association between PFS, a specific measure of a regimen's curative potential, and any clinical factor, although there was a trend toward a higher probability of disease progression among patients with multiple extranodal sites. In contrast, when the Cox model was applied to the subgroup of patients with drug-resistance markers, bcl-2 was significantly associated with a higher risk of progression, even after adjustment for modified IPI (P2 = .04). Among all patients, the modified IPI was the only factor associated with OS (P2 = .015), whereas in the subgroup with drug-resistance markers, there was a trend toward an association of p53 with OS (P2 = .06), a result confirmed by likelihood ratio testing.
Cox models for PFS and OS
| Variable4-150 . | Variable estimate . | P2 . | Relative risk (95% CI) . |
|---|---|---|---|
| PFS: all patients (n = 50) | |||
| No. of extranodal sites (2-5 vs 0-1) | 0.88 | .08 | 2.42 (0.90-6.50) |
| PFS: patients with marker data (n = 29) | |||
| Bcl-2 positive vs bcl-2 negative | 1.35 | .05 | 3.86 (0.99-14.96) |
| PFS: Bcl-2 status adjusted for IPI score (n = 29) | |||
| Bcl-2 positive vs bcl-2 negative | 1.49 | .04 | 4.42 (1.10-17.69) |
| Modified IPI score (3-5 vs 0-2) | 0.64 | .33 | 1.90 (0.52-6.89) |
| OS: all patients (n = 50) | |||
| Modified IPI score (3-5 vs 0-2) | 1.60 | .015 | 4.96 (1.36-18.06) |
| OS: patients with marker data (n = 33) | |||
| Modified IPI score (3-5 vs 0-2) | 1.62 | .04 | 5.07 (1.04-24.62) |
| P53 positive vs p53 negative | 1.27 | .06 | 3.54 (0.92-13.54) |
| Variable4-150 . | Variable estimate . | P2 . | Relative risk (95% CI) . |
|---|---|---|---|
| PFS: all patients (n = 50) | |||
| No. of extranodal sites (2-5 vs 0-1) | 0.88 | .08 | 2.42 (0.90-6.50) |
| PFS: patients with marker data (n = 29) | |||
| Bcl-2 positive vs bcl-2 negative | 1.35 | .05 | 3.86 (0.99-14.96) |
| PFS: Bcl-2 status adjusted for IPI score (n = 29) | |||
| Bcl-2 positive vs bcl-2 negative | 1.49 | .04 | 4.42 (1.10-17.69) |
| Modified IPI score (3-5 vs 0-2) | 0.64 | .33 | 1.90 (0.52-6.89) |
| OS: all patients (n = 50) | |||
| Modified IPI score (3-5 vs 0-2) | 1.60 | .015 | 4.96 (1.36-18.06) |
| OS: patients with marker data (n = 33) | |||
| Modified IPI score (3-5 vs 0-2) | 1.62 | .04 | 5.07 (1.04-24.62) |
| P53 positive vs p53 negative | 1.27 | .06 | 3.54 (0.92-13.54) |
CI indicates confidence interval; and IPI, International Prognostic Index.
Variables with greatest significance in Cox proportional hazards model analysis. The Cox model included all individual IPI prognostic factors, combined high IPI risk categories (3-5) versus low modified IPI risk categories (0-2), and marker data where available.
Toxicity
Toxicity was assessed during 318 cycles (Table6). The dose-adjustment paradigm was designed to produce a period of shallow neutropenia, thus resulting in ANC nadirs of between 0.499 × 109/L and 0.1 × 109/L in 34% of cycles, with more severe neutropenia (ANC < 0.1 × 109/L) in 15% of cycles. Thrombocytopenia was less common and was only dose limiting (platelet count < 25 × 109/L) in 7% of cycles. Most important, the clinical effect of the hematopoietic toxicity was limited, with no cases of hemorrhage and only an 8% rate of fever with neutropenia. Gastrointestinal toxicity was generally mild. Nausea or vomiting were rare during the chemotherapy infusions, but patients did receive prophylactic antiemetics with cyclophosphamide, which was associated with mild emesis in 7% of cycles. Stomatitis was uncommon and occurred only during later cycles and at the higher dose levels. In general, vincristine toxicity was manageable and infrequently required dose reductions. The cases of serious constipation that occurred tended to be most severe in the first cycle; to reduce this side effect, patients received prophylactic bowel therapy. Serious (≥ grade 3) motor and sensory toxicity was observed in only 2 and 4 patients, respectively, and was nearly resolved during the year after treatment. A troubling side effect for patients was fatigue, which increased markedly in the later cycles. Fatigue was more severe during the first 2 weeks of each cycle and improved dramatically in the first month after treatment was completed. There were no cardiac complications or treatment-related deaths.
Toxicity of EPOCH chemotherapy in 50 patients
| Variable . | Value . |
|---|---|
| No. (%) of cycles | 318 (100) |
| Hospitalization | |
| No. (%) cycles with hospitalization for fever and neutropenia | 26 (8) |
| No. (%) cycles with hospitalization for other reason | 15 (5) |
| Hematologic toxicity | |
| No. (%) cycles with ANC less than 0.1 × 109/L | 47 (15) |
| No. (%) cycles with ANC 0.1 to 0.5 × 109/L | 109 (34) |
| No. (%) cycles with platelet count less than 25 × 109/L | 21 (7) |
| No. (%) cycles with platelet count 26 to 50 × 109/L | 40 (13) |
| Toxicity at least grade 25-150 | |
| Gastrointestinal | |
| No. (%) cycles with nausea or vomiting | 19 (6) |
| No. (%) cycles with stomatitis | 39 (12) |
| No. (%) cycles with constipation | 13 (4) |
| Neurologic | |
| No. (%) patients with sensory effects | 14 (28) |
| No. (%) patients with motor effects | 6 (12) |
| No. (%) patients with fatigue | 13 (26) |
| No. (%) patients with cardiac effects | 0 (0) |
| No. (%) of treatment-related deaths | 0 (0) |
| Variable . | Value . |
|---|---|
| No. (%) of cycles | 318 (100) |
| Hospitalization | |
| No. (%) cycles with hospitalization for fever and neutropenia | 26 (8) |
| No. (%) cycles with hospitalization for other reason | 15 (5) |
| Hematologic toxicity | |
| No. (%) cycles with ANC less than 0.1 × 109/L | 47 (15) |
| No. (%) cycles with ANC 0.1 to 0.5 × 109/L | 109 (34) |
| No. (%) cycles with platelet count less than 25 × 109/L | 21 (7) |
| No. (%) cycles with platelet count 26 to 50 × 109/L | 40 (13) |
| Toxicity at least grade 25-150 | |
| Gastrointestinal | |
| No. (%) cycles with nausea or vomiting | 19 (6) |
| No. (%) cycles with stomatitis | 39 (12) |
| No. (%) cycles with constipation | 13 (4) |
| Neurologic | |
| No. (%) patients with sensory effects | 14 (28) |
| No. (%) patients with motor effects | 6 (12) |
| No. (%) patients with fatigue | 13 (26) |
| No. (%) patients with cardiac effects | 0 (0) |
| No. (%) of treatment-related deaths | 0 (0) |
ANC indicates absolute neutrophil count.
According to the National Cancer Institute's common toxicity criteria.
Dose adjustment and pharmacokinetics
The dose-adjustment paradigm normalized drug-dose rates to hematopoietic toxicity in an effort to reduce interpatient variation in drug concentrations. Most patients required dose escalations to achieve an ANC nadir, with 29% of cycles having at least 144% of the entry-dose level and 5% having below the entry level (Figure3A). Cycle 1 was associated with greater hematopoietic toxicity than cycle 2 in 22% of patients in whom the drug dose could not be escalated until cycle 3. For all cycles, the mean (±SD) DIs for the escalated agents were equivalent to the second dose level and were 296 ± 59 mg/m2 per week for cyclophosphamide, 80 ± 14 mg/m2 per week for etoposide, and 16 ± 2.8 mg/m2 per week for doxorubicin. The vincristine DI was administered at 96% of the full dose and was 0.51 ± 0.06 mg/m2 per week. Of note, because the cycle length was 98% of the predicted value, virtually all the variation in DI was due to differences in dose rate and not cycle length.
Dose-adjustment map and correlation between etoposide DI and age.
(A) Map of dose levels achieved, according to cycle, for each patient. The percentage of cycles administered at each dose level is shown at right. Overall, 56% of cycles had escalation above and 5% had reduction below the starting-dose level. (B) Correlation between etoposide DI and age (r = −0.48;P2 = .0004) shows that older patients could not tolerate as high a dose rate as younger patients, possibly because of decreased hematopoietic tolerance or altered pharmacokinetics.
Dose-adjustment map and correlation between etoposide DI and age.
(A) Map of dose levels achieved, according to cycle, for each patient. The percentage of cycles administered at each dose level is shown at right. Overall, 56% of cycles had escalation above and 5% had reduction below the starting-dose level. (B) Correlation between etoposide DI and age (r = −0.48;P2 = .0004) shows that older patients could not tolerate as high a dose rate as younger patients, possibly because of decreased hematopoietic tolerance or altered pharmacokinetics.
The strategy of normalizing dose rate allows an opportunity to examine the influence of clinical factors on DI more accurately. This analysis revealed a moderately strong inverse correlation (Spearman nonparametric analysis) between the DI of all adjusted agents and patient age but no correlation with any other IPI risk factor, the IPI index itself, or PFS or OS (data not shown). Specifically, the correlation coefficients for the etoposide and doxorubicin DIs and age were r = −0.48 (P2 = .0004; Figure 3B) and r = −0.48 (P2 = .0004), respectively. In contrast, there was no correlation between any clinical factor, including age, and vincristine DI, which was not dose adjusted.
The inverse correlation between DI and age could be explained by decreased hematopoietic tolerance or decreased drug metabolism in older patients. To examine this issue, we analyzed the preliminary pharmacokinetics of etoposide and doxorubicin, measured in 23 and 12 patients, respectively, who received dose-adjusted EPOCH. We found a moderately strong inverse correlation (Spearman nonparametric analysis) between age and clearance of free etoposide (r = −0.45;P2 = .034) and doxorubicin (r = −0.54;P2 = .08) (data not shown), indicating that older patients had higher serum levels than younger patients at similar dose rates (Figure 4A).27Additionally, in patients who underwent analysis of preliminary pharmacokinetics during multiple cycles, clearance of free etoposide increased during each cycle, with an average increase of 56% between cycles 1 and 6 (P2 = .015 after application of a Bonferroni adjustment for multiple comparisons; Figure4B).
Free-etoposide clearance, according to age and cycle number.
(A) Free-etoposide clearance measured in 23 patients during several cycles decreased with age (r = −0.45;P2 = .034), suggesting that the lower dose rate in older patients may have been due to decreased drug clearance. (B) Free-etoposide clearance increased with cycle number (paired comparison of cycles 1 and 6 in 12 patients;P2 = .015 after applying a Bonferroni adjustment for multiple comparisons), indicating that at fixed dose rates, serum levels of free etoposide would fall cycle after cycle. The number of patient samples is shown above the y-axis.
Free-etoposide clearance, according to age and cycle number.
(A) Free-etoposide clearance measured in 23 patients during several cycles decreased with age (r = −0.45;P2 = .034), suggesting that the lower dose rate in older patients may have been due to decreased drug clearance. (B) Free-etoposide clearance increased with cycle number (paired comparison of cycles 1 and 6 in 12 patients;P2 = .015 after applying a Bonferroni adjustment for multiple comparisons), indicating that at fixed dose rates, serum levels of free etoposide would fall cycle after cycle. The number of patient samples is shown above the y-axis.
Discussion
Dose-adjusted EPOCH chemotherapy evolved from the hypothesis that incremental improvements in CHOP chemotherapy through optimization of drug selection, schedule, and pharmacokinetics would improve the outcome in patients with aggressive lymphomas. Overall, 92% of the patients treated with EPOCH in this series had a CR, with similar rates in patients at low and high risk. More important, dose-adjusted EPOCH produced a PFS probability of 70% and an OS probability of 73% at the median follow-up time of 62 months (Figure 1A). These results may be better than those achieved with CHOP or CHOP-based regimens.3 Fisher et al3 (R. Fisher, personal communication, March 2000) conducted a randomized comparison between CHOP and 3 later-generation regimens and observed comparable CR rates of between 44% and 56% and overall PFS and OS rates of approximately 44% and 52%, respectively, at 3 years, with evidence of continued disease progression at 5 years. Attempts to improve outcome through dose escalation have yielded more toxicity without clear benefits. A randomized study comparing standard with escalated doxorubicin doses in a bleomycin-doxorubicin-cyclophosphamide-vincristine-prednisone regimen showed no significant differences in CR (∼60%), PFS (∼52%) and OS at a median follow-up time of 65 months.28 A trial of double-dose cytarabine-bleomycin-vincristine-mechlorethamine therapy produced a CR of 69%, PFS of 58%, and OS of 73% at 4 years, and only 15% of patients were at high-intermediate or high risk.29A study of high-dose CHOP in patients at high risk had a favorable PFS of 69%, but the 20-month median follow-up was too short to provide confidence in the durability of the outcome.30
Selection bias does not appear to account for the favorable results achieved with dose-adjusted EPOCH in the current study. The proportion of patients at high risk in our series (44%) was comparable to that in the Fisher study (44%) and the IPI validation series (38%).3,22 There were more patients with low-risk disease (38%) in our series than in the Fisher study (22%), which partly reflects our inclusion of patients with bulky stage I thymic lymphomas. The IPI, however, is not predictive in thymic lymphomas, and no difference in outcome was observed between patients at low risk and those at low-intermediate risk in our study (Table 3).31Of note, among the patients with thymic lymphomas, dose-adjusted EPOCH alone achieved a 92% CR, which required negative gallium 67 in patients with residual abnormalities, compared to 67Ga persistence in 66% of patients with thymic lymphomas treated with methotrexate-doxorubicin-cyclophosphamide-vincristine-prednisone-bleomycin (MACOP-B).32
The lack of an association between the IPI or any individual risk factor and outcome suggests that dose-adjusted EPOCH has a cell-kill profile that is different from that of CHOP-based regimens. Because clinical prognostic factors are surrogate measures of drug resistance, we assessed known mechanisms of drug resistance, including tumor proliferation and p53 and bcl-2 expression, and outcome with dose-adjusted EPOCH. Patients with lymphomas with high tumor proliferation rates (≥ 80%) had somewhat higher, although not significantly higher, rates of PFS and OS than those with tumors with lower proliferation rates (Figure 2B). In contrast, CHOP-based regimens were found to be less effective in treating rapidly proliferating lymphomas. Miller et al26 reported a 1-year survival rate of 18% in patients with tumor proliferation rates above 80% measured by Ki-67 analysis and a rate of 82% in patients with lower rates. Bauer et al25 reported median survival times of 7 and 39 months, respectively, in patients with lymphomas with high (≥ 80%) and low (< 80%) proliferative activity. These results suggest that infusion schedules may be more effective than bolus schedules against rapidly dividing tumors and are consistent with our previous results and findings of studies in in vitro models that showed an increased sensitivity of dividing cells to DNA-damaging agents.15,24 Mutation of p53, a cause of drug resistance in vitro, was assessed through the surrogate of overexpression and was significantly associated with decreased OS and moderately decreased PFS.15
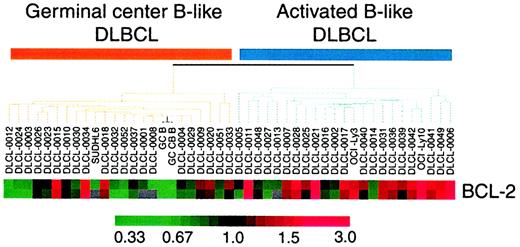
The relation between expression of bcl-2 and outcome was of interest to us because of our previous work showing that large B-cell lymphomas may comprise 2 diseases with complementary DNA microarray-expression patterns corresponding to a germinal-center B-like and activated B-like origin.33 Of clinical relevance was the finding that patients with activated B-like lymphoma had a significantly shorter survival and would be at high risk of treatment failure. An analysis in the microarray database revealed at least 4-fold higher levels of bcl-2 messenger RNA expression in 70% of activated B-like lymphomas (P2 = .003) compared with germinal-center B-like lymphomas (Figure 5) and suggests that bcl-2 protein expression may be a marker for and potential mechanism of drug failure in this poor-prognosis group.15,33 34 In the current study, bcl-2 expression was the only factor associated with PFS (Figure 2A), suggesting that pathobiologic characteristics may be the important indicators of outcome with dose-adjusted EPOCH, rather than clinical factors, many of which reflect tumor volume.
Gene-expression patterns of bcl-2 on complementary DNA analysis in germinal center B-like and activated B-like large B-cell lymphomas.
Maximum red and green results indicate expression levels 3 fold above and below baseline values, respectively. This array shows a significant (P2 = .003) association between increased bcl-2 expression and the activated B-like genotype, and these results suggest that bcl-2 protein expression may identify tumors with the activated B-cell genotype.
Gene-expression patterns of bcl-2 on complementary DNA analysis in germinal center B-like and activated B-like large B-cell lymphomas.
Maximum red and green results indicate expression levels 3 fold above and below baseline values, respectively. This array shows a significant (P2 = .003) association between increased bcl-2 expression and the activated B-like genotype, and these results suggest that bcl-2 protein expression may identify tumors with the activated B-cell genotype.
Our dose-adjustment strategy was developed to normalize interpatient drug plasma concentrations and resulted in dose escalations in most patients (Figure 3A). Potentially important is our finding that younger patients required higher dose rates than older patients to achieve the targeted ANC nadir (Figure 3B). Analyses of the pharmacokinetics of free etoposide and doxorubicin showed higher clearance rates in younger patients and provide an explanation for the higher dose rate (Figure4A). Unexpectedly, we also observed that free-etoposide clearance increased during multiple cycles, indicating that at constant dose rates, plasma concentrations of this agent would actually decrease with successive cycles (Figure 4B). With dynamic dose adjustment, DI was maintained with clinical toxicity that was no greater than that observed with the CHOP regimen.3 The targeted neutropenia level was achieved in 49% of cycles, whereas hospital admissions for fever and neutropenia occurred in only 8% of cycles. Rates of gastrointestinal side effects were low and did not affect the ability to increase the dose. Patients were able to receive 96% DI of vincristine with no serious long-term neurologic side effects.
These observations raise questions regarding the optimal treatment of large B-cell lymphomas. Although the importance of DI in aggressive lymphomas is only weakly supported by clinical studies, the validity of dose response is incontrovertible.35,36 The practical clinical application of DI, however, is confounded by the relative importance of cycle time and dose rate, both of which contribute to the calculation of DI and the design of treatment regimens, and by the use of a single standard dose. Indeed, use of a standard dose tests the hypothesis that the dose is superior to reduced doses, when in fact many patients receiving a full dose may not be getting a sufficient amount of the therapeutic agent, whereas some patients receiving a reduced dose may be getting an optimal amount, based on a pharmacologic model. The alternative strategy of starting at a high dose likely produces more optimal doses but frequently results in excess toxicity.30 Our preliminary pharmacologic data suggest that, at least for etoposide and doxorubicin, dynamic dose adjustment is necessary to achieve some interpatient equivalency in plasma concentrations and possibly to compensate for changes in drug clearance. This dose-adjustment principle is likely to be particularly important for continuous-infusion schedules because of the need to exceed threshold concentrations.8 Nevertheless, even with bolus schedules, cytotoxicity has been correlated with the area under the curve (AUC), and dynamic dose adjustment would maximize AUC.37 However, irrespective of schedule optimization, dynamic dose adjustment with bolus administration may produce unacceptable toxicity, such as cardiac damage from high peak concentrations of doxorubicin.38
Our results suggest that dose rate could have important implications in the treatment of curable lymphomas, particularly in assessments of the relative merits of infusion-based regimens. Gaynor et al39reported that the infusional CHOP-based regimen consisting of cyclophosphamide, dexamethasone, infusional doxorubicin, and vincristine (CVAD) produced results equivalent to those of CHOP in patients with aggressive lymphomas. However, the estimated delivered dose of vincristine for an average-sized patient (eg, 1.7 m2 of body-surface area) in the CVAD study was approximately 2.9-fold lower than that administered in the current study, and larger patients had even lower relative doses because of the vincristine cap—doses below those shown in a phase II study to be effective.40 Of relevance is evidence showing that vincristine reduces nuclear accumulation of p53 and expression of mdm2 and p21 and may play an important role in modulation of the apoptotic response.41 In addition, the absence of dynamic dose adjustment in the CVAD study likely contributed to widely variable plasma concentrations of doxorubicin.7
The possible importance of dose rate in infusion-based regimens may also partly account for the poor results achieved in a randomized study comparing EPOCH and CHOP in the treatment of aggressive lymphomas.42 In that study, EPOCH was not dose adjusted and doses significantly lower than those in the current study would have been administered; however, the researchers provided no DI data. Although most patients in the study had favorable prognoses (72% had low-risk disease), the event-free and OS rates in patients treated with EPOCH were 30% and 42%, respectively, at a median of 27 months—results that were significantly worse than those in the CHOP arm of that study and in the current study of EPOCH.
Ultimately, the efficacy of a treatment regimen can only be determined accurately within defined disease entities. Virtually all published trials of CHOP-based regimens for treating aggressive lymphomas included many histologic types, some of which are essentially incurable (such as peripheral T-cell and mantle cell lymphomas) and others that have extremely good prognoses (such as anaplastic lymphoma kinase–positive anaplastic lymphomas).9 Although this variable is mitigated by the fact that most cases are of the large B-cell type, some large B-cell lymphoma cases are likely to have low-grade elements with a different natural history.43 The current trial, however, included only patients with large B-cell lymphomas, none of whom had low-grade disease. These differences make it difficult to compare our results with those in previous series but should not deter comparisons with future studies in patients with large B-cell lymphoma.33
Our findings suggest that dose-adjusted EPOCH may represent an improved method of treating large B-cell lymphomas. However, patients with bcl-2–expressing tumors, corresponding with an activated B-like origin, had a relatively low PFS (50%) compared with patients with bcl-2 negative tumors (82%) and require further improvements in treatment. In studies in vitro, rituximab was found to down-regulate bcl-2 expression and sensitize lymphoma cells to cytotoxic agents, and this agent is currently being tested with dose-adjusted EPOCH in patients with previously untreated bcl-2–positive large B-cell lymphomas.44 45
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wyndham H. Wilson, Medicine Branch, National Cancer Institute, Building 10, Room 12N/226, 9000 Rockville Pike, Bethesda, MD 20892.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal