Erythropoietin (EPO) and its receptor (EPOR) are critical for definitive erythropoiesis, as mice lacking either gene product die during embryogenesis with severe anemia. Here we demonstrate that mice expressing just one functional allele of the EpoR have lower hematocrits and die more frequently than do wild-type littermates on anemia induction. Furthermore, EpoR+/−erythroid colony-forming unit (CFU-E) progenitors are reduced both in frequency and in responsiveness to EPO stimulation. To evaluate the interaction between EPO and granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin 3 (IL-3),GM-CSF−/− orIL-3−/− mice were interbred withEpoR+/− mice. Deletion of either GM-CSF or IL-3 also leads to reduction in CFU-E numbers and hematocrits but does not significantly alter steady-state erythroid burst-forming unit numbers. These results suggest EpoR haploinsufficiency and promotion of in vivo erythropoiesis by GM-CSF and IL-3.
Introduction
Erythropoietin (EPO) and the EPO receptor (EPOR) are indispensable for the proliferation and survival of erythroid colony-forming unit (CFU-E) progenitors and their terminal differentiation into mature erythrocytes.1-3 Mice lacking either Epo or EpoR die at about embryonic day 13 with defects in definitive erythropoiesis and cardiac development.1-4 Furthermore, both types of knockout mice exhibit identical phenotypes, suggesting that no other ligands or receptors can replace EPO or the EPOR.1 5 In the steady state, both heterozygotes exhibit normal hematologic parameters, viability, and fertility. However, the compensatory capacity of one copy of Epo or EpoR in stress or emergency situations remained to be studied.
In concert with EPO, other cytokines have been implicated in regulating erythropoiesis. Early experiments suggest that interleukin 3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) primarily act on early multipotent progenitors to enhance the formation of the erythroid burst-forming unit (BFU-E) in the presence of EPO.6,7 In addition, ectopic expression of βc (the subunit common to receptors for GM-CSF and IL-3) enhances EPO responsiveness in Ba/F3 cells transfected withEpoR, and a direct interaction between EPOR and βc has been described by coimmunoprecipitation.8 In UT-7 cells, however, GM-CSF induces a rapid down-regulation of EpoR messenger RNA and, thus, opposes EPO activity.9 Furthermore, in vitro studies reveal that EPO counteracts bone marrow CFU-GM colony growth, whereas colony-stimulating factors may inhibit BFU-E colony growth.10-12
Although GM-CSF and IL-3 stimulate proliferation and differentiation of hematopoietic cells in vitro,13 gene inactivation studies suggest that neither factor is essential for steady-state hematopoiesis. Instead, GM-CSF is important in pulmonary homeostasis,14,15 whereas IL-3 plays a physiologically relevant role in delayed-type hypersensitivity16 and the generation of mast cells or basophils in response to parasites.17 Baseline hematopoiesis in mice lacking βc, βc and βIL-3, or GM-CSF and IL-3, is substantially intact, although modest changes in eosinophil homeostasis have been observed, suggesting that the normalcy of hematopoiesis in the GM-CSF or IL-3 single knockout is not due to compensation by one cytokine for the lack of the other.18-20
To assess whether both EpoR alleles are required for animals to compensate for erythroid perturbation and to analyze the nature and extent of physiologically relevant interplay between the lineage-dominant EPO and the early-acting GM-CSF and IL-3 in erythropoiesis, GM-CSF– or IL-3–deficient mice were interbred withEpoR heterozygous mutant mice and subjected to hemolysis. Our results suggest that (1) EpoR is haploinsufficient in response to phenylhydrazine (PHZ) treatment and (2) GM-CSF and IL-3 promote, rather than oppose, EPO activity in vivo.
Study design
Mice
PHZ administration and hematocrit measurement
PHZ (ICN Biomedicals, Aurora, OH), which causes erythrocyte lysis in vivo,21 was injected intraperitoneally (60 mg/kg). For hematocrit measurements, mice anesthetized with Avertin (Sigma Chemical, St Louis, MO; 0.015 mL 2.5% stock/g) were bled from the retro-orbital plexus (20-40 μL) with the use of heparin-coated capillary tubes, which were then spun in a microcentrifuge.
Progenitor cell assays
Cells were isolated from femurs or spleens, washed thrice in Iscoves modified Dulbecco media supplemented with 2% fetal calf serum, and counted according to Wu et al.1 Nucleated cells were plated at 100 000/mL in methylcellulose (0.9% wt/vol) (M3236; StemCell Technologies, Vancouver, BC) supplemented with 15% fetal calf serum (Omega, Tarzana, CA) and recombinant hEPO (Amgen, Thousand Oaks, CA) at the indicated concentrations. For BFU-E analysis, 1 U/mL rhEPO and 100 ng/mL stem cell factor were used. Colonies were scored according to Wu et al.1
Statistical analysis
Statistical analysis was performed by using single-factor analysis of variance.
Results and discussion
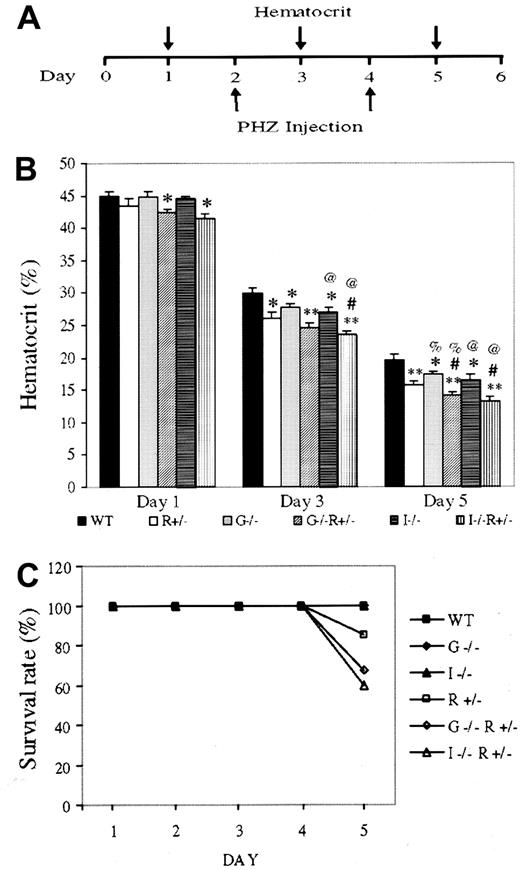
Anemia was induced in 6 groups of mice matched for genetic background, sex, age, and weight, with more than 30 animals per group: (1) wild type (WT), (2) EpoR+/−, (3)GM-CSF−/−, (4)GM-CSF−/−, EpoR+/−, (5) IL-3−/−, and (6)IL-3−/−, EpoR+/−. Two PHZ injections were administered 48 hours apart, and hematocrit was recorded from blood collected on alternate days (Figure1A). The differences in hematocrit among genotypes were highly reproducible and enhanced by the degree of anemia (Figure 1B). WT mice had the highest hematocrits, followed byGM-CSF−/− and IL-3−/−mice, followed by EpoR+/− mice. Deleting one allele of EpoR has a significantly greater effect on hematocrit than deleting 2 alleles of GM-CSF, suggesting that EPO/EPOR plays a more dominant role in erythropoiesis.IL-3−/−, EpoR+/− andGM-CSF−/−, EpoR+/−mice had the lowest hematocrits, and the day 5 hematocrits are likely overestimates, because mice in these 2 groups had the highest mortality (Figure 1C). Indeed, hematocrits of below 15% are often lethal, even in WT mice.
Anemia induction and lethality in gene-targeted mice.
(A) Schematic of anemia induction regime. (B) Hematocrits of 10- to 12-week-old, weight-matched female mice having undergone the aforementioned treatment. Results (average ± SEM) are based on 5 separate experiments using at least 5 mice per genotype per experiment. Compared with same-day WT hematocrit, * and ** denoteP < .05 and < .005, respectively. # representsP < .05 forIL-3−/−;EpoR+/− orGM-CSF−/−;EpoR+/−, when compared with EpoR+/− hematocrit; % and @ represent P < .05 when comparing WT mice with mice lacking GM-CSF or IL-3, respectively. (C) Survival rates after PHZ injection. Results are based on the cumulative studies of at least 30 mice per genotype.
Anemia induction and lethality in gene-targeted mice.
(A) Schematic of anemia induction regime. (B) Hematocrits of 10- to 12-week-old, weight-matched female mice having undergone the aforementioned treatment. Results (average ± SEM) are based on 5 separate experiments using at least 5 mice per genotype per experiment. Compared with same-day WT hematocrit, * and ** denoteP < .05 and < .005, respectively. # representsP < .05 forIL-3−/−;EpoR+/− orGM-CSF−/−;EpoR+/−, when compared with EpoR+/− hematocrit; % and @ represent P < .05 when comparing WT mice with mice lacking GM-CSF or IL-3, respectively. (C) Survival rates after PHZ injection. Results are based on the cumulative studies of at least 30 mice per genotype.
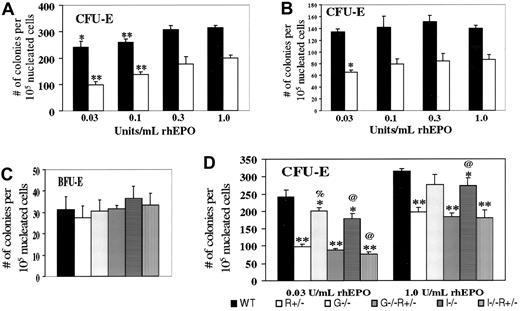
We next examined bone marrow erythroid progenitor cells from the aforementioned mice without PHZ treatment. In addressing the haploinsufficiency of EpoR, various concentrations of EPO were used (Figure 2A). CFU-E–derived colonies occurred for WT cells at a 60% greater frequency than forEpoR+/− cells at the highest EPO concentration (1 U/mL) but at a 150% greater frequency at the lowest EPO concentration tested (0.03 U/mL). Similar trends were noted with spleen-derived erythroid progenitors (Figure 2B). These results suggest that CFU-E progenitors are not only fewer but also less responsive to EPO in EpoR+/− mice compared with WT mice. BFU-E numbers did not vary between WT andEpoR+/− (Figure 2C), in agreement with previous findings that EPO promotes the transition from the BFU-E to CFU-E stage.1 2
In vitro colony formation of erythroid progenitors from mice of the indicated genotypes.
Numbers refer to scored colonies/105 nucleated bone marrow cells from the femurs (A,C,D) or spleens (B) of 10- to 12-week-old female mice. Results are based on 3 sets of independent experiments per study. (A) CFU-E frequencies, for either WT (black) or EpoR+/−(white) bone marrow cells, grown in the indicated concentration of rhEPO. * and ** denote P < .05 and < .005, respectively, when each value is compared with the same genotype value with 1.0 U/mL EPO. In comparing WT withEpoR+/−, P < .002 at each EPO concentration. (B) CFU-E frequencies, spleen. Statistical analysis was performed and indicated as in part A. (C) BFU-E frequencies, bone marrow. (D) CFU-E frequencies, bone marrow. Compared with WT values at the same EPO concentration, * and ** denote P < .05 and < .005, respectively. % and @ represent P < .05 when comparing WT mice with mice lacking GM-CSF or IL-3, respectively.
In vitro colony formation of erythroid progenitors from mice of the indicated genotypes.
Numbers refer to scored colonies/105 nucleated bone marrow cells from the femurs (A,C,D) or spleens (B) of 10- to 12-week-old female mice. Results are based on 3 sets of independent experiments per study. (A) CFU-E frequencies, for either WT (black) or EpoR+/−(white) bone marrow cells, grown in the indicated concentration of rhEPO. * and ** denote P < .05 and < .005, respectively, when each value is compared with the same genotype value with 1.0 U/mL EPO. In comparing WT withEpoR+/−, P < .002 at each EPO concentration. (B) CFU-E frequencies, spleen. Statistical analysis was performed and indicated as in part A. (C) BFU-E frequencies, bone marrow. (D) CFU-E frequencies, bone marrow. Compared with WT values at the same EPO concentration, * and ** denote P < .05 and < .005, respectively. % and @ represent P < .05 when comparing WT mice with mice lacking GM-CSF or IL-3, respectively.
We then tested the effects of disrupting either GM-CSF or IL-3 on BFU-E and CFU-E rates. BFU-E frequencies do not vary significantly (Figure2C), although with BFU-E values so small, significant changes might not be discernible. In contrast, CFU-E frequencies were reduced, either on WT or EpoR+/− background, especially at the minimal EPO concentration (0.03 U/mL) (Figure 2D), at which the ratio is 0.4 in EpoR+/−versus WT, 0.44 in EpoR+−,GM-CSF−/− versusGM-CSF−/−, and 0.44 inEpoR+/−;IL-3−/−versus IL-3−/−. Thus, we conclude that GM-CSF and IL-3 support, rather than oppose, steady-state CFU-E progenitor formation in vivo and may potentially do so in an additive manner with EPO.
Together, our results indicate that both copies of EpoR are required for emergent erythropoiesis and that responsiveness of CFU-E progenitors to EPO stimulation depends on EPOR dosage. Whereas the reduction in CFU-E frequency in EpoR+/− mice is dramatic, the effect on hematocrit is relatively mild, suggestive of compensation for reduced CFU-E levels in the animal. These results also highlight the importance of physiologically challenging genetically engineered mice to discern functional deficiencies. Furthermore, we have shown that GM-CSF and IL-3 appear to promote erythropoiesis in vivo. The role of GM-CSF or IL-3 in erythropoiesis and the haploinsufficiency of EpoR have clinical implications for EPO therapy in combination with other growth factors or for individuals with defective EPOR function.22
We thank Dr Harvey Lodish for supporting the initial phase of this study.
Supported in part by the Medical Scientist Training Program (A.G.J.), the Leukemia and Lymphoma Society (G.D.), the Howard Hughes Medical Institute (H.W.), and the V Foundation and CA74886.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hong Wu, Howard Hughes Medical Institute and Department of Molecular and Medical Pharmacology, UCLA School of Medicine, CHS 23-214, Los Angeles, CA 90095-1735; e-mail:hwu@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal