Multicentric Castleman disease (MCD) is a distinct type of lymphoproliferative disorder associated with inflammatory symptoms and interleukin 6 (IL-6) dysregulation. In the context of human immunodeficiency virus (HIV) infection, MCD is associated with Kaposi sarcoma–associated herpesvirus, also called human herpesvirus type 8 (KSHV/HHV8). Within a prospective cohort study on 60 HIV-infected patients with MCD, and a median follow-up period of 20 months, 14 patients developed KSHV/HHV8-associated non-Hodgkin lymphoma (NHL): 3 “classic” KSHV/HHV8+ Epstein-Barr virus–positive (EBV+) primary effusion lymphoma (PEL), 5 KSHV/HHV8+ EBV− visceral large cell NHL with a PEL-like phenotype, and 6 plasmablastic lymphoma/leukemia (3/3 KSHV/HHV8+ EBV−). The NHL incidence observed in this cohort study (101/1000 patient-years) is about 15-fold what is expected in the general HIV+ population. MCD-associated KSHV/HHV8+ NHL fell into 2 groups, suggesting different pathogenesis. The plasmablastic NHL likely represents the expansion of plasmablastic microlymphoma from the MCD lesion and progression toward aggressive NHL. In contrast, the PEL and PEL-like NHL may implicate a different original infected cell whose growth is promoted by the cytokine-rich environment of the MCD lesions.
Introduction
Kaposi sarcoma–associated herpesvirus, also called human herpesvirus type 8 (KSHV/HHV8), has been identified in a limited subset of lymphoproliferative disorders.1,2 Among these are primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD).3,4 MCD is characterized by lymphadenopathy with angiofollicular hyperplasia and plasma cell infiltration.5In the context of human immunodeficiency virus (HIV) infection, MCD may present as a distinct devastating lymphoproliferative disorder and the most effective therapy remains low-dose chemotherapy.6Virtually all HIV+ patients with MCD and nearly 50% of HIV− patients are infected with KSHV/HHV8.4,7The dysregulated production of human interleukin 6 (hIL-6) has been considered as a pivotal factor in the pathogenesis of the disease.8-11 A viral homologue of IL-6 (KSHV/HHV8-vIL6) exhibits many of the biologic activities of hIL-6.12-14Furthermore, when expressed constitutively in mice, vIL-6 can induce symptoms that resemble human MCD.15 In HIV-associated MCD, clinical symptoms are associated with high levels of plasma hIL-6 and IL-10, accompanied with a 1.7 log increment of KSHV/HHV8 copy numbers over what is observed during clinical remission in peripheral blood mononuclear cells (PBMCs).16 Recent data have shown that, in MCD lymph nodes, the KSHV/HHV8-infected cells are predominantly present within the mantle zone or coalescent in small sheets of large plasmablastic cells exhibiting a restricted monotypic λ phenotype.17 These cells are EBV− and molecular studies performed on microdissected sheets of plasmablasts show that the infected cell population is usually multiclonal and does not harbor somatic mutation in the rearranged immunoglobulin gene, suggesting that the cells originated from naive B cells.18These data suggest that the MCD lesion is constituted by KSHV/HHV8-associated microscopic plasmablastic lymphomas that can sometimes progress toward aggressive non-Hodgkin lymphoma (NHL).
Since 1990, a cohort study of 60 HIV-infected patients with MCD was conducted in a single institution. Data on the first 20 patients were reported in a clinical series in 1995.6 By August 2001, 14 of the 60 patients had developed NHL. This incidence is about 15-fold what is expected in the HIV+ population.
Patients and methods
Patients
Sixty HIV-infected patients, with histologically proven Castleman disease, were included in a single-institution prospective cohort study from 1990 through 2001. Patients receiving chemotherapy were seen every 2 weeks, whereas patients off therapy were seen every 3 months. Kaposi sarcoma was diagnosed in 42 patients. At entry, 36 patients were on antiretroviral therapy. The mean (± SD) CD4 cell count was 148 (± 242) × 106/L and the median plasma HIV RNA level was 4.7 log copies/mL. The frequency of a positive Coombs test was not different in the patients who developed NHL (3 of 10) than in the whole cohort population (14 of 46). All patients except one received intermittent low-dose chemotherapy, vinblastine or etoposide, every 2 to 3 weeks, at the onset of recurrent clinical symptoms.
Pathology and morphology
Paraffin block sections were stained with hematoxylin and eosin. Smears or cytospin preparations of cells isolated from effusion, blood, or bone marrow were stained with May-Grümwald-Giemsa and examined by light microscopy.
Immunophenotyping analysis
Immunophenotyping analysis was performed using monoclonal antibodies directed against CD3, CD4, CD8, CD5, CD7, CD20, CD79a, CD30, CD45, HLA-DR, and the immunoglobulin light chains, κ and λ.
KSHV/HHV8 immunohistochemistry
Immunostaining for KSHV/HHV8 latent nuclear antigen 1 (LNA-1) encoded by viral open reading frame (ORF) 73 was carried out with mouse monoclonal antibody LN53 (Advanced Biotechnologies, Columbia, MD) using the streptavidin-biotin method preceded by heat retrieval of antigen as previously described.19
Viral genome analysis and genotypic analysis
Detection of EBV and KSHV/HHV8 DNA sequences was performed on extracted DNA using primer sets and polymerase chain reaction (PCR) amplification as previously described.4 Gene rearrangement studies were performed in patient 6, using Southern blot analysis of genomic DNA digested with EcoRI and BgI II. Probe for the joining region of the immunoglobulin heavy chain gene (JH) was used as previously described.20
PCR and sequence analysis of the rearranged IGgenes
Where indicated, the rearranged Ig heavy chain was amplified from the framework 3 (Fr3) to the joining (J) regions with consensus primers using seminested protocols as described previously.21 All samples were analyzed in duplicate. PCR products were purified from 1% agarose gel using QIA Quick Gel Extraction Kit (Qiagen, West Sussex, United Kingdom), then ligated into the pCR4-TOPO TA cloning vector (Invitrogen Life Technologies, Paisley, United Kingdom) and transformed into TOP10 competent cells (Invitrogen Life Technologies). The transformed cells were selected on LB-ampicillin agar plates. Colonies were screened using PCR with vector primers (T7 and T3). The PCR products showing the expected insert size were sequenced in both directions using an ABI 377 DNA sequencer with dRhodamine dye terminators (ABI, Foster City, CA). Up to 16 PCR clones from each sample were sequenced. The sequences were aligned using Sequence Navigator software (ABI) and the variable (V), diversity (D), and joining (J) segments were identified by sequence comparison to the V base using online DNAPLOT (MRC Center for Protein Engineering,http://www.mcr-cpe.cam.ac.uk/imt-doc/vbase-home-page.html).
A primer specific to tumor clone was designed from the VDJ joining sequence of the tumor-derived IG gene. The specificity of the primer was confirmed by searching the GenBank database using the BLASTn program (http://www.ncbi.nlm.nih.gov/BLAST). By combination of the clone-specific primer with the consensus Fr3 primer, a clone-specific PCR was designed and used for detection of NHL cells from the original MCD lesion.
EBV RNA in situ hybridization
EBV RNA (EBER) in situ hybridization was carried out with a PCR-generated DNA probe labeled with digoxigenin, followed by incubation with antidigoxigenin-AP (Boehringer Mannheim, Mannheim, Germany) and visualization with 5-bromo-4-chloro-3-inodolyl-phosphate (BCIP) and nitroblue tetrazolium (NBT).18
Results
Incidence of NHL and patient characteristics
Over a median follow-up period of 20 months, 14 patients developed NHL, 0 to 76 months after MCD diagnosis. Data on patient 9 have been reported previously.17 The estimated 2-year probability for developing NHL after a diagnosis of MCD was 24.3% (95% CI, 10.9%-37%; Figure 1A). At the time of NHL diagnosis, the median CD4 cell count was 248 × 106/L, 6 of 9 patients had a plasma HIV RNA level below 2.7 log copies/mL, and 9 of the 14 patients had clinical Kaposi sarcoma. None of the baseline HIV-associated covariates (ie, initial CD4 cell count, plasma HIV RNA, Kaposi sarcoma) was found to be predictive of NHL occurrence (Table 1).
NHL in HIV-associated MCD.
(A) Incidence of NHL in 60 HIV-infected patients with MCD. (B) Overall survival from NHL diagnosis in 14 patients with MCD- associated NHL.
NHL in HIV-associated MCD.
(A) Incidence of NHL in 60 HIV-infected patients with MCD. (B) Overall survival from NHL diagnosis in 14 patients with MCD- associated NHL.
Major characteristics of 14 HIV-infected patients with MCD who developed NHL
| Patient . | At MCD diagnosis . | At NHL diagnosis . | |||||
|---|---|---|---|---|---|---|---|
| CD4+ T cells (× 106/L) . | HIV-RNA (copies/mL) . | Delay for NHL (mo) . | Antiretroviral therapy . | CD4+ T cells (× 106/L) . | HIV-RNA (copies/mL) . | KS . | |
| 1 | 104 | ND | 5 | — | 64 | ND | Yes |
| 2 | 216 | ND | 2 | — | 216 | ND | Yes |
| 3 | 30 | 1 599 000 | 3 | d4T-3TC-NVP | 64 | 2 500 | Yes |
| 4 | 125 | ND | 76 | d4T-3TC-RTV | 280 | 334 000 | No |
| 5 | 373 | 255 000 | 23 | AZT-3TC-IDV | 688 | < 500 | No |
| 6 | 1126 | 362 000 | 0 | — | 1126 | 362 000 | No |
| 7 | 68 | 5 300 | 18 | ABC-ddI-RTV-SQV | 114 | < 50 | No |
| 8 | 171 | 230 000 | 60 | AZT-3TC-RTV-SQV | 887 | < 20 | Yes |
| 9 | 144 | ND | 69 | d4T-3TC-NFV-NVP | 800 | 400 | No |
| 10 | 119 | ND | 7 | — | 8 | ND | Yes |
| 11 | 227 | ND | 23 | — | 410 | ND | Yes |
| 12 | 27 | ND | 6 | d4T-3TC-IDV | ND | < 50 | Yes |
| 13 | 89 | 16 700 | 6 | AZT-3TC-RTV-SQV | 348 | < 500 | Yes |
| 14 | 267 | 650 200 | 2 | — | 75 | 650 200 | Yes |
| Patient . | At MCD diagnosis . | At NHL diagnosis . | |||||
|---|---|---|---|---|---|---|---|
| CD4+ T cells (× 106/L) . | HIV-RNA (copies/mL) . | Delay for NHL (mo) . | Antiretroviral therapy . | CD4+ T cells (× 106/L) . | HIV-RNA (copies/mL) . | KS . | |
| 1 | 104 | ND | 5 | — | 64 | ND | Yes |
| 2 | 216 | ND | 2 | — | 216 | ND | Yes |
| 3 | 30 | 1 599 000 | 3 | d4T-3TC-NVP | 64 | 2 500 | Yes |
| 4 | 125 | ND | 76 | d4T-3TC-RTV | 280 | 334 000 | No |
| 5 | 373 | 255 000 | 23 | AZT-3TC-IDV | 688 | < 500 | No |
| 6 | 1126 | 362 000 | 0 | — | 1126 | 362 000 | No |
| 7 | 68 | 5 300 | 18 | ABC-ddI-RTV-SQV | 114 | < 50 | No |
| 8 | 171 | 230 000 | 60 | AZT-3TC-RTV-SQV | 887 | < 20 | Yes |
| 9 | 144 | ND | 69 | d4T-3TC-NFV-NVP | 800 | 400 | No |
| 10 | 119 | ND | 7 | — | 8 | ND | Yes |
| 11 | 227 | ND | 23 | — | 410 | ND | Yes |
| 12 | 27 | ND | 6 | d4T-3TC-IDV | ND | < 50 | Yes |
| 13 | 89 | 16 700 | 6 | AZT-3TC-RTV-SQV | 348 | < 500 | Yes |
| 14 | 267 | 650 200 | 2 | — | 75 | 650 200 | Yes |
KS indicates Kaposi sarcoma; d4T, stavudine; 3TC, lamivudine; NFV, nelfinavir; NVP, nevirapine; RTV, ritonavir; AZT, zidovudine; IDV, indinavir; ABC, abacavir; ddI, didanosine; SQV, saquinavir; and ND, not determined.
Pathology
Major NHL characteristics are summarized in Table2. Three patients (nos. 1-3) developed classic PEL 2 to 5 months after MCD diagnosis. The large/anaplastic cells exhibited a non-B non-T activated phenotype (CD20−, CD3−, CD45+, CD30+ in all 3 cases). The 3 patients with PEL were positive for KSHV/HHV8 and EBV by PCR analysis.
NHL characteristics in 14 HIV-infected patients with MCD
| Patient . | Site of diagnosis . | Morphology . | Phenotype . | KSHV/HHV8 . | EBV . |
|---|---|---|---|---|---|
| 1 | Pleura | LC/PEL | Non-B, non-T | + PCR | + PCR |
| 2 | Pericardium | LC/PEL | Non-B, non-T | + PCR | + PCR |
| 3 | Pleura, peritoneum | LC/PEL | Non-B, non-T | + PCR | + PCR |
| 4 | Skin, BM, GI, CNS | LC | Non-B, non-T | + IHC | − EBER |
| 5 | Cerebral | LC | Non-B, non-T | + IHC | − EBER |
| 6 | Rectal | LC | Non-B, non-T | + IHC | ND |
| 7 | GI, BM | LC/anaplastic | Non-B, CD3+ (R-JH) | + IHC | − EBER |
| 8 | GI, pleura, peritoneum | LC | CD20-, μλ+, CD3− | + IHC, + PCR | − EBER, − PCR |
| 9 | Nodal | Plasmablastic | CD20+, μλ+ | + IHC | − EBER |
| 10 | Blood, spleen | Plasmablastic | ND | ND | ND |
| GI | LC/anaplastic | Non-B, CD3+, CD30+ | + IHC | − EBER | |
| 11 | Blood | Plasmablastic | ND | ND | ND |
| 12 | Blood | Plasmablastic | ND | + IHC | − EBER |
| 13 | Blood | Plasmablastic | ND | ND | ND |
| 14 | Nodal, spleen | Plasmablastic | CD20+, μλ+ | + IHC | − EBER |
| Patient . | Site of diagnosis . | Morphology . | Phenotype . | KSHV/HHV8 . | EBV . |
|---|---|---|---|---|---|
| 1 | Pleura | LC/PEL | Non-B, non-T | + PCR | + PCR |
| 2 | Pericardium | LC/PEL | Non-B, non-T | + PCR | + PCR |
| 3 | Pleura, peritoneum | LC/PEL | Non-B, non-T | + PCR | + PCR |
| 4 | Skin, BM, GI, CNS | LC | Non-B, non-T | + IHC | − EBER |
| 5 | Cerebral | LC | Non-B, non-T | + IHC | − EBER |
| 6 | Rectal | LC | Non-B, non-T | + IHC | ND |
| 7 | GI, BM | LC/anaplastic | Non-B, CD3+ (R-JH) | + IHC | − EBER |
| 8 | GI, pleura, peritoneum | LC | CD20-, μλ+, CD3− | + IHC, + PCR | − EBER, − PCR |
| 9 | Nodal | Plasmablastic | CD20+, μλ+ | + IHC | − EBER |
| 10 | Blood, spleen | Plasmablastic | ND | ND | ND |
| GI | LC/anaplastic | Non-B, CD3+, CD30+ | + IHC | − EBER | |
| 11 | Blood | Plasmablastic | ND | ND | ND |
| 12 | Blood | Plasmablastic | ND | + IHC | − EBER |
| 13 | Blood | Plasmablastic | ND | ND | ND |
| 14 | Nodal, spleen | Plasmablastic | CD20+, μλ+ | + IHC | − EBER |
BM denotes bone marrow; GI, gastrointestinal tract; CNS, central nervous system; LC, large cells; IHC, immunohistochemistry; EBER, EBV RNA in situ hybridization; and ND, not determined.
Five patients (nos. 4-8) developed, 0 to 76 months after MCD diagnosis, extranodal NHL whose morphologic and phenotypical characteristics were very similar to those observed in PEL. Patient 5 developed primary central nervous system large cell lymphoma (PCNSL; Figure2). In 4 of these patients, including the one with PCNSL, the cells were large and shared a non-B non-T phenotype (CD20−, CD3−, CD45+; Figure 3). One of these tumors (in patient 8) was associated with peritoneal and pleural effusions. In this case, the malignant cells, although CD20−, expressed a μ λ surface immunoglobulin. In patient 7, who presented with intestinal and bone marrow involvement, the cells were large/anaplastic CD20− cells. Interestingly, these cells were CD3+ on immunohistochemistry and CD3− by flow cytometry, suggesting an illegitimate and incomplete expression of the CD3 T-cell marker on the cell surface. This lymphoma was confirmed to be of clonal B-cell origin by immunoglobulin heavy chain rearrangement Southern blot study (data not shown).
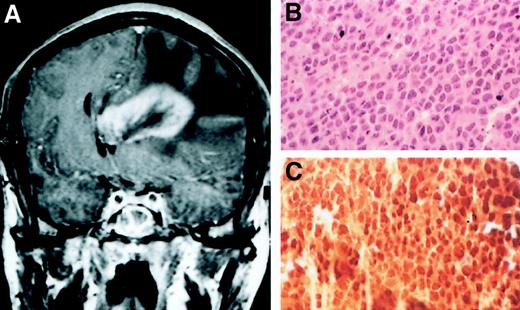
Primary cerebral lymphoma 23 months after MCD diagnosis in patient 5.
(A) Cerebral CT scan; (B) large cell NHL; (C) positive staining with the KSHV/HHV8 LNA-1 specific monoclonal antibody. Original magnification ×400 in panels B and C.
Primary cerebral lymphoma 23 months after MCD diagnosis in patient 5.
(A) Cerebral CT scan; (B) large cell NHL; (C) positive staining with the KSHV/HHV8 LNA-1 specific monoclonal antibody. Original magnification ×400 in panels B and C.
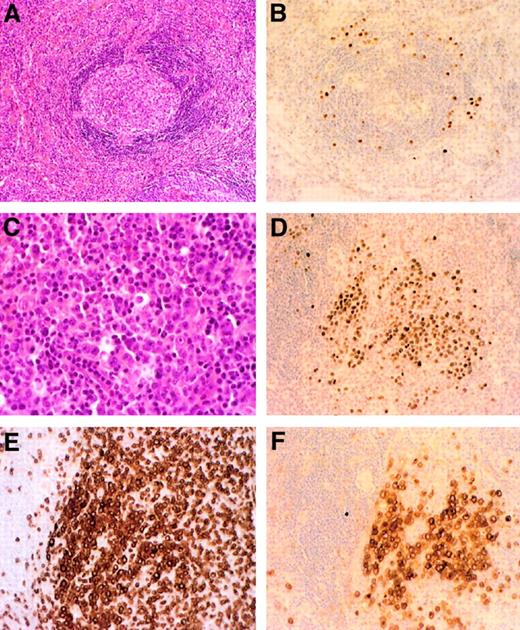
Systemic NHL 18 months after MCD diagnosis in patient 7.
Initial lymph node biopsy shows the typical Castleman lesion (A) and the positive staining with the KSHV/HHV8 LNA-1 specific monoclonal antibody (B). Postmortem spleen analysis reveals diffuse infiltration with large/anaplastic cells (C), with positive staining with the KSHV/HHV8 LNA-1 antibody (D), an illegitimate expression of the T-cell CD3 marker (E), and the expression of the CD30 marker (F). Original magnification ×200 in panels A, B, D-F; ×400 in C.
Systemic NHL 18 months after MCD diagnosis in patient 7.
Initial lymph node biopsy shows the typical Castleman lesion (A) and the positive staining with the KSHV/HHV8 LNA-1 specific monoclonal antibody (B). Postmortem spleen analysis reveals diffuse infiltration with large/anaplastic cells (C), with positive staining with the KSHV/HHV8 LNA-1 antibody (D), an illegitimate expression of the T-cell CD3 marker (E), and the expression of the CD30 marker (F). Original magnification ×200 in panels A, B, D-F; ×400 in C.
Six patients (nos. 9-14) developed plasmablastic NHL with nodal or splenic involvement in 3 (nos. 9, 10, 14). In 4 of them (nos. 10-13), the evolution was marked by a leukemic phase and a rapidly fatal outcome. The white blood cell (WBC) count ranged from 12 500 to 38 000 × 106/L, with 34% to 85% plasmablasts (Figure4). In patients 10 and 11, a blood smear examination performed 9 and 14 days, respectively, before the blast crisis was normal. In patient 10, a huge splenomegaly was associated with the fulminant emergence of circulating plasmablasts. In this case, a distinct lymphomatous process was diagnosed on an intestinal biopsy. The cells were anaplastic large CD20−, CD3+ cells on immunohistochemistry and suggested a second co-occurring lymphoma in this patient.
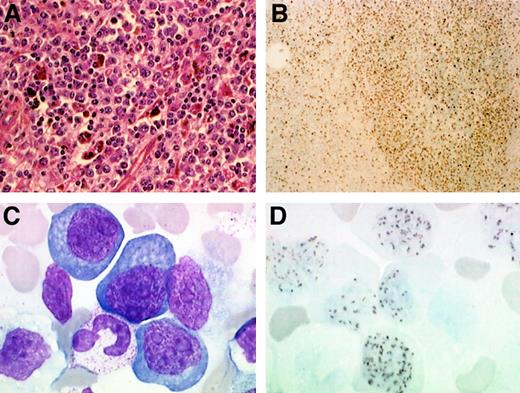
Plasmablastic lymphoma complicating HIV-associated MCD in patients 12 and 14.
In patient 14, spleen analysis (A) shows a diffuse infiltration with plasmablastic cells, which stained positive with a KSHV/HHV8 specific LNA-1 monoclonal antibody (B). In patient 12, a blood smear (C) shows leukemic plasmablastic cells and nuclear staining of the circulating plasmablastic cells with a KSHV/HHV8 specific LNA-1 monoclonal antibody (D). Original magnification ×400 in panel A; ×100 in panel B; and ×2400 in panels C and D.
Plasmablastic lymphoma complicating HIV-associated MCD in patients 12 and 14.
In patient 14, spleen analysis (A) shows a diffuse infiltration with plasmablastic cells, which stained positive with a KSHV/HHV8 specific LNA-1 monoclonal antibody (B). In patient 12, a blood smear (C) shows leukemic plasmablastic cells and nuclear staining of the circulating plasmablastic cells with a KSHV/HHV8 specific LNA-1 monoclonal antibody (D). Original magnification ×400 in panel A; ×100 in panel B; and ×2400 in panels C and D.
The KSHV/HHV8 immunohistochemical study using the LN53 antibody directed against the KSHV/HHV8 LNA-1 latent antigen was positive in all 9 cases studied. In 8 of these patients, including the PCNSL case, EBER in situ hybridization was negative.
Detection of NHL cells from the original MCD lesion by PCR
In patients 3 and 8, the search for NHL cells in the original MCD lesion was carried out. Fr3-JH PCR was performed from DNA samples prepared from both the original MCD and subsequent NHL lesions. In patient 8, the NHL showed 2 PCR bands but in patient 3 failed to amplify, whereas the original MCD lesion in both patients displayed a polyclonal pattern. In patient 8, the Castleman lesion was diagnosed on the spleen tissue and splenectomy had been performed 5 years before NHL was diagnosed. The PCR product from the NHL in patient 8 was cloned and sequenced. Two clonal rearranged immunoglobulins were revealed, one being a functional rearrangement and the other containing a stop codon. Clone-specific primer was designed from the functional rearrangedIG gene and used in conjunction with the Fr3 consensus primer for detection of the tumor clone in the original MCD lesion. A PCR product of predicted size (64 base pairs) was seen from DNA samples prepared from the corresponding NHL but not from those prepared from a range of unrelated lymphoid tissues, confirming the specificity of the clone-specific PCR used. DNA samples prepared from the original MCD lesion including one frozen and one paraffin-embedded spleen tissue were subjected to clone-specific PCR. Despite the fact that up to 15 different amounts of template DNA were used for PCR, clone-specific PCR did not yield any expected PCR product from the MCD lesion.
Clinical outcome
Seven patients were treated with low-dose (n = 3) or standard (n = 4) CHOP-derived chemotherapy regimens. The 4 patients who presented with a fulminant leukemic disease received no therapy and died within 1 week. The overall median survival from NHL diagnosis is 1 month (Figure 1B). Only patients 3 and 6 survived, and both of them have received an intensive chemotherapy regimen. The overall 1-year probability for survival after NHL diagnosis was below 10%. Patient 6 remained in complete remission for both NHL and MCD at month 25 (Table3).
Clinical outcome in 14 HIV-infected patients with MCD and NHL
| Patient . | Staging . | WBC × 106/L . | Hb g/dL . | Platelets × 109/L . | Therapy . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | Pleura | 2 300 | 8.5 | 124 | VP16 | Failure | Death wk 3 |
| 2 | Pericardium | 3 200 | 7.8 | 154 | ACVBP | PR | Death mo 7 |
| 3 | Pleura, peritoneum | 10 800 | 10.6 | 565 | CHOP-MTX | CR | Alive wk 5 |
| 4 | Skin, BM, GI, CNS | 6 900 | 8.3 | 413 | EDX-VP16 | PR | Death mo 5 |
| 5 | Cerebral | 7 800 | 13.4 | 265 | Steroids | Failure | Death wk 3 |
| 6 | Rectal | 3 200 | 12 | 187 | ACVBP | CR | Alive mo 25 |
| 7 | GI, BM | 2 500 | 7.8 | 35 | Low-dose CHOP | Failure | Death wk 7 |
| 8 | GI, pleura, peritoneum | 9 000 | 11.7 | 854 | CHOP-MTX | Failure | Death wk 3 |
| 9 | Nodal | 15 800 | 17 | 34 | None | Progression | Death wk 1 |
| 10 | Blood, spleen, GI | 12 500 | 8.2 | 68 | None | Progression | Death wk 1 |
| 11 | Blood | 15 600 | 9.9 | 154 | None | Progression | Death wk 1 |
| 12 | Blood | 38 000 | 8.5 | 228 | None | Progression | Death wk 1 |
| 13 | Blood | 22 600 | 7.1 | 12 | None | Progression | Death wk 1 |
| 14 | Nodal, spleen | 7 500 | 10.8 | 82 | None | Progression | Death wk 2 |
| Patient . | Staging . | WBC × 106/L . | Hb g/dL . | Platelets × 109/L . | Therapy . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | Pleura | 2 300 | 8.5 | 124 | VP16 | Failure | Death wk 3 |
| 2 | Pericardium | 3 200 | 7.8 | 154 | ACVBP | PR | Death mo 7 |
| 3 | Pleura, peritoneum | 10 800 | 10.6 | 565 | CHOP-MTX | CR | Alive wk 5 |
| 4 | Skin, BM, GI, CNS | 6 900 | 8.3 | 413 | EDX-VP16 | PR | Death mo 5 |
| 5 | Cerebral | 7 800 | 13.4 | 265 | Steroids | Failure | Death wk 3 |
| 6 | Rectal | 3 200 | 12 | 187 | ACVBP | CR | Alive mo 25 |
| 7 | GI, BM | 2 500 | 7.8 | 35 | Low-dose CHOP | Failure | Death wk 7 |
| 8 | GI, pleura, peritoneum | 9 000 | 11.7 | 854 | CHOP-MTX | Failure | Death wk 3 |
| 9 | Nodal | 15 800 | 17 | 34 | None | Progression | Death wk 1 |
| 10 | Blood, spleen, GI | 12 500 | 8.2 | 68 | None | Progression | Death wk 1 |
| 11 | Blood | 15 600 | 9.9 | 154 | None | Progression | Death wk 1 |
| 12 | Blood | 38 000 | 8.5 | 228 | None | Progression | Death wk 1 |
| 13 | Blood | 22 600 | 7.1 | 12 | None | Progression | Death wk 1 |
| 14 | Nodal, spleen | 7 500 | 10.8 | 82 | None | Progression | Death wk 2 |
Hb indicates hemoglobin; BM, bone marrow; GI, gastrointestinal tract; CNS, central nervous system; PR, partial response; and CR, complete remission.
Discussion
In the present cohort study of HIV-infected patients with MCD, the incidence of NHL (101/1000 patient-years) is about 15-fold higher than that observed in the general HIV+population and that observed in a French national cohort study where it peaked in 1994 (9/1000 patient-years) and slowly decreased to 4/1000 patient-years in the past 2 years.22,23 An increased incidence of NHL was previously observed in HIV-infected patients with Kaposi sarcoma, another KSHV/HHV8-associated disease.24,25In contrast, NHL occurrence was not increased in patients with KSHV/HHV8 asymptomatic infection.26 27 These data suggest that the development of the MCD lesion per se increases the risk of NHL.
Acquired immunodeficiency syndrome (AIDS)–associated MCD has been recently considered as a new type of lymphoproliferative disorder associated with the presence of large plasmablastic KSHV/HHV8+ EBV− cells in the mantle zone of lymphoid organs. Molecular and phenotypic studies have suggested that these cells, derived from naive B cells, were restricted for the expression of μ λ immunoglobulin chains and could form sheets of cells in the mantle zone as well as in the interfollicular zone. These microlymphomas, although monotypic, are polyclonal or at least multiclonal.18 Thus, KSHV/HHV8 may infect IgM+ naive B cells and drive these cells to differentiate into plasmablasts without undergoing the germinal center reaction. vIL-6 is produced in KSHV/HHV8+ cells from MCD lesions,28 and may play a role in the plasmacytic differentiation of the cells. These foci of microlymphomas may have a spontaneous smoldering evolution or be controlled by the immune system or low-dose chemotherapy for months.
In the present series, 3 patients developed “classic” KSHV/HHV8+ EBV+ PELs. The association of both diseases, MCD and PEL, has been recently reported and may suggest a higher risk of developing PEL in patients with MCD.29 Five patients developed a distinct type of lymphoma that shared morphologic and phenotypical characteristics of “classic” PEL. These KSHV/HHV8+“extracavitary” tumors differed from “classic” PELs because they were extranodal tumors but not body-cavity based. Extraserous involvement of PEL has already been reported as well as visceral localizations of anaplasticlike KSHV/HHV8+NHL.30-32 The illegitimate expression of T-cell markers has already been reported in some PEL cells.31 33 In contrast with more than 85% of the HIV-associated “classic” PELs, these tumors were EBV−, suggesting that, in the pathogenesis of PEL, the dual infection with KSHV/HHV8 and EBV may be involved in the peculiar tropism of the tumor.
Almost half of the NHLs observed in the present series were plasmablastic lymphomas. The cells were medium/large cells with plasmacytic differentiation and prominent nucleoli. These cells were very similar to the KSHV/HHV8+ plasmablastic cells observed in the mantle zone of the MCD lesions. This similarity associated with the nodal or splenic localization of these lymphoma suggests that they originated from the MCD lesion itself. In some MCD lesions, the KSHV/HHV8+ plasmablastic cells may form confluent sheets suggesting a diagnosis of microlymphoma. These microlymphomas are considered as multiclonal B-cell populations with uncertain malignant capacity. One may speculate about a secondary oncogenic event in one of these clones or a further decline in the immune control of these plasmablasts leading to this aggressive and sometimes fulminant lymphoproliferative disease. The clonality of the leukemic phase observed in some patients has not been assessed.
Cells from PEL and plasmablastic lymphoma are clearly distinct, with PEL cells representing postgerminal center cells that have undergone an intense somatic mutation process on the immunoglobulin gene hypervariable region, and plasmablastic lymphoma cells, naive unmutated pregerminal center cells.18 34 The nature of the B cell infected with KSHV/HHV8 may therefore trigger the morphologic, phenotypical, and some of the clinical characteristics of the KSHV/HHV8-associated lymphoproliferation. The EBV coinfection of the cells, which is present in most “classic” PELs, is absent in plasmablastic lymphoma. Interestingly, among the NHL with PEL-like cells observed in this series, only 3 were dually KSHV/HHV8 and EBV coinfected and presented as “classic” PEL. In contrast, all KSHV/HHV8+ EBV− PEL-like cases presented with extranodal visceral involvement.
These data suggest that KSHV/HHV8 is associated with various types of B-cell lymphoproliferative disorders and that the incidence of KSHV/HHV8-associated NHL is increased in patients with MCD. In such patients, half of the emerging lymphomas are very similar to the microlymphomas observed in the MCD lesion itself and may represent the expansion of a microscopic plasmablastic lymphoma toward aggressive NHL. Although we failed to demonstrate the presence of NHL cells in the original MCD lesion, this does not exclude the presence of the tumor clone in the MCD lesion. The case we examined showed only scattered KSHV+ cells in the MCD-involved spleen. These KSHV+ cells are polyclonal. Thus, all representative tissue specimens from the spleen need to be examined to be absolutely sure whether the NHL clone is present in the original MCD lesion. Because the clone-specific PCR is sensitive, our results suggest that the NHL clone was at least not present in a significant proportion, if it was, in the original MCD lesion. The presence of the same cellular clone (identical CDR3) has already been detected as a dominant clone in the lymphomatous lesion and as minor cell populations in distinct Castleman microlymphoma lesions from the same patient (patient 118). The other half may originate with a distinct pathophysiology, involving a different original infected cell. The progression toward PEL or PEL-like tumor may be facilitated by the peculiar cytokine environment of the MCD lesion and in particular the high levels of hIL-6 and IL-10, both involved in MCD pathogenesis as well as in PEL cell lines growth.35 36 In this series, the clinical characteristics of the tumors correlated with the presence of EBV in the cells of the 3 primary effusion lymphoma cases and with absence of detectable EBV coinfection in the other PEL-like extranodal tumors.
The high incidence of KSHV/HHV8+ NHL in HIV-infected patients with MCD may therefore be explained both by the expansion or burst out of plasmablastic microlymphomas, which were previously present in the MCD lesion, and by an increased occurrence of PEL or PEL-like tumors originating from a distinct B-cell population that find an optimal cytokine-rich environment in the MCD lesions.
We thank Agnes Perus and François Sigaux for the Southern blot analysis and Françoise Picard, Jacqueline Mikol, and Antoine Moulignier for providing some of the samples.
N.D. is a recipient of a grant from SIDACTION and Association pour la Recherche sur le Cancer (ARC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric Oksenhendler, Service d'Immunologie et d'Hématologie, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75010, Paris, France; e-mail:eric.oksenhendler@sls.ap-hop-paris.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal