Abstract
Temozolomide (TZM) is a DNA-methylating agent that has recently been introduced into various clinical trials for treatment of solid or hematologic neoplasias, including brain lymphomas. In the current study, we have investigated whether the antitumor activity of TZM could be selectively enhanced at the central nervous system (CNS) site by intracerebral injection of a poly(ADP-ribose) polymerase (PARP) inhibitor. Mice were injected intracranially with lymphoma cells. The PARP inhibitor NU1025 (1 mg/animal) was delivered intracerebrally, whereas TZM was given as a single or a fractionated dose of 200 mg/kg by intraperitoneal administration. Results indicated that this drug combination significantly enhanced the survival of tumor-bearing mice and that this fractionated modality of treatment was the most effective schedule. Increased survival time was related to a marked reduction of tumor growth, as evidenced by histologic studies. Treatment with TZM alone was ineffective. This is the first report exploring in vivo the combination of TZM with PARP inhibitor for intracerebral neoplasias.
Introduction
Temozolomide (TZM) is a methylating agent that crosses the blood–brain barrier and is indicated for malignant gliomas and metastatic melanomas.1-3 Moreover, the drug has recently shown promising antitumor activity in a patient affected by primary brain lymphoma4 and is under phase 2 clinical trials for leptomeningeal metastasis from leukemia and lymphoma. However, the antitumor activity of TZM is strongly affected by the functional status of DNA repair systems, involved either in the removal of methyl adducts from O6G or in the apoptotic signaling triggered by O6methylG–C/T mispairs. In particular, TZM is effective against tumor cells that are characterized by low levels of O6-alkylguanine DNA alkyltransferase (OGAT) and a functional mismatch repair system (MR).5-7
Resistant leukemia cells become susceptible to TZM when this drug is combined with inhibitors of poly(ADP-ribose) polymerase (PARP), a component of the base excision repair system.8-10 In this case cytotoxicity is caused by interruption of the repair process of N-methylpurines generated by TZM. In the current study, we have investigated whether the antitumor activity of TZM could be selectively potentiated at the CNS site through the use of intracerebral injection of PARP inhibitor.
Study design
In vitro studies
The murine lymphoma cell line L5178Y of DBA/2 (H-2d/H-2d) origin (ATCC, Manassas, VA) was cultured in RPMI-1640 containing 10% fetal calf serum and antibiotics. Inhibition of PARP was obtained by treating cells (105 cells/mL) with 8-hydroxy-2-methylquinazolin-4[3H]-1 (NU1025; Calbiochem, Darmstadt, Germany), at a concentration (25 μM) that abrogates PARP activity.11,12 Cells were then exposed to TZM (7.5-125 μM) (Schering-Plough, Kenilworth, NJ) and were cultured for 3 days. Cell growth was evaluated by counting viable cells in quadruplicate, and apoptosis was assessed by flow cytometry analysis of DNA content.13 Long-term survival was analyzed by colony-formation assay.9
In vivo studies
Male B6D2F1 (C57BL/6 × DBA/2) mice were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) in 0.9% NaCl solution (10 mL/kg intraperitoneally). L5178Y cells (104 in 0.03 mL RPMI-1640) were then injected intracranially, through the center-middle area of the frontal bone to a 2-mm depth, using a 0.1-mL glass microsyringe and a 27-gauge disposable needle.14 To evaluate tumor cell growth, brains were fixed in 10% phosphate-buffered formaldehyde, and histologic sections (5 μm) were cut along the axial plane, stained with hematoxylin-eosin, and analyzed by light microscopy.
TZM was dissolved in dimethyl-sulfoxide (40 mg/mL), diluted in saline (5 mg/mL), and administered intraperitoneally on day 2 after tumor injection at 100 mg/kg or 200 mg/kg, doses commonly used for in vivo preclinical studies.15-17 Because cytotoxicity induced by TZM and PARP inhibitors can be improved by fractionated modality of treatment,9 in selected groups a total dose of 200 mg/kg TZM was divided in 2 doses of 100 mg/kg given on days 2 and 3.
NU1025 was dissolved in polyethylene glycol-400 (40% in saline) and was injected intracranially at the maximal deliverable dose (1 mg/mouse, 0.03 mL) or, in selected groups, intraperitoneally (0.3 mL) on day 2 after tumor challenge, 1 hour before TZM administration. Control mice were injected with drug vehicles.
Mice were monitored for mortality for 90 days. Median survival time (MST) was determined, and percentage of increase in lifespan (ILS) was calculated as [MST (days) of treated mice/MST (days) of control mice] −1] × 100.18 Efficacy of treatments was evaluated by comparing survival curves between treated and control groups.
To assess the ability of different treatments to reduce tumor growth, histologic examination of the brains was performed using additional animals not considered for analysis of survival. Mice were killed at different time points after tumor challenge, selected within the MST range of untreated tumor-bearing animals. Areas of tumor infiltration were measured by histomorphometry, using an automated image analyzer system (Quantimet 520; Leica, Cambridge, United Kingdom).
Drug toxicity was evaluated by treating intact mice (10/group) with the compounds under investigation or with vehicles only. Weights and survival times of the mice were recorded for 3 weeks. Animal care was in compliance with international guidelines.19
Statistical analysis
Survival curves were generated by the Kaplan-Meier product-limit estimate,20 and statistical differences between the various groups were evaluated by log-rank analysis (software Primer of Biostatistics; McGraw-Hill, New York, NY)
Results and discussion
In vitro chemosensitivity of L5178Y cells to TZM ± PARP inhibitor
Lymphoma cell counts performed 72 hours after treatment showed that the IC50 (confidence limit) of TZM was 44 μM (35-58 μM) when used alone and 16 μM (12-26 μM) when combined with NU1025. Long-term colony assay and apoptosis analysis also showed a more pronounced growth inhibition and cytotoxicity in cells exposed to NU1025+TZM compared with those treated with TZM only (Table1). Overall the results indicated that the inhibition of PARP markedly enhanced the antitumor activity of TZM, consistent with previous findings in human leukemia cells.8 9
Influence of NU1025 on apoptosis and colony formation in L5178Y cells treated with TZM
| In vitro treatment . | Apoptosis, % . | Colony formation, % . | |
|---|---|---|---|
| Exp 1 . | Exp 2 . | ||
| CTR | 5 ± 1 | — | — |
| TZM 15 μM | 9 ± 2 | 92 | 88 |
| TZM 30 μM | 13 ± 2 | 58 | 55 |
| NU1025 | 4 ± 1 | 100 | 100 |
| NU1025 + TZM 15 μM | 27 ± 2 | 56 | 51 |
| NU1025 + TZM 30 μM | 37 ± 3 | 17 | 15 |
| In vitro treatment . | Apoptosis, % . | Colony formation, % . | |
|---|---|---|---|
| Exp 1 . | Exp 2 . | ||
| CTR | 5 ± 1 | — | — |
| TZM 15 μM | 9 ± 2 | 92 | 88 |
| TZM 30 μM | 13 ± 2 | 58 | 55 |
| NU1025 | 4 ± 1 | 100 | 100 |
| NU1025 + TZM 15 μM | 27 ± 2 | 56 | 51 |
| NU1025 + TZM 30 μM | 37 ± 3 | 17 | 15 |
Apoptosis was assessed by flow cytometry analysis of the sub-G1 DNA content on day 1 after treatment. Data are expressed as percentage of apoptotic cells and represent the mean of 3 independent experiments, ± SE, calculated after angular transformation of the percentage values.
For colony formation, untreated or drug-treated cells were cultured in flat-bottomed, 96-well plates (10 cells/well), and after 2 weeks colonies were counted. Results are expressed as percentages of colonies formed by drug-treated cells with respect to those generated by untreated controls, and they derive from 2 independent experiments (two 96-well plates per group of treatment in each experiment).
Intraperitoneal TZM and intracranial PARP inhibitor enhance survival of mice with intracerebral lymphoma
Toxicologic studies showed that no drug-related death occurred in mice treated with TZM (100 or 200 mg/kg) or with NU1025 ± TZM and that the maximal weight loss was 12%. All mice recovered initial body weight 1 week after treatment.
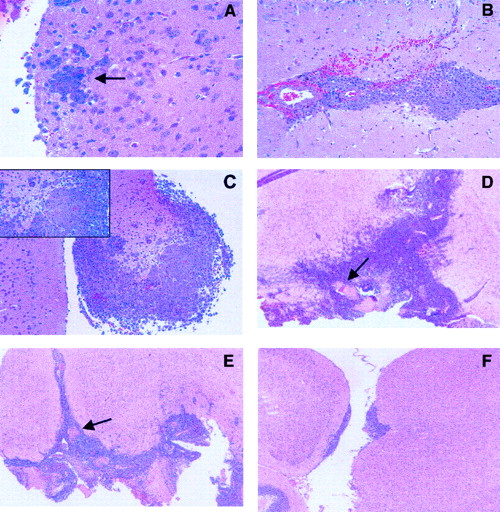
Histologic studies were carried out to analyze tumor growth in the brain of untreated animals. Results indicated that lymphoma cells were microscopically evident 2 days after challenge (Figure1A) and that tumor infiltration in brain parenchyma progressively increased during the following days (Figure 1B-C). Moreover, intracranial injection of L5178Y cells was fatal in 12 to 21 days, and macroscopic evidence of tumor was observed in additional mice killed when moribund.
Growth characteristics of intracerebral L5178Y lymphoma cells.
(A-F) Histologic section stained with hematoxylin-eosin. (A-C) Tumor cell growth in untreated animals. (A) Section obtained from mice killed 2 days after tumor challenge, demonstrates early neoplastic infiltration of the brain. Arrow points to lymphoma cells surrounding a capillary vessel (original magnification, 40 ×). (B) Extensive infiltration, associated with small hemorrhages, is observed in mice killed 4 days after challenge (original magnification, 25 ×). (C) Massive neoplastic infiltration, with brain damage (inset), is detected in mice killed 6 days after challenge (original magnification, 10 ×, inset 40 ×). (D-F) Effects of drug treatment on tumor cell growth. Histologic analysis of untreated or drug-treated mice was performed on day 15 after tumor challenge in additional mice that were not considered for the evaluation of survival (original magnification, 4 ×). Arrows point to hemorrhage and necrosis. (D) Untreated control: massive neoplastic infiltration of the brain. (E) 100 mg/kg TZM on days 2 and 3: diffuse brain infiltration. (F) 100 mg/kg NU1025 + TZM on days 2 and 3: focal collections of neoplastic cells, localized mostly under the leptomeninges, with initial infiltration of the brain tissue. There is no evidence of hemorrhage or necrosis. Tumor cell growth was analyzed by quantitative morphometry of histologic sections (n = 15, 5 animals/group). Results, expressed as mean area (mm2± SE) of tumor infiltration, were as follows: untreated control, 6.82 ± 2; TZM, 5.52 ± 1; NU1025 + TZM, 0.058 ± 0.05. Statistical analysis according to unpaired Student's ttest: NU1025 + TZM versus untreated controls,P = .00104; NU1025 + TZM versus TZM,P = .000108; TZM versus untreated controls,P = .115. Tumor growth inhibition induced by NU1025 + TZM or by TZM was 99% (range, 98%-99.9%) and 20% (range, 12%-49%), respectively.
Growth characteristics of intracerebral L5178Y lymphoma cells.
(A-F) Histologic section stained with hematoxylin-eosin. (A-C) Tumor cell growth in untreated animals. (A) Section obtained from mice killed 2 days after tumor challenge, demonstrates early neoplastic infiltration of the brain. Arrow points to lymphoma cells surrounding a capillary vessel (original magnification, 40 ×). (B) Extensive infiltration, associated with small hemorrhages, is observed in mice killed 4 days after challenge (original magnification, 25 ×). (C) Massive neoplastic infiltration, with brain damage (inset), is detected in mice killed 6 days after challenge (original magnification, 10 ×, inset 40 ×). (D-F) Effects of drug treatment on tumor cell growth. Histologic analysis of untreated or drug-treated mice was performed on day 15 after tumor challenge in additional mice that were not considered for the evaluation of survival (original magnification, 4 ×). Arrows point to hemorrhage and necrosis. (D) Untreated control: massive neoplastic infiltration of the brain. (E) 100 mg/kg TZM on days 2 and 3: diffuse brain infiltration. (F) 100 mg/kg NU1025 + TZM on days 2 and 3: focal collections of neoplastic cells, localized mostly under the leptomeninges, with initial infiltration of the brain tissue. There is no evidence of hemorrhage or necrosis. Tumor cell growth was analyzed by quantitative morphometry of histologic sections (n = 15, 5 animals/group). Results, expressed as mean area (mm2± SE) of tumor infiltration, were as follows: untreated control, 6.82 ± 2; TZM, 5.52 ± 1; NU1025 + TZM, 0.058 ± 0.05. Statistical analysis according to unpaired Student's ttest: NU1025 + TZM versus untreated controls,P = .00104; NU1025 + TZM versus TZM,P = .000108; TZM versus untreated controls,P = .115. Tumor growth inhibition induced by NU1025 + TZM or by TZM was 99% (range, 98%-99.9%) and 20% (range, 12%-49%), respectively.
To determine whether PARP inhibitor, injected at the site of tumor burden, might increase the antitumor activity of TZM administered systemically, animals were treated with NU1025 intracranially and with TZM intraperitoneally. Drug treatment was started on day 2 after challenge, when neoplastic infiltration of the brain tissue was evident in histologic sections. TZM, as a single agent, did not significantly increase MST with respect to control (Table2). Noteworthy, intracranial injection of NU1025, immediately before the administration of 100 or 200 mg/kg TZM, significantly increased lifespans with respect to controls or to groups treated with TZM only. When TZM was fractionated, the ILS obtained with this schedule was higher than that observed when NU1025 was combined with a single injection of TZM (statistical comparison of survival curves: NU1025 intracranially + TZM 100 mg/kg × 2 vs NU1025 + TZM 200 mg/kg; P = .023). When PARP inhibitor was given intraperitoneally in combination with TZM, no increase in lifespan was observed.
Influence of NU1025 on survival of animals treated with TZM
| Treatment . | No. mice . | MST (range) . | ILS† (vs CTR) (%) . | P‡(vs CTR) . | ILS2-153 (vs TZM) (%) . | P‡ (vs TZM) . |
|---|---|---|---|---|---|---|
| CTR | 14 | 17 (12-21) | — | — | — | — |
| NU 1025 ip | 10 | 17 (15-19) | — | NS | — | — |
| NU1025 ic | 14 | 16 (12-21) | — | NS | — | — |
| TZM (100 mg/kg) | 14 | 18 (15-21) | 6 | NS | — | — |
| TZM (200 mg/kg) | 14 | 18 (15-19) | 6 | NS | — | — |
| TZM (100 mg/kg) twice* | 14 | 19 (15-22) | 12 | NS | — | — |
| NU1025 ip + TZM (100 mg/kg) twice* | 10 | 17 (15-20) | — | NS | — | — |
| NU1025 ic + TZM (100 mg/kg) | 14 | 23 (19-> 90)2-155 | 35 | < .0001 | 28 | < .0001 |
| NU1025 ic + TZM (200 mg/kg) | 14 | 23 (15-> 90)2-155 | 35 | < .0001 | 28 | .0002 |
| NU1025 ic + TZM (100 mg/kg) twice* | 14 | 27 (19-> 90)2-155 | 59 | < .0001 | 42 | < .0001 |
| Treatment . | No. mice . | MST (range) . | ILS† (vs CTR) (%) . | P‡(vs CTR) . | ILS2-153 (vs TZM) (%) . | P‡ (vs TZM) . |
|---|---|---|---|---|---|---|
| CTR | 14 | 17 (12-21) | — | — | — | — |
| NU 1025 ip | 10 | 17 (15-19) | — | NS | — | — |
| NU1025 ic | 14 | 16 (12-21) | — | NS | — | — |
| TZM (100 mg/kg) | 14 | 18 (15-21) | 6 | NS | — | — |
| TZM (200 mg/kg) | 14 | 18 (15-19) | 6 | NS | — | — |
| TZM (100 mg/kg) twice* | 14 | 19 (15-22) | 12 | NS | — | — |
| NU1025 ip + TZM (100 mg/kg) twice* | 10 | 17 (15-20) | — | NS | — | — |
| NU1025 ic + TZM (100 mg/kg) | 14 | 23 (19-> 90)2-155 | 35 | < .0001 | 28 | < .0001 |
| NU1025 ic + TZM (200 mg/kg) | 14 | 23 (15-> 90)2-155 | 35 | < .0001 | 28 | .0002 |
| NU1025 ic + TZM (100 mg/kg) twice* | 14 | 27 (19-> 90)2-155 | 59 | < .0001 | 42 | < .0001 |
NS indicates not significant.
Sequential treatment with TZM (100 mg/kg) on days 2 and 3.
ILS of drug-treated mice was calculated (see “Study design”) comparing their MST with those of control animals injected with drug vehicles only (CTR).
P was calculated comparing survival curves (see “Statistical analysis”) of drug-treated groups versus CTR, or comparing survival curves of mice treated with the drug combination versus mice treated with the corresponding dose and schedule of TZM.
ILS of mice treated with NU1025 intracranially (ic) + TZM was calculated comparing their MST with those of mice treated with TZM only. In particular, ILS of animals treated with NU1025 + 100 mg/kg or 200 mg/kg TZM was calculated comparing their MST with those of animals treated with 100 mg/kg or 200 mg/kg TZM, respectively. ILS of animals treated with NU1025 + 100 mg/kg TZM twice was calculated comparing their MST with those of animals treated with TZM (100 mg/kg) twice.
In each of the groups treated with NU1025 intracranially and TZM intraperitoneally (ip), one animal survived more than 90 days.
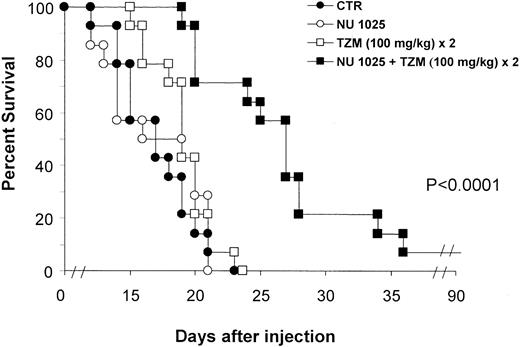
Figure 2 shows the survival curves of groups treated with the fractionated schedule. The increase in survival detected in the NU1025 + TZM group was indeed related to a statistically significant reduction of tumor growth, as evidenced by histologic studies (Figure 1D-F). On the contrary, a remarkable infiltration of lymphoma cells in the surrounding brain tissue was observed in controls or in mice treated with TZM only.
Combined treatment with TZM intraperitoneally and PARP inhibitor intracranially increases survival of tumor-bearing mice.
Animals (14 per group) were inoculated intracranially with L5178Y lymphoma cells (day 0). Treatment was performed by intracranial injection of NU1025 (1 mg/mouse) or intraperitoneal injection of TZM (100 mg/kg) on day 2 after tumor challenge. An additional dose of TZM was administered on day 3. Control animals (CTR) were treated with drug vehicles only (ie, intracranial polyethylene glycol + intraperitoneal dimethyl sulfoxide) on day 2. Combined treatment with intracranial NU1025 and TZM (100 mg/kg) twice significantly increased survival of tumor-bearing mice with respect to control or TZM-treated groups (P < .0001). Differences between survival curves of mice treated with TZM or NU1025 and survival curve of controls were not statistically significant.
Combined treatment with TZM intraperitoneally and PARP inhibitor intracranially increases survival of tumor-bearing mice.
Animals (14 per group) were inoculated intracranially with L5178Y lymphoma cells (day 0). Treatment was performed by intracranial injection of NU1025 (1 mg/mouse) or intraperitoneal injection of TZM (100 mg/kg) on day 2 after tumor challenge. An additional dose of TZM was administered on day 3. Control animals (CTR) were treated with drug vehicles only (ie, intracranial polyethylene glycol + intraperitoneal dimethyl sulfoxide) on day 2. Combined treatment with intracranial NU1025 and TZM (100 mg/kg) twice significantly increased survival of tumor-bearing mice with respect to control or TZM-treated groups (P < .0001). Differences between survival curves of mice treated with TZM or NU1025 and survival curve of controls were not statistically significant.
The current results demonstrate that intracerebral treatment with NU1025 before the systemic administration of TZM is a new, safe, and effective approach to increase antitumor activity of the methylating agent in the brain. Moreover, fractionated doses of TZM, combined with NU1025, induced a more pronounced antitumor effect compared with an equivalent single dose of TZM + NU1025. It can be speculated that interruption of the repair of N-methylpurines generated by TZM, through the use of PARP inhibitor, might increase tumor cell susceptibility to subsequent TZM administration. It is unlikely that OGAT depletion and elevated O6-methylguanine damage might contribute to the increased efficacy of fractionated schedule because L5178Y cells express low levels of OGAT activity (6.3 fmol/mg protein).
When NU1025 was administered intraperitoneally in combination with TZM, no increase in survival was observed. This is likely because of the negative impact of the blood–brain barrier on the distribution of NU1025 into the brain. On the other hand, the intralesional route could be an inadequate strategy against brain lymphoma, considering that the pattern of this malignancy is often diffuse and multifocal. Therefore, formulations of PARP inhibitor that permeate the blood–brain barrier or innovative techniques for drug delivery that provide homogeneous distribution of the compounds into the brain parenchyma21 should be explored.
Noteworthy, PARP inhibitors render neoplastic cells susceptible to TZM even in the case of tumors with defective MR or high OGAT levels.8,22 Although OGAT-dependent resistance could be reversed by OGAT inhibitors (ie, O6-benzylguanine23), mutated forms of OGAT enzyme24 might escape inactivation. Furthermore, O6-benzylguanine does not potentiate the activity of TZM in MR-deficient tumors.6 25 Therefore, the use of PARP inhibitors combined with TZM could represent a novel strategy for the control of malignancies localized at the CNS site.
Supported by grants from the Italian Association for Cancer Research and from the Italian Ministry of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lucio Tentori, Department of Neuroscience, University of Rome Tor Vergata, Via di Tor Vergata 135, 00133 Rome, Italy; e-mail: tentori@uniroma2.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal