Abstract
Neutrophils are continuously released from the bone marrow (BM), and this release is accelerated during inflammation. This study compared the mechanical properties of mature neutrophils within the BM and the circulating blood, as well as the role of microtubule rearrangement in the release of neutrophils from the BM in rats. Neutrophils isolated from the BM were stiffer than neutrophils in the circulating blood, using magnetic twisting cytometry. BM neutrophils also contained more F-actin within the submembrane region than circulating neutrophils when examined using confocal microscopy, suggesting that mature quiescent neutrophils within the BM are stiffer than circulating neutrophils because of increased formation of F-actin beneath the plasma membrane. Complement protein 5 fragments or formylmethionyl-leucylphenylalanine (fMLP) induced a stiffening response within 2 minutes that was greater in circulating than in BM neutrophils. This stiffening required F-actin formation within the submembrane region but not microtubule rearrangement in both circulating and BM neutrophils. fMLP-induced shape changes were more pronounced in circulating than in BM neutrophils, which showed fewer and smaller pseudopods and fewer membrane irregularities. In vivo, fMLP induced neutropenia, sequestration of neutrophils within the pulmonary capillaries, and release of neutrophils from the BM. Studies using colchicine demonstrated that rearrangement of microtubules was not required for any of these processes but was required for normal trafficking of neutrophils through the pulmonary capillaries.
Introduction
During the acute inflammatory response within the lungs, as occurs during bacteria pneumonia or acute respiratory distress syndrome, many neutrophils sequester in the microvasculature and emigrate into the lungs. The inflammatory reaction also results in the production of many mediators, some of which induce the release of neutrophils from the bone marrow (BM).1,2 These newly recruited neutrophils constitute a large fraction of the neutrophils that are accumulating during these inflammatory processes.1-3
Previous studies have demonstrated that the initial sequestration of circulating neutrophils within the pulmonary capillary bed in response to inflammatory mediators does not require any of the commonly used adhesion molecules.4-6 We and others have postulated that changes in the mechanical properties of neutrophils are important in the process of sequestration.5-10 In the normal uninflamed lung, most neutrophils must become elongated to pass through the capillary bed into the venules. Inflammatory stimuli induce neutrophils to become stiffer and less able to change their shape.7-10We postulate that this stiffening prevents neutrophils from deforming and passing through the many capillaries that are narrower than spherical neutrophils.
Because large numbers of neutrophils are released from the BM into the venous circulation where they travel first to the lungs, the mechanical properties of these neutrophils are likely to be critical in determining how they traffic through the pulmonary capillary bed. Lichtman11 compared the stiffness of myelocytes and circulating neutrophils by using micropipette assays and demonstrated that the myelocytes were stiffer than the circulating neutrophils. However, the mechanical properties of mature neutrophils within the BM pool have not been examined. This study tested the hypothesis that these mature but not yet released neutrophils are stiffer than circulating neutrophils. The mechanical properties and cytoskeletal structure of BM and circulating neutrophils were examined, as well as the effect of an inflammatory stimulus. The hypothesis that microtubule rearrangements are important in the release of neutrophils from the BM and in the deformations required for neutrophil transit time through the normal pulmonary capillary bed was also tested. The mechanical properties of neutrophils were evaluated by measuring the apparent stiffness of neutrophils by using magnetic twisting cytometry, which measures the rotation of magnetic beads bound to neutrophils in response to a torque.12-16 The release of neutrophils from the BM was quantitated by using a previously described technique based on the observation that intramuscular formylmethionyl-leucylphenylalanine (fMLP) and C5a induce neutropenia, followed by release of neutrophils from the BM into the venous blood.1 Because the inflammatory stimulus induces immediate sequestration of neutrophils within the pulmonary capillaries, the difference between neutrophil counts in the venous and arterial blood represents a measure of BM release.1
Materials and methods
Reagents
Cytochalasin D, colchicine, zymosan yeast A, and fMLP, L-α lysophosphatidylcholine, fluorescein isothiocyanate (FITC)–phalloidin, and rhodamine-phalloidin were purchases from Sigma Chemical (St Louis, MO). Neutrophil isolation medium (NIH.2) and erythrocyte lysing buffer (E-LYSE) were obtained from Cardinal Associates (Santa Fe, NM). Murine antirat CD45 monoclonal antibodies were purchased from Pharmingen (San Diego, CA). Ferromagnetic beads measuring 4.5 μm in diameter, to which goat antimouse immunoglobulin G Fc antibodies were bound covalently, were purchased from Spherotech (Libertyville, IL).
Isolation of neutrophils
Rat neutrophils were isolated from whole rat blood using a 2-component step gradient. Lewis male rats were anesthetized using ketamine hydrochloride (160 mg/kg intramuscularly). The inferior vena cava was exposed through a midline laparotomy, and blood was drawn using 0.5 M EDTA as the anticoagulant. The gradient was prepared by layering NIM.2B onto NIM.2A, following the manufacturer's protocol. The blood was layered on top of the NIM.2B and centrifuged at 900g for 45 minutes. The neutrophils localized to the interface of the NIM.2B and NIM.2A. This cell band was collected and washed with Hanks balanced salt solution (0.4 mM Na2HPO4, 5.7 mM KC1, 137 mM NaCl, and 4.2 mM NaHCO3, pH 7.4) twice. E-LYSE was used for the hypotonic lysis of the residual red blood cells. Finally, the neutrophils were resuspended in an isotonic buffer containing 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCI, 137 mM NaCl, and 5.5 mM glucose (pH 7.4) at a concentration of 2.0 × 106 neutrophils/mL. The purity of neutrophils isolated from the circulating blood was more than 95%.
Isolation of BM neutrophils
The femurs were removed and the ends of the bones were clipped. The marrow contents were removed by flushing the BM cavity with phosphate-buffered saline (PBS, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCI, and 137 mM NaCl, pH 7.4) containing 2 mM EDTA. After disrupting clumps using a pipette, the cells were centrifuged at 500g for 10 minutes. The supernatant containing the platelets was discarded. The resuspended pellet was layered onto a NIM gradient as described for circulating neutrophils, and subsequent steps in this protocol were followed except that no lysis step was included. This procedure resulted in a population of cells consisting of 75% mature neutrophils, 5% bands, and 20% nucleated precursors. In the studies using magnetic twisting cytometry (please see below), the ferromagnetic beads were bound to neutrophils through CD45. Because the purity of neutrophils isolated from the BM was only 75%, immunohistochemistry was performed on cytospins from the BM isolation to determine which cells expressed CD45. These studies showed that more than 90% of the CD45+cells were mature neutrophils with segmented nuclei. Therefore, the stiffness was measured using magnetic twisting cytometry and CD45-bound beads were primarily that of mature neutrophils, despite the low purity of the isolation.
Magnetic twisting cytometry
Magnetic twisting cytometry quantitates the apparent stiffness of cells by measuring the angular rotation of ferromagnetic beads bound to cellular surfaces in response to an applied magnetic torque.12-16 Goat anti–mouse immunoglobulin G Fc was covalently linked to ferromagnetic microbeads measuring 4.5 μm in diameter. These beads were coated with murine monoclonal antibodies against rat CD45 by incubating the beads with the antibody at a concentration of 1 μg/106 beads at 4°C for 30 minutes. The beads were washed in PBS 3 times. Isolated neutrophils (2.0 × 105/well) and antibody-coated microbeads (3.5 × 105/well) were combined, placed in nonadhesive plastic wells (Immunon 2; Dynatech Laboratories, Chantilly, VA), and gently centrifuged at 150g for 4 minutes. After unbound beads were washed away, each well was placed in the magnetic twisting cytometer, and the biomechanical properties of the neutrophils were measured as previously described.15,16 In brief, the bound beads were exposed to a brief (10-μsecond) but strong (1000 G) magnetic field to be magnetized in the horizontal direction. After 20 seconds, the beads were twisted by using a much weaker (30 G) but continuous (1 minute) vertical magnetic field. This twisting field was not strong enough to change the magnetic alignment of the beads, but it caused the beads to rotate. However, bead rotation was opposed by forces developed within the cell to which the bead is bound. The magnitude of the magnetic vector in the horizontal direction (remnant magnetic field) was measured by an in-line magnetometer. From this value, the average bead rotation (angular strain) was calculated.12-16
In vitro studies of mechanical properties and F-actin
The mechanical properties of BM and circulating neutrophils were compared. Quiescent neutrophils were compared with those treated with either complement fragments generated by activation of plasma using zymosan yeast A (20% final concentration) or fMLP (10−6M) for 2, 5, 10, and 15 minutes. The role of the microtubules in the stiffening response was determined by pretreating BM and circulating neutrophils with colchicine (50 μM) or buffer for 15 minutes. The role of F-actin assembly was evaluated by pretreating neutrophils with cytochalasin D (10 μM in 0.1% dimethyl sulfoxide) or vehicle for 5 minutes. Each study was repeated by using the BM and circulating neutrophils from 5 rats.
To measure the F-actin content and distribution, isolated BM and circulating neutrophils were fixed with paraformaldehyde in PBS (pH 7.3, 3.2% final concentration) for 30 minutes. After washing, the cells were incubated with L-α lysophosphatidylcholine (final concentration 200 μg/mL) and FITC-phalloidin (final concentration 3.3 × 10−7 M) for 60 minutes in the dark. After washing, the F-actin content was measured by flow cytometry (n = 5 rats) or by placing 105 BM or circulating neutrophils per well of a 96-well plate and quantitating FITC by using a fluorescent plate reader (n = 5 rats).
To determine the distribution of F-actin within neutrophils, the cells were similarly stained with rhodamine-labeled phalloidin and examined by a confocal microscopy (n = 3 rats). Prepared BM or circulating neutrophils were examined using a Leica TCSNT confocal laser scanning microscope (Leica, Exton, PA) fitted with air-cooled Argon and Krypton lasers. Fields of view were selected and brought into view under bright-field imaging conditions. Confocal micrographs of emission spectra (> 590 nm) were recorded under fluorescent imaging mode by using an excitation wavelength of 568 nm. Randomly selected images of 100 mature neutrophils from the blood or the BM with and without exposure to fMLP in each rat (n = 3 rats in each group) were examined and collected from a ×100 oil objective lens with 0.08-μm pixel size. Micrographs were examined by using ImageSpace software (Molecular Dynamics, Sunnyvale, CA) with pseudocolor thermal gradient map applied.
In vivo studies of pulmonary sequestration and BM release of neutrophils induced by fMLP
The release of neutrophils from the BM was quantitated using a previously described technique, based on the difference between the venous and arterial neutrophil counts in animals receiving infusions of inflammatory mediators,1 as well as by determining the number of mature-appearing neutrophils within the BM. Lewis male rats (0.25-0.30 kg) were anesthetized by intramuscular injection of ketamine hydrochloride (160 mg/kg) and acepromazine maleate (3.65 mg/kg). A catheter was placed in the tail vein, and the animals received heparin (200 U/kg intravenously [IV]). To collect samples of blood entering the lungs (venous blood), a 24-space gauge catheter was placed through the right external jugular vein into the superior vena cava near the right atrium. To collect samples of blood exiting the lungs (arterial blood), a 24-space gauge catheter was placed through the left carotid artery into the aorta. The location of catheter tips was confirmed at autopsy.
Rats were pretreated with either colchicine (1 mg/kg IV) or saline (n = 5 rats in each group). After 15 minutes, an infusion of fMLP (0.01 mg/mL IV at a rate of 0.3 mg/kg/mL for 15 minutes) was begun. Blood samples were drawn simultaneously from the venous and arterial catheters before and at 5, 10, 15, 20, 30, 40, 50, 60, 90, and 120 minutes after the start of the fMLP infusion. The total circulating leukocyte counts were determined by using a hemacytometer, and the neutrophil counts were determined by using blood smears stained with Leukostat (Fisher, Pittsburgh, PA). The circulating neutrophil counts in the venous and arterial blood samples were corrected for changes in hematocrit and were compared at each time point.
IV injection of colchicine causes a decrease in the circulating neutrophil counts, which recover by 2 hours, and a second injection does not cause a similar decrease.17 Therefore, a second series of studies were performed in which a second dose of colchicine (1 mg/kg IV) or saline was injected 2 hours after the first injection so that the circulating neutrophil counts were similar to those in control animals. Blood samples were obtained simultaneously from the venous and arterial lines at the same time points as in studies using a single dose.
In a third series of studies, the effect of fMLP on the numbers of neutrophils in the BM, blood, and pulmonary capillaries was determined, and the role of the microtubules was examined. Rats were prepared similarly to the first study described above and pretreated with either colchicine (1 mg/kg IV) or saline for 15 minutes. After a 15-minute infusion of fMLP or saline (n = 5 in each group), blood samples were obtained simultaneously from the arterial and venous lines, and saturated potassium chloride was injected into the aorta root through the arterial catheter to stop the heart. The chest was rapidly opened, the base of the heart was tied to maintain the pulmonary blood volumes, and the lungs were inflated with glutaldehyde (6%) at 22 cm H2O pressure. The femurs were removed and fixed in 10% formalin. Histologic sections of peripheral lung tissue (5-6 μm thick) were cut from paraffin-embedded tissue blocks and stained with hematoxylin and eosin. The BM samples were decalcified by using Decal (Fisher, Pittsburgh, PA) and embedded in plastic (Immunobed; Polysciences, Warrington, PA). Sections 1 to 2 μm thick were cut and stained with hematoxylin and eosin.
To quantitate the number of neutrophils that were sequestered in pulmonary capillaries, the number of neutrophils in 10 randomly selected fields at × 1000 magnification were counted, and neutrophil sequestration was expressed as the number of intracapillary neutrophils per 10 high-power fields.18 To quantitate the volume fraction of BM occupied by mature neutrophils, point-counting techniques were used. A grid containing 100 points was projected onto the microscopic field by using a camera lucida. The fraction of points lying on mature neutrophils was counted in 5 microscopic fields. The data were expressed as the volume fraction of BM occupied by mature neutrophils.
Statistics
Data are presented as means ± SEM. One-way analyses of variance with post hoc tests to correct for multiple comparisons andt tests were used as appropriate to compare apparent stiffness, amount of F-actin, circulating neutrophil counts, and volume fractions of neutrophils in organs.
Results
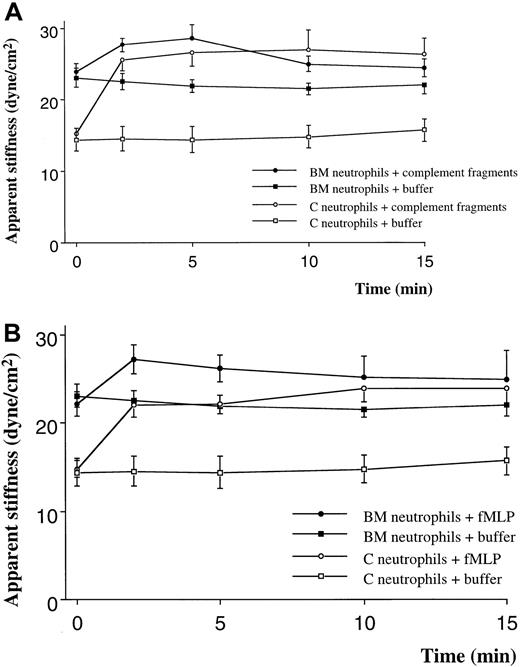
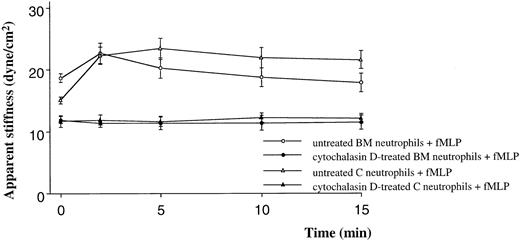
Quiescent neutrophils isolated from the BM were stiffer than quiescent neutrophils from the circulating blood, isolated using similar techniques (Figure 1A,B). Stimulation with either C5 fragments or fMLP induced a stiffening of both BM and circulating neutrophils. This increase in stiffness was present by 2 minutes and persisted throughout the 15-minute study. The increase in apparent stiffness of BM neutrophils was significantly less than that of circulating neutrophils (Figure 1A,B). Pretreatment of neutrophils with colchicine did not alter the stiffness of quiescent neutrophils isolated from either site. In addition, the increase in stiffness induced by fMLP was not inhibited by this pretreatment (Figure 2). In contrast, pretreatment with cytochalasin D reduced the stiffness of quiescent neutrophils isolated from either the BM or the circulating blood (Figure3). In fact, the stiffness of BM neutrophils was reduced to the level observed in circulating neutrophils. In addition, pretreatment with cytochalasin D completely inhibited the increase in stiffness induced by fMLP in both BM and circulating neutrophils (Figure 3).
The effect of complement fragments (A) and fMLP (B) on the apparent stiffness of neutrophils isolated from the BM or the circulating blood.
The apparent stiffness of BM neutrophils before stimulation (time 0) was greater than that of neutrophils in the circulating blood (P < .01). Either complement fragments or fMLP induced an increase in apparent stiffness within 2 minutes (P < .01). n = 5 rats in each group. BM indicates bone marrow; C, circulating.
The effect of complement fragments (A) and fMLP (B) on the apparent stiffness of neutrophils isolated from the BM or the circulating blood.
The apparent stiffness of BM neutrophils before stimulation (time 0) was greater than that of neutrophils in the circulating blood (P < .01). Either complement fragments or fMLP induced an increase in apparent stiffness within 2 minutes (P < .01). n = 5 rats in each group. BM indicates bone marrow; C, circulating.
The effect of pretreatment with colchicine on the apparent stiffness of quiescent neutrophils or neutrophils stimulated with fMLP.
Pretreatment of neutrophils with colchicine (50 μM) did not alter the apparent stiffness of quiescent neutrophils isolated from either the BM or the circulating blood. Colchicine also did not prevent the increase in stiffness induced by fMLP. n = 5 rats in each group. BM indicates bone marrow; C, circulating.
The effect of pretreatment with colchicine on the apparent stiffness of quiescent neutrophils or neutrophils stimulated with fMLP.
Pretreatment of neutrophils with colchicine (50 μM) did not alter the apparent stiffness of quiescent neutrophils isolated from either the BM or the circulating blood. Colchicine also did not prevent the increase in stiffness induced by fMLP. n = 5 rats in each group. BM indicates bone marrow; C, circulating.
The effect of cytochalasin D on the apparent stiffness of quiescent neutrophils and neutrophils stimulated with fMLP.
Pretreatment with cytochalasin D (10 μM) decreased the apparent stiffness of quiescent neutrophils (time 0). This decrease was greater for neutrophils isolated from the BM than the circulating blood, and there was no significant difference in the stiffness of neutrophils isolated from the BM or the circulating blood after this pretreatment. In addition, cytochalasin D completely inhibited the increase in stiffness induced by fMLP (P < .05). n = 5 rats in each group. BM indicates bone marrow; C, circulating.
The effect of cytochalasin D on the apparent stiffness of quiescent neutrophils and neutrophils stimulated with fMLP.
Pretreatment with cytochalasin D (10 μM) decreased the apparent stiffness of quiescent neutrophils (time 0). This decrease was greater for neutrophils isolated from the BM than the circulating blood, and there was no significant difference in the stiffness of neutrophils isolated from the BM or the circulating blood after this pretreatment. In addition, cytochalasin D completely inhibited the increase in stiffness induced by fMLP (P < .05). n = 5 rats in each group. BM indicates bone marrow; C, circulating.
Figure 4 shows representative images from the confocal microscopy, using rhodamine-phalloidin to localize F-actin. The neutrophils isolated from the circulating blood were primarily round and contained F-actin distributed either centrally or in small amounts near the periphery of the cell (Figure 4A). Occasional cells appeared activated and contained actin within pseudopods. On stimulation with fMLP, the shape became markedly distorted, with numerous pseudopods and irregularities to the plasma membrane (Figure4B). In addition, no F-actin was present within the central regions of the cell but was localized to the submembrane region. fMLP induced both an increase in the amount of F-actin, as well as a change in its distribution.
Representative images from confocal microscopy using rhodamine-phalloidin to delineate F-actin.
Neutrophils isolated from circulating blood (A) were spherical, and the small amount of F-actin was located within the central regions of these cells. Occasional neutrophils showed pseudopod formation and contained F-actin within these pseudopods. On stimulation with fMLP (B), their shape became markedly distorted, with numerous pseudopods and irregularities to the plasma membrane. F-actin was no longer observed within the central regions of the neutrophils. Increased amounts of F-actin were present in the submembrane region, forming a shell beneath the membrane. Quiescent neutrophils isolated from the BM (C) were spherical. The amount of F-actin was greater than in the quiescent neutrophils isolated from the circulating blood. Although these cells contained a small amount of F-actin in the central regions, similar to the circulating neutrophils, the increase in F-actin was located primarily in the submembrane region. Stimulation of BM neutrophils with fMLP (D) induced only small changes in shape that were minimal compared with those observed in the circulating blood. As in the circulating neutrophils, the BM neutrophils showed a marked increase in F-actin within the submembrane region and no identifiable F-actin within the central region. n = 3 rats in each group.
Representative images from confocal microscopy using rhodamine-phalloidin to delineate F-actin.
Neutrophils isolated from circulating blood (A) were spherical, and the small amount of F-actin was located within the central regions of these cells. Occasional neutrophils showed pseudopod formation and contained F-actin within these pseudopods. On stimulation with fMLP (B), their shape became markedly distorted, with numerous pseudopods and irregularities to the plasma membrane. F-actin was no longer observed within the central regions of the neutrophils. Increased amounts of F-actin were present in the submembrane region, forming a shell beneath the membrane. Quiescent neutrophils isolated from the BM (C) were spherical. The amount of F-actin was greater than in the quiescent neutrophils isolated from the circulating blood. Although these cells contained a small amount of F-actin in the central regions, similar to the circulating neutrophils, the increase in F-actin was located primarily in the submembrane region. Stimulation of BM neutrophils with fMLP (D) induced only small changes in shape that were minimal compared with those observed in the circulating blood. As in the circulating neutrophils, the BM neutrophils showed a marked increase in F-actin within the submembrane region and no identifiable F-actin within the central region. n = 3 rats in each group.
In striking contrast to circulatory neutrophils, quiescent BM neutrophils contained more F-actin within the submembrane region (Figure 4C). Similar amounts of F-actin were observed within the central region of BM and circulating neutrophils (Figure 4C). In addition, virtually all BM neutrophils were round. Stimulation with fMLP induced only minimal changes in shape that consisted primarily of small irregularities in the plasma membrane and occasional pseudopods that were smaller in size than those seen in the circulating blood (Figure 4D). In addition, the central regions of the cells contained almost no F-actin, and there was a redistribution to the submembrane region, as well as an increase in the amount of F-actin, similar to the changes observed in neutrophils from the circulating blood.
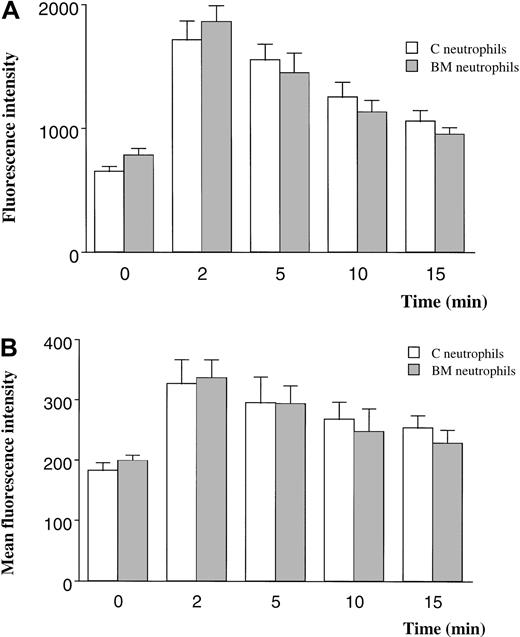
The F-actin content of BM and circulating neutrophils was quantitated using 2 techniques. When F-actin was labeled with FITC-phalloidin and measured with the use of a fluorescent plate reader, neutrophils from the BM contained 20% more F-actin than neutrophils from the circulating blood (Figure 5A). The F-actin content increased 180% in response to fMLP in neutrophils from either site (Figure 5A).
F-actin content in quiescent and fMLP-stimulated neutrophils measured by using FITC-phalloidin and a fluorescent plate reader (A) or flow cytometry (B).
Measurements using a fluorescent plate reader demonstrated 20% more F-actin in BM than in circulating neutrophils (P < .05) and a 180% increase after exposure to fMLP for 2 minutes (A). In contrast, the F-actin content of quiescent neutrophils isolated from the BM was similar to that of neutrophils from the circulating blood when measured by flow cytometry. Stimulation with fMLP induced an increase in F-actin content that was similar in neutrophils isolated from either site. n = 5 rats in each group. C indicates circulating; BM, bone marrow.
F-actin content in quiescent and fMLP-stimulated neutrophils measured by using FITC-phalloidin and a fluorescent plate reader (A) or flow cytometry (B).
Measurements using a fluorescent plate reader demonstrated 20% more F-actin in BM than in circulating neutrophils (P < .05) and a 180% increase after exposure to fMLP for 2 minutes (A). In contrast, the F-actin content of quiescent neutrophils isolated from the BM was similar to that of neutrophils from the circulating blood when measured by flow cytometry. Stimulation with fMLP induced an increase in F-actin content that was similar in neutrophils isolated from either site. n = 5 rats in each group. C indicates circulating; BM, bone marrow.
In contrast, the F-actin content of quiescent BM and circulating neutrophils was similar when measured using flow cytometry (Figure 5B). The F-actin content increased by 80% after stimulation with fMLP in both BM and circulating neutrophils.
In vivo, IV infusion of fMLP induced a decrease in the circulating neutrophil counts (Figure6A), as demonstrated previously.1 At early time points of 5 minutes, there was no difference between the arterial and venous counts. However, by 10 minutes there was a significant increase in the number of neutrophils in the venous sample entering the lungs. The arterial counts did not increase, indicating that the newly released neutrophils were immediately sequestered within the lung (Figure 6A). This venous-arterial gradient in neutrophil concentration persisted for 20 minutes. This difference was considered to be a measure of BM release.1
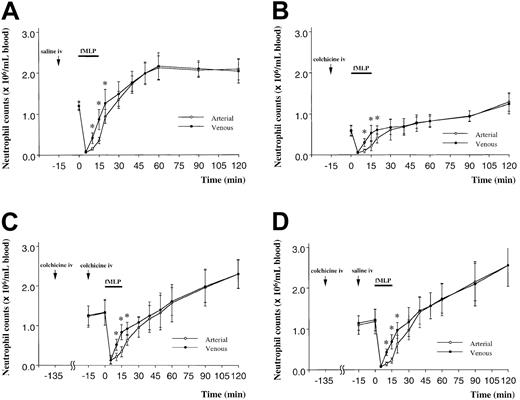
The effect of pretreating rats with colchicine on BM release induced by infusion of fMLP in rats.
The circulating neutrophil counts in rats pretreated with saline decreased in both arterial and venous samples within 5 minutes of infusion (A). By 10 minutes, there was a significant increase in the number of neutrophils within the venous blood sample. This increase was not observed in the arterial blood sample. This venous-arterial gradient in neutrophil concentration measured BM release.1Pretreatment with colchicine induced a fall in the circulating neutrophil counts to approximately 50% of baseline value (B). However, infusion of fMLP induced a similar fall in the circulating neutrophil counts and a similar venous-arterial gradient (B) as observed in animals that did not receive colchicine. When rats received a second dose of colchicine 2 hours after the first, no fall in circulating neutrophil counts occurred (C). This dosing regimen also did not inhibit the fMLP-induced decrease in circulating neutrophil counts or the venous-arterial gradient compared with animals given saline (D). n = 5 rats in each group; *, significantly greater than values in the arterial blood at this time point, P < .05.
The effect of pretreating rats with colchicine on BM release induced by infusion of fMLP in rats.
The circulating neutrophil counts in rats pretreated with saline decreased in both arterial and venous samples within 5 minutes of infusion (A). By 10 minutes, there was a significant increase in the number of neutrophils within the venous blood sample. This increase was not observed in the arterial blood sample. This venous-arterial gradient in neutrophil concentration measured BM release.1Pretreatment with colchicine induced a fall in the circulating neutrophil counts to approximately 50% of baseline value (B). However, infusion of fMLP induced a similar fall in the circulating neutrophil counts and a similar venous-arterial gradient (B) as observed in animals that did not receive colchicine. When rats received a second dose of colchicine 2 hours after the first, no fall in circulating neutrophil counts occurred (C). This dosing regimen also did not inhibit the fMLP-induced decrease in circulating neutrophil counts or the venous-arterial gradient compared with animals given saline (D). n = 5 rats in each group; *, significantly greater than values in the arterial blood at this time point, P < .05.
Pretreatment of rats with colchicine decreased the circulating neutrophil counts to approximately 50% of the values observed without colchicine (Figure 6B compared with Figure 6A). Infusion of fMLP induced a fall in circulating neutrophil counts that was similar in size to that observed in animals that did not receive colchicine (Figure 6B). In addition, colchicine did not prevent the increase in neutrophil counts observed in the venous blood at 10 to 20 minutes after initiation of the infusion. The venous-arterial gradient was similar with and without colchicine.
In a second series of studies, rats received a second injection of colchicine 2 hours after the initial injection. In contrast to the first injection, this second injection did not induce a fall in the circulating neutrophil counts. This pretreatment regimen did not inhibit the fall in the circulating counts induced by infusion of fMLP or the development of a venous-arterial difference in circulating neutrophil counts (Figures 6C,D). Neither 1 nor 2 doses of colchicine inhibited the venous-arterial differences observed between 10 and 20 minutes of fMLP infusion.
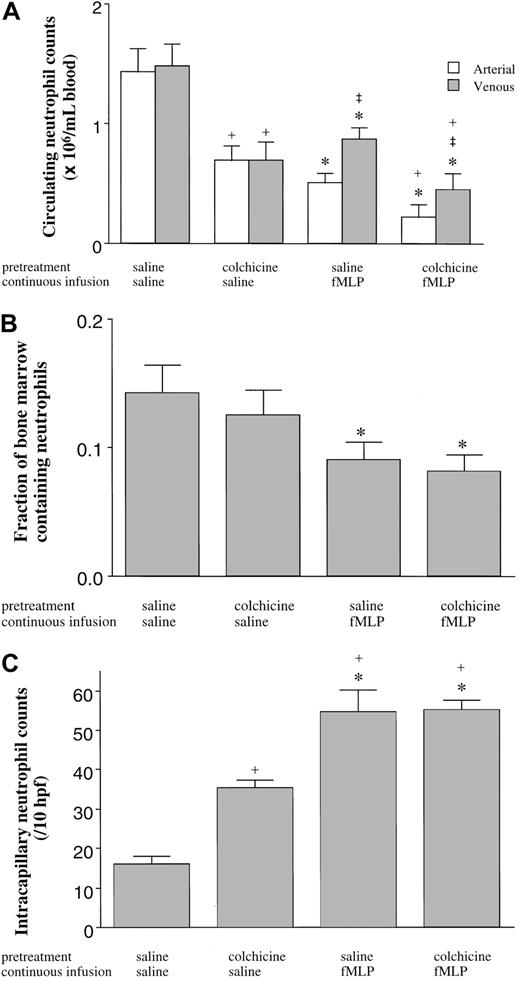
The third series of experiments quantitated the numbers of neutrophils in the BM, blood, and lungs in rats pretreated with colchicine or saline and given a 15-minute infusion of fMLP or saline. In saline-pretreated rats, fMLP induced a fall in the circulating neutrophil counts (Figure 7A), as described above. fMLP also induced a release of mature neutrophils from the BM, as shown by a decrease in the volume fraction of the BM cavity occupied by neutrophils (Figure 7B) and by the increase in neutrophils within the venous blood compared with arterial counts (Figure 7A). Infusion of fMLP also induced an increase in neutrophil sequestration within the pulmonary capillaries (Figure 7C). Inhibition of microtubule rearrangement using colchicine had no effect on the fMLP-induced fall in the circulating neutrophil counts, on the increased neutrophil counts in the venous compared with arterial blood (Figure 7a), on the release of neutrophils from the BM (Figure 7B), or on the sequestration of neutrophils within the pulmonary capillaries (Figure 7C). Interestingly, however, colchicine itself did cause a fall in the circulating neutrophil counts in animals given infusions of saline (Figure 7B) as observed previously (Figure 6), and this fall in the circulating counts was accompanied by an increase in neutrophil sequestration within the pulmonary capillaries (Figure 7C).
The effect of fMLP on the distribution of neutrophils in the blood (A), the BM (B), and the pulmonary capillaries (C).
Neither the fMLP-induced fall in the circulating neutrophil counts nor the venous-arterial gradient was inhibited by pretreatment with colchicine (A). fMLP induced a release of mature neutrophils from the BM as demonstrated by the decrease in the volume fraction of the BM cavity occupied by neutrophils (B), which was not inhibited by pretreatment of rats with colchicine (B). fMLP induced neutrophil sequestration within the pulmonary capillaries, and this sequestration was not inhibited by colchicine (C). Colchicine itself induced a decrease in the number of neutrophils circulating within the blood, which was accompanied by an increase in the number of neutrophils sequestered within the capillaries. n = 5 rats in each group; *, significantly different from animals given an infusion of saline compared with fMLP (P < .05); ‡, significantly different from arterial counts (P < .05); and +, significantly different from saline pretreated rats (P < .05).
The effect of fMLP on the distribution of neutrophils in the blood (A), the BM (B), and the pulmonary capillaries (C).
Neither the fMLP-induced fall in the circulating neutrophil counts nor the venous-arterial gradient was inhibited by pretreatment with colchicine (A). fMLP induced a release of mature neutrophils from the BM as demonstrated by the decrease in the volume fraction of the BM cavity occupied by neutrophils (B), which was not inhibited by pretreatment of rats with colchicine (B). fMLP induced neutrophil sequestration within the pulmonary capillaries, and this sequestration was not inhibited by colchicine (C). Colchicine itself induced a decrease in the number of neutrophils circulating within the blood, which was accompanied by an increase in the number of neutrophils sequestered within the capillaries. n = 5 rats in each group; *, significantly different from animals given an infusion of saline compared with fMLP (P < .05); ‡, significantly different from arterial counts (P < .05); and +, significantly different from saline pretreated rats (P < .05).
Discussion
The acute inflammatory response results in the release of mature as well as immature neutrophils from the BM. The studies presented in this study demonstrate that neutrophils in the BM are stiffer than neutrophils in the circulating blood, when evaluated by measuring the angular rotation of ferromagnetic beads in response to an applied torque. This increased stiffness appears to be due to differences in cytoskeletal structure, as demonstrated by an increase in F-actin within the submembrane region of the BM neutrophils and by the ability of cytochalasin D to reduce the stiffness to that of circulating neutrophils. The increase in F-actin is not due to differential activation of BM compared with circulating neutrophils by the neutrophil isolation procedures, because similar differences were observed in neutrophils in whole blood or BM suspensions fixed immediately without isolation (data not shown).
Both BM and circulating neutrophils responded to inflammatory mediators by increasing their apparent stiffness. The increase was less in BM than in circulating neutrophils, and the maximum stiffness was similar in neutrophils from both sites. This fMLP-induced stiffening response was dependent on cytoskeletal reorganization but not on rearrangement of microtubules, because cytochalasin D completely prevented the increase in stiffness in neutrophils from either site, whereas colchicine had no effect.
Although neutrophils from either site responded by an increase in stiffness, the shape changes were quite different. The neutrophils from the circulating blood became markedly irregular within just 2 minutes, forming many pseudopods and numerous invaginations and irregularities in the membrane. The submembrane F-actin formed a discontinuous rim within the submembrane region. Pseudopods contained large quantities of F-actin, particularly at their periphery. In contrast, the neutrophils within the BM had fewer and smaller shape changes, with fewer irregularities of the membrane, fewer pseudopods, and a more continuous rim of F-actin. Although the mechanism for the observed difference in this response to fMLP is not clear, these data suggest the hypothesis that a more continuous rim of F-actin may result in fewer pseudopods and irregularities. Other investigators have suggested that pseudopods form at sites of weakness within the submembrane region, allowing cytoplasmic protrusions to occur.19 Our observations would support this postulate.
These studies used a previously described approach to measure the release of neutrophils from the BM. This approach involves infusing an inflammatory mediator, in this study fMLP, to induce neutrophil sequestration. Inflammatory mediators such as fMLP, C5a, and interleukin 8 induce the release of neutrophils from the BM within 5 to 7 minutes of infusion.1,2 This release from the BM results in an increase in the circulating neutrophil counts within the venous blood. Because the inflammatory stimulus induces neutrophil sequestration immediately on entry into the pulmonary microvasculature, the venous-arterial difference in circulating neutrophil counts is a measure of BM release.1 In this study, fMLP induced the release of neutrophils from the BM, as previously described.1 Pretreatment of the rats with colchicine to inhibit rearrangement did not prevent this increase in neutrophil counts within the venous blood or the decrease in the BM pool of mature neutrophils when measured morphologically. These studies suggest that release of neutrophils from the BM is not dependent on microtubular rearrangement, as measured by either the venous-arterial difference in the blood or neutrophil numbers in the BM.
The role of microtubular rearrangements in neutrophil kinetics and trafficking through uninflamed pulmonary capillaries is surprising. Microtubular rearrangements appear important in neutrophil transit through the lungs of normal rats, as demonstrated by the colchicine-induced decrease of 60% in the circulating neutrophils and increase of 94% in the number of marginated neutrophils within the pulmonary capillaries. This shift toward a larger marginated pool, presumably because of longer transit times of neutrophils when microtubular rearrangements cannot occur, was not accompanied by any change in the number of BM neutrophils. These data suggest that microtubular rearrangements are important in the normal traffic of neutrophils through the pulmonary circulation. They also suggest that the release of neutrophils from the BM is not due solely to a decrease in circulating neutrophil counts but requires some additional stimulus.
However, in contrast to the role of microtubular reassembly in normal margination and trafficking, microtubular rearrangements appear to play no role in either fMLP-induced neutrophil sequestration or in BM release when studied after either 1 or 2 doses of colchicine. Microtubular rearrangements have also been shown not to be required for neutrophil sequestration induced by infusion of complement fragments in rabbits.8 These studies contrast with previous studies from our laboratory which showed that neutrophil emigration from the pulmonary capillaries into the alveolar spaces does require microtubular rearrangement, because pretreatment with colchicine virtually completely inhibited neutrophil migration into the lungs of rabbits with pneumonia induced by instillation of Streptococcus pneumoniae.17 Therefore, the microtubular system appears to play critical roles in the normal transit of neutrophils through the pulmonary microvasculature and in emigration of neutrophils from the microvasculature into the tissue, but it is not required for either the stiffening response or the sequestration with the pulmonary capillary bed.
Neutrophils are an important aspect of host defense during acute respiratory distress syndrome and pneumonia. These acute inflammatory injuries induce the release of neutrophils from the BM. Our studies suggest that these neutrophils are stiffer and likely sequester more readily within the pulmonary capillary bed. Sato et al20 21have demonstrated that neutrophils newly released from the BM in fact do sequester better and do not emigrate as well as circulating neutrophils. Together, these studies suggest that intrinsic differences in the mechanical properties of BM neutrophils compared with circulating neutrophils may play an important role in the response of these neutrophils to inflammatory stimuli.
The initial dose of colchicine caused the circulating neutrophil counts to decrease. Over 2 hours these counts increased, and a second dose of colchicine caused no change. This dosing regimen allowed us to examine the effect of colchicine on the release of neutrophils from the BM when the circulating counts in the colchicine-pretreated and control rats were similar. Previous studies have also demonstrated this effect in rabbits.17 However, the reason for this lack of change in the circulating neutrophil counts after the second injection is unclear. Presumably, the initial dose of colchicine blocked microtubular rearrangement, causing a lengthening of the transit time of neutrophils through the pulmonary microcirculation and subsequent fall in the circulating counts. This immediate effect was followed by an increase in the circulating counts as equilibrium between the marginated and circulating pools was re-established over time. If a blocking concentration were still present 2 hours later, then the second dose of colchicine would have no effect, as observed. The circulating half-life of colchicine when used clinically in patients with gout and other problems appears to be far longer than the 2 hours required for the circulating neutrophil counts to recover in our studies, as patients receive this drug only twice a day.
The increase in F-actin observed in BM neutrophils compared with circulating ones by using either a plate reader or confocal microscopy, as well as the functional observations about F-actin assembly using cytochalasin D, contrast with the data obtained using flow cytometry in which circulating and BM neutrophils show similar quantities of F-actin. We suggest that this discrepancy may represent a technical problem in accurately quantitating F-actin by using flow cytometry. F-actin is an intracellular protein forming a dense band in the submembrane region. It appears likely that flow cytometry underestimates an increase in F-actin, because this technique is best suited to quantitate proteins expressed on the plasma membrane. This possibility is supported by the observation that flow cytometry measured only an 80% increase in F-actin on exposure of neutrophils to fMLP, whereas this increase measured 180% when measured with the use of a fluorescent plate reader.
This study demonstrates that mature neutrophils in the BM are stiffer than those in the circulating blood, most likely because of differences in the F-actin cytoskeleton. Neutrophils in either site become stiffer on exposure to fMLP or complement fragments for less than 2 minutes. The inflammatory response within the lungs or at other sites induces the release of large numbers of neutrophils, both mature and immature, from the BM into the venous circulation whereby the first capillaries they reach are the pulmonary bed. In the absence of a systemic inflammatory stimulus that produces circulating mediators, we suggest that these newly released neutrophils may have longer pulmonary transit times and may sequester with the pulmonary capillaries more readily than neutrophils that have been circulating. During localized inflammatory responses, this change in kinetics may alter their response. Whether the newly released neutrophils are more capable of inducing endothelial damage remains to be determined.
We thank Lester Kobzik and Amy Imrich for their help with the flow cytometry and the fluorescent plate reader; Kathryn Marino, Sabrina Bhagwan, and Irving Meluleni for preparation of the histologic sections; Huifang Lu for the protocol for isolating bone marrow neutrophils; and Virginia Ehrbar for preparation of the manuscript. We also thank Qin Wang and Jeffrey J. Fredberg for their help with interpretation of the data obtained using magnetic twisting cytometry and for numerous invaluable discussions.
Supported by HL 48160, HL 52446, and a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Claire M. Doerschuk, Rainbow Babies and Children's Hospital, Room 787, 11100 Euclid Ave; Cleveland, OH 44106; e-mail: cmd22@po.cwru.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal