Abstract
Combretastatin A–4-phosphate (CA-4-P) is a tubulin-binding compound currently in clinical trial as a tumor vascular-targeting agent. In endothelial cells, CA-4-P is known to cause microtubule depolymerization, but little is known about its subsequent effects on cell morphology and function. Here, we demonstrate that within minutes of endothelial cell exposure to CA-4-P, myosin light chain (MLC) was phosphorylated, leading to actinomyosin contractility, assembly of actin stress fibers, and formation of focal adhesions. These cytoskeletal alterations appeared to be a consequence of Rho activation, as they were abolished by either the Rho inhibitor C3 exoenzyme or Rho-kinase inhibitor Y-27632. In response to CA-4-P, some cells rapidly assumed a blebbing morphology in which F-actin accumulated around surface blebs, stress fibers misassembled into a spherical network surrounding the cytoplasm, and focal adhesions appeared malformed. Blebbing was associated with decreased cell viability and could be inhibited by Rho/Rho-kinase inhibitors or by blocking the CA-4-P–mediated activation of stress-activated protein kinase-2/p38. The extracellular-regulated kinases 1 and 2 (ERK-1/2) were shown to protect against blebbing since blebbing was attenuated on ERK-1/2 stimulation and was up-regulated by specific inhibition of ERK-1/2 activation. The use of MLC kinase (MLCK) and myosin adenosine triphosphatase inhibitors led us to propose a role for MLCK and myosin activity independent of MLC phosphorylation in regulating the blebbing process. CA-4-P–mediated contractility and blebbing were associated with a Rho-dependent increase in monolayer permeability to dextrans, suggesting that such functional changes may be important in the rapid response of the tumor endothelium to CA-4-P in vivo.
Introduction
The tumor endothelial network is an important target for tumor therapy and is exploited either to deliver anticancer drugs to modify the tumor microenvironment or to hinder its own development and function.1,2 Whereas antiangiogenic approaches aim to prevent the growth of the neovasculature, antivascular approaches aim to cause a rapid and catastrophic shutdown of the already existing tumor vascular system, thus leading to secondary tumor cell death.1,2 Combretastatin A–4 (CA-4), originally isolated from the South African bush Combretum caffrum, is a tubulin-binding agent that resembles colchicine in structure.3 CA-4 and its more soluble derivative CA-4-disodium-phosphate (CA-4-P)4 act as effective antivascular agents, causing the rapid and selective shutdown of animal tumor blood vessels.5-7 In contrast to colchicine and other tubulin-binding agents, CA-4-P is active well below its maximum tolerated dose and is currently in clinical trials as a tumor vascular-targeting agent.
Microtubules and microfilaments are cytoskeletal elements responsible for coordinating many cellular processes, including movement, adhesion, and contraction. Cell contractility can lead to gap formation and changes in permeability in endothelial cell monolayers.8 Endothelial contraction is attributed to the activation of actin-nonmuscle myosin interactions regulated by myosin light chain (MLC)9 and can be triggered by signals generated by growth factors and cytokines as well as stress-inducing agents.8,10 Signal-mediated transient increases in intracellular calcium lead to the activation of Ca++/calmodulin-dependent myosin light chain kinase (MLCK) that phosphorylates MLC on Thr-18 and Ser-19.9Phosphorylated MLC activates actinomyosin interactions and myosin adenosine triphosphatase (ATPase) activity, leading to increased contractility.9 MLC can also be phosphorylated by activation of the guanosine triphosphatase Rho, a known regulator of actin dynamics.11-14 On growth factor or cytokine stimulation, guanosine triphosphate–bound Rho activates the serine/threonine kinase, Rho-kinase, that phosphorylates the myosin-binding subunit of MLC phosphatase, inactivating it and thereby preventing MLC dephosphorylation.11-14 In addition, Rho-kinase has also been reported to phosphorylate MLC directly in some cell systems, thus acting as a novel MLCK enzyme.15Microtubule disruption was previously shown to activate Rho, triggering contractility and formation of stress fibers and focal adhesions, structures that link the cytoskeleton to integrins and the extracellular matrix.16-19 Actin stress fibers, which consist of bundles of actin and myosin filaments, not only have contractile properties but also contribute to the maintenance of the integrity of the endothelium by enhancing cellular adhesion to the substratum.19-21
The extracellular-regulated kinases 1 and 2 (ERK-1/2) and the stress-activated protein kinases (SAPK), members of the mitogen-activated protein kinase (MAPK) family interact with cytoskeletal targets in many cells, including endothelial cells.22-25 In particular, SAPK-2/p38 was shown to mediate changes in actin dynamics, leading to stress fiber formation in endothelial cells by activation of MAPKAP kinase 2-3, that phosphorylates heat shock protein 27 (HSP27) and releases its inhibitory function on F-actin polymerization.23,24Whereas SAPK-2/p38 is generally thought to transduce signals generated by stressing and proapoptotic agents, ERKs generate signals that promote growthlike and prosurvival responses.26
Rearrangements of the actin cytoskeleton and myosin force generation are involved in dynamic apoptotic membrane blebbing that occurs during the execution phase of apoptosis.27,28 “Early” membrane blebbing, which shares some cytoskeletal features of apoptotic blebbing, has been described to occur immediately after exposure of cells to stressing agents, and, thus, long before hallmarks of apoptosis would be expected to become apparent.25 29 Early membrane blebbing as well as apoptotic blebbing in the endothelium could have serious implications on monolayer integrity and barrier function.
The purpose of this study was to examine the mechanisms and signaling pathways involved in the effects of CA-4-P on the cytoskeleton of human endothelial cells. We report that CA-4-P–induced microtubule breakdown caused Rho/Rho-kinase–mediated reorganization of actin, leading to actinomyosin contractility, assembly of stress fibers and focal adhesions, and, under primarily postconfluent conditions, early membrane blebbing. Early membrane blebbing shared cytoskeletal features with apoptotic blebbing but was associated with necrosis rather than the onset of an apoptotic pathway. We provide evidence suggesting that early membrane blebbing resulted from the F-actin polymerization activity generated by Rho/Rho-kinase and was regulated by activated SAPK-2/p38 and ERKs as well as by MLCK and myosin activity. We further show that endothelial contraction and membrane blebbing were linked with increased permeability to macromolecules.
Materials and methods
Materials
CA-4-P was obtained from OXiGENE (Watertown, MA) and CA-4-Ptrans-isomer (t-CA-4-P) was kindly provided by Dr G. R. Pettit (Arizona State University, Tempe, AZ).4 Rho-kinase inhibitor Y-27632 was supplied by the Welfide Corporation (Osaka, Japan).30 Cytochalasin D, 2,3-butanedione 2-monoxime (BDM), phorbol-12,13-dibutyrate (PDBu), SB203580, PD98059, ML-7, ML-9, and HA1077 were purchased from Calbiochem (Nottingham, United Kingdom). Taxol was from Bristol-Myers Squibb Pharmaceuticals (Hounslow, United Kingdom). Antiphosphospecific MLC rabbit polyclonal antibody was a generous gift from Dr James Staddon, (EISAI London Research Laboratories, United Kingdom).31 Anti–β-tubulin monoclonal, (clone TUB.2.1), anti–ERK-1/2 rabbit polyclonal, antiactivated (diphosphorylated) ERK1/2 monoclonal, antihuman vinculin monoclonal (clone hVIN-1), fibronectin, heparin, and mammalian protease inhibitor cocktail were from Sigma (Poole, United Kingdom). Anti–phosphospecific-SAPK-2/p38 rabbit polyclonal was from New England Biolabs (Hitchin, United Kingdom). Recombinant C3 exoenzyme ofClostridium botulinum was expressed in Escherichia coli BL21 as a glutathione-S-transferase fusion protein from a pGEX-2T plasmid vector, kindly provided by Dr Michael Seckl (Imperial College, London, United Kingdom).32 Expressed protein was purified on glutathione Sepharose beads (Amersham-Pharmacia Biotech, Little Chalfont, United Kingdom) and released by cleavage with α-thrombin (ERL, Swansea, United Kingdom) as described.33
Cell culture
Human umbilical vein endothelial cells (HUVECs) from pooled donors (TCS CellWorks, Botolph Clayton, United Kingdom) were grown on gelatin-coated culture dishes in M199 supplemented with 20% fetal calf serum, 4 mM L-glutamine, 20 μg/mL endothelial cell growth supplement, and 80 μg/mL heparin. Confluent monolayers were obtained by plating cells at a density of 104 cells/cm2 with medium changes every 24 hours for 4 to 6 days. To obtain sparse cultures, cells were plated at 5.0 × 103 cells/cm2 and were used 48 hours after plating. Cells were used between the first and fourth passages. Cell viability was evaluated by trypan blue exclusion assay by using trypsinized cells or by monitoring propidium iodide (PI) uptake by cells in situ via fluorescence microscopy.
F-actin visualization and immunofluorescence microscopy
Cells were cultured on Permanox Lab-Tek chamber slides (Life Technologies, Paisley, United Kingdom) coated with 10 μg/mL human fibronectin. To visualize F-actin, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline, permeabilized with 0.1% Triton X-100, incubated with 5 U/mL Texas Red–conjugated phalloidin (Molecular Probes, Leiden, The Netherlands), and mounted in Vectashield with DAPI (Vector Laboratories, Peterborough, United Kingdom). Immunofluorescence localization of vinculin or phosphorylated MLC was performed simultaneously with F-actin staining. Primary antibodies were followed by sequential incubations with biotin-labeled antimouse/antirabbit immunoglobulin G and fluorescein isothiocyanate (FITC)–labeled avidin D (Vector Laboratories), the latter added together with phalloidin. For microtubule staining, cells were fixed in methanol for 20 minutes at −20°C and incubated with anti–β-tubulin antibody. Fluorescence and phase contrast images were taken with a Nikon Eclipse TE200 inverted microscope and a cooled CCD camera (Cohu, San Diego, CA) and processed by using Adobe Photoshop software. Quantification of blebbing was performed by counting such phalloidin-stained cells in 9 random microscope fields and expressing these counts as a proportion of total DAPI-stained nuclei in identical fields.
Assessment of endothelial microtubule sensitivity to CA-4-P–mediated disassembly
When cells are rapidly lysed using Pipes-EGTA–based buffers containing detergents, in vivo assembled cytoplasmic microtubules remain preserved in their polymerized state and are detergent insoluble.34 A differential extraction procedure of detergent-soluble and insoluble tubulin was used to assess the sensitivity of endothelial microtubules to disassembly by CA-4-P. Cells were extracted for 3 minutes at 30°C in lysis buffer (80 mM Pipes-KOH, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 0.2% TritonX-100, 10% glycerol, containing protease inhibitor cocktail) as described previously.35 Supernatants containing detergent-soluble tubulin and the detergent-insoluble polymerized cytoskeleton extracted with 1% sodium dodecylsulfate were suspended in Laemmli sample buffer36 and analyzed by Western blotting for β-tubulin.
Western blotting analysis
Cell lysates were prepared as described before.37Equal amounts of protein (Bio-Rad microassay system; Bio-Rad, Hemel Hempstead, United Kingdom) were separated on Novex Tris-Glycine gels (Invitrogen, Groningen, The Netherlands) and transferred to nitrocellulose membranes, and immunoreactive bands were visualized by enhanced chemiluminescence (Amersham-Pharmacia Biotech).
Measurement of endothelial permeability
Diffusion of FITC-coupled dextran (mean mol wt 40 kd) through endothelial monolayers was determined as previously described.38 Cells were plated on fibronectin-coated culture inserts (3-μm pore size; 0.3 cm2) set into 24-well companion plates (BD Biosciences, Oxford, United Kingdom) and grown to confluency for 6 days. At the start of the experiment cells were preincubated with specific inhibitors, followed by CA-4-P for a further 15 minutes before medium in the upper compartment was replaced with FITC-coupled dextran. Samples were collected from the lower compartment, and fluorescence was monitored in an LS30 Fluorimeter (Perkin Elmer). Results were expressed as a percentage of fluorescence passing through monolayers disrupted with 5 mM EGTA.
Statistical analysis
Data were analyzed by using a standard analysis of variance, followed by the Tukey-Kramer honest significance difference test for multiple comparisons (JMP Statistics for the Apple Macintosh). A value of P < .05 was considered significant.
Results
CA-4-P disrupts the endothelial microtubule cytoskeleton and mediates changes in endothelial cell morphology
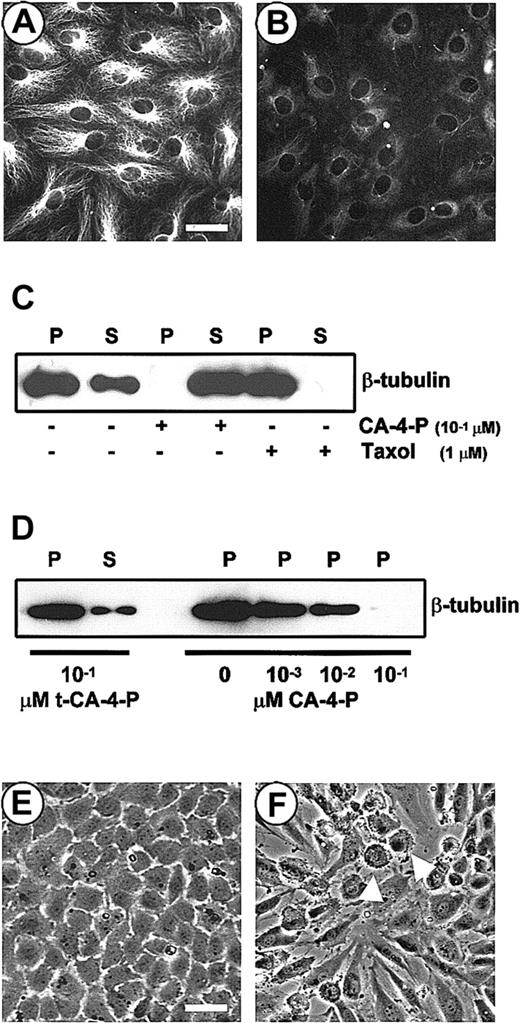
The first phase of experiments focused on the effects of CA-4-P on endothelial cell microtubules. Although the precise mechanism by which CA-4-P blocks tubulin polymerization remains unclear, one hypothesis is that CA-4-P associates with tubulin dimers and renders them incompetent for assembly.39 Immunofluorescence staining confirmed that 1 μM CA-4-P caused the disruption of microtubules within 30 minutes (Figure 1A,B).40 Microtubule sensitivity to CA-4-P was evaluated by exposing cells to varying amounts of CA-4-P or the microtubule-stabilizing agent Taxol as a control.41 Cytoskeletal detergent-insoluble polymerized (P) or dimeric detergent-soluble (S) fractions were differentially extracted and analyzed by Western blotting. As shown in Figure 1C, 0.1 μM CA-4-P mediated the complete disruption of microtubules evidenced by the absence of polymerized tubulin (P) and an increase in the soluble form (S). Taxol, as expected, caused complete tubulin polymerization (Figure 1C), whereas the inactive t-CA-4-P that does not bind tubulin failed to induce any significant effect (Figure 1D). A detectable decrease in polymerized tubulin was obtained with as low as 1 nM CA-4-P (Figure 1D). These effects of CA-4-P were paralleled by changes in morphology, as the cobblestone monolayer (Figure 1E) was extensively and rapidly disrupted, cells retracted, and intercellular gaps were formed (Figure 1F). In addition, some cells assumed a “blebbing morphology” (Figure 1F).
CA-4-P disrupts the endothelial microtubule cytoskeleton and alters endothelial cell morphology.
(A,B) Immunostaining of endothelial cells with an anti–β-tubulin antibody before (A) and after (B) exposure to CA-4-P (1 μM, 30 minutes). Bar = 60 μm. (C,D) Western blotting analysis of soluble (S) and polymeric (P) tubulin fractions. Cells were exposed to either CA-4-P (30 minutes) or Taxol (30 minutes; C) or t-CA-4-P (30 minutes) or indicated amounts of CA-4-P (30 minutes; D). Equal aliquots of protein (30 μL) were run into the gel. (E,F) Phase contrast images of endothelial cells before (E) or after (F) exposure to CA-4-P (1 μM, 30 minutes). Arrowheads indicate blebbing cells. Bar = 90 μm.
CA-4-P disrupts the endothelial microtubule cytoskeleton and alters endothelial cell morphology.
(A,B) Immunostaining of endothelial cells with an anti–β-tubulin antibody before (A) and after (B) exposure to CA-4-P (1 μM, 30 minutes). Bar = 60 μm. (C,D) Western blotting analysis of soluble (S) and polymeric (P) tubulin fractions. Cells were exposed to either CA-4-P (30 minutes) or Taxol (30 minutes; C) or t-CA-4-P (30 minutes) or indicated amounts of CA-4-P (30 minutes; D). Equal aliquots of protein (30 μL) were run into the gel. (E,F) Phase contrast images of endothelial cells before (E) or after (F) exposure to CA-4-P (1 μM, 30 minutes). Arrowheads indicate blebbing cells. Bar = 90 μm.
CA-4-P stimulates actin stress fiber formation and membrane blebbing and increases monolayer permeability via Rho/Rho-kinase
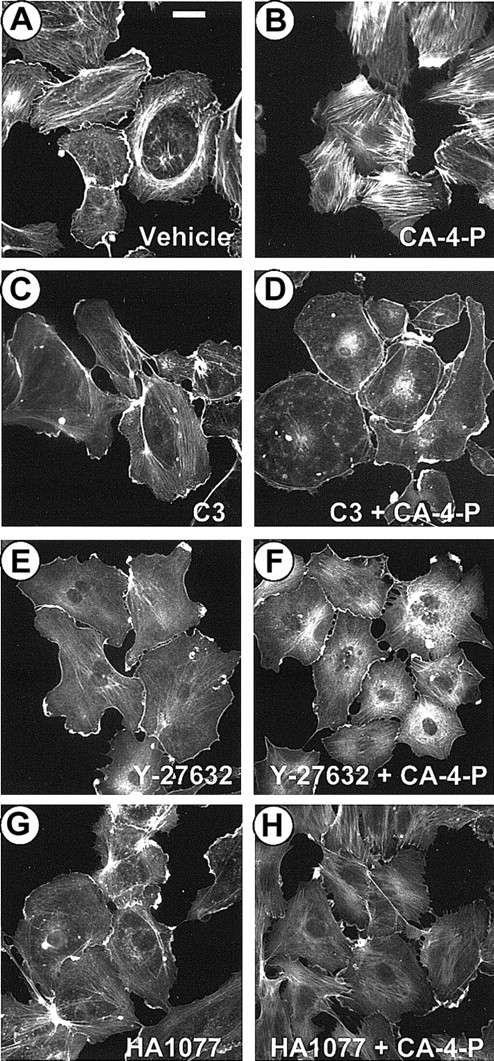
CA-4-P–mediated morphologic changes are indicative of actin cytoskeleton alterations, and the next phase of experiments focused on F-actin distribution analysis. Sparse cultures were exposed to CA-4-P, fixed, and stained with Texas Red–conjugated phalloidin. F-actin in control cells was concentrated in peripheral bands and along the cell margin, forming ruffles with few fine filaments traversing the cells (Figure 2A). CA-4-P led to the formation of abundant actin stress fibers across the cell body by 30 minutes (Figure 2B). A concentration of 1 μM CA-4-P induced optimal stress fiber formation within 30 to 60 minutes; therefore, these conditions were used for most subsequent experiments. The inactive t-CA-4-P (0.1-10 μM) failed to induce any significant actin reorganization (data not shown). To determine whether the actin response to CA-4-P was due to the activation of Rho and Rho-kinase, cells were pretreated with the selective Rho inhibitor C3 exoenzyme from Clostridium botulinum33 (24 hours, 10 μg/mL; Figure 2C,D) or Rho-kinase inhibitors Y-2763230 (30 minutes, 10 μM; Figure 2E,F) and HA107742 (30 minutes, 10 μM; Figure2G,H) before CA-4-P addition. All inhibitors reduced microfilament assembly in resting cells to a small extent and completely blocked CA-4-P–mediated actin stress fiber formation. C3 blocked all actin polymerization activity, whereas Rho-kinase inhibitors allowed some polymerization to occur (Figure 2F,H). These data indicate that CA-4-P induced these cytoskeletal rearrangements through activation of Rho and Rho-kinase.
CA-4-P stimulates actin stress fiber formation via Rho/Rho-kinase.
Sparsely plated endothelial cells were pretreated with either vehicle control (30 minutes; A,B) or C3 exoenzyme (24 hours, 10 μg/mL; C,D) or Y-27632 (30 minutes, 10 μM; E,F) or HA1077 (30 minutes, 10 μM; G,H) and then were exposed to 1 μM CA-4-P for 30 minutes (B,D,F,H). Actin was stained with Texas Red–conjugated phalloidin as described in “Materials and methods,” and cells were examined by fluorescence microscopy. Bar = 30 μm.
CA-4-P stimulates actin stress fiber formation via Rho/Rho-kinase.
Sparsely plated endothelial cells were pretreated with either vehicle control (30 minutes; A,B) or C3 exoenzyme (24 hours, 10 μg/mL; C,D) or Y-27632 (30 minutes, 10 μM; E,F) or HA1077 (30 minutes, 10 μM; G,H) and then were exposed to 1 μM CA-4-P for 30 minutes (B,D,F,H). Actin was stained with Texas Red–conjugated phalloidin as described in “Materials and methods,” and cells were examined by fluorescence microscopy. Bar = 30 μm.
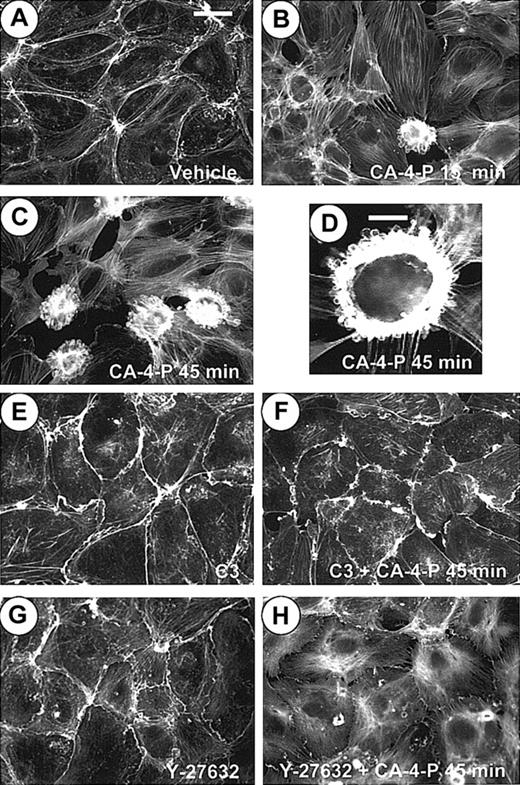
The response of confluent monolayers (Figure3A) to CA-4-P was heterogeneous. Many cells formed stress fibers and retracted, and intercellular gaps were formed (Figure 3B,C). In addition, there were regions where cells assumed a “blebbing” morphology. In these rounded cells, instead of forming transcytoplasmic stress fibers, F-actin accumulated as a dense spherical band separating the blebs from the cytoplasm and also collected in the perimeter of surface blebs (Figure 3B-D). Blebbing was quantified, and data from all subsequent experiments are summarized in Figure 4. Time course studies revealed that blebbing was complete within 1 hour of treatment (not shown) when it was evident in 16% ± 4.5% of cells (Figure 4). C3 exoenzyme (Figure 3F) or Y-27632 (Figure 3H) completely blocked stress fiber formation and also blocked the appearance of blebbing cells, suggesting that actin reorganization generated by Rho/Rho-kinase is a prerequisite for blebbing (Figure 4). It should be noted that some ruffling, an indication of Rac activation, appeared in the presence of C3 (Figure3E,F) and is likely to be an indirect effect of inhibiting Rho and independent of CA-4-P, as it did not occur when cells were exposed to Y-27632 and CA-4-P. A very low concentration of cytochalasin D (10 nM), just sufficient to inhibit microfilament assembly without affecting the filaments in uninduced cells, blocked the formation of blebs, further confirming the role of actin reorganization (images not shown; Figure4).
CA-4-P stimulates early membrane blebbing in confluent endothelial cells by Rho/Rho-kinase.
Confluent endothelial cultures were pretreated with either vehicle control (A-D) or C3 exoenzyme (24 hours, 10 μg/mL; E,F) or Y-27632 (30 minutes, 10 μM; G,H) and then were exposed to 1 μM CA-4-P for either 15 minutes (B) or 45 minutes (C,D,F,H). Actin was stained with Texas Red–conjugated phalloidin as described in “Materials and methods,” and cells were examined by fluorescence microscopy. Bars for panels A-C and panels E-H = 40 μm. Panel D represents a higher magnification of a characteristic blebbing cell. Bar for panel D = 15 μm.
CA-4-P stimulates early membrane blebbing in confluent endothelial cells by Rho/Rho-kinase.
Confluent endothelial cultures were pretreated with either vehicle control (A-D) or C3 exoenzyme (24 hours, 10 μg/mL; E,F) or Y-27632 (30 minutes, 10 μM; G,H) and then were exposed to 1 μM CA-4-P for either 15 minutes (B) or 45 minutes (C,D,F,H). Actin was stained with Texas Red–conjugated phalloidin as described in “Materials and methods,” and cells were examined by fluorescence microscopy. Bars for panels A-C and panels E-H = 40 μm. Panel D represents a higher magnification of a characteristic blebbing cell. Bar for panel D = 15 μm.
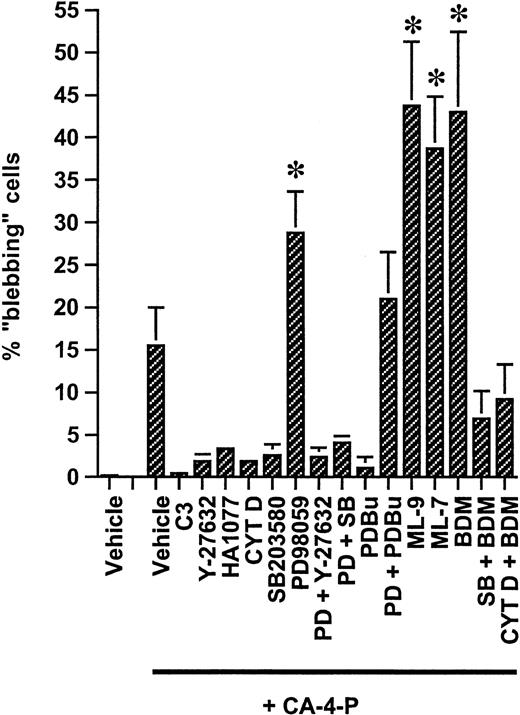
Regulation of CA-4-P–induced membrane blebbing.
Confluent endothelial cultures grown on Permanox slides were pretreated with either C3 exoenzyme (24 hours, 10 μg/mL) or for 30 minutes with all other specified inhibitors/activators used either singly or in combination at the following concentrations: Y-27632, 10 μM; HA1077, 10 μM; cytochalasin D, 10 nM; SB203580, 10 μM; PD98059, 25 μM; PDBu, 200 nM; ML-7, 10 μM; ML-9, 10 μM; and BDM, 5 mM. Cells were then exposed to CA-4-P (60 minutes, 1 μM), stained with Texas Red–conjugated phalloidin, and mounted in Vectashield with DAPI. Blebbing was quantified by counting phalloidin-stained blebbing cells in 9 random microscope fields per treatment and expressing these as a proportion of total numbers of DAPI-stained nuclei in identical fields. Values are means ± SD from 4 independent experiments using HUVEC cultures at passages 2 to 3. *P < .05 compared with vehicle + CA-4-P.
Regulation of CA-4-P–induced membrane blebbing.
Confluent endothelial cultures grown on Permanox slides were pretreated with either C3 exoenzyme (24 hours, 10 μg/mL) or for 30 minutes with all other specified inhibitors/activators used either singly or in combination at the following concentrations: Y-27632, 10 μM; HA1077, 10 μM; cytochalasin D, 10 nM; SB203580, 10 μM; PD98059, 25 μM; PDBu, 200 nM; ML-7, 10 μM; ML-9, 10 μM; and BDM, 5 mM. Cells were then exposed to CA-4-P (60 minutes, 1 μM), stained with Texas Red–conjugated phalloidin, and mounted in Vectashield with DAPI. Blebbing was quantified by counting phalloidin-stained blebbing cells in 9 random microscope fields per treatment and expressing these as a proportion of total numbers of DAPI-stained nuclei in identical fields. Values are means ± SD from 4 independent experiments using HUVEC cultures at passages 2 to 3. *P < .05 compared with vehicle + CA-4-P.
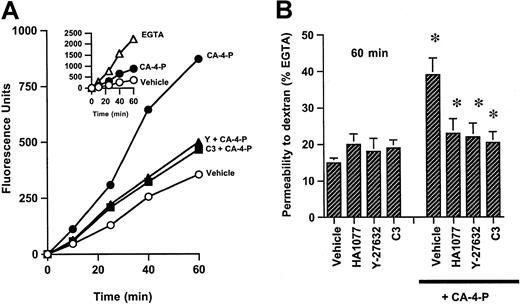
Endothelial cell contraction, membrane blebbing, and intercellular gap formation would be expected to increase endothelial permeability to macromolecules.8 Indeed, CA-4-P induced a time-dependent increase in transendothelial diffusion of dextran compared with control monolayers (Figure 5). C3 exoenzyme, Y-27632, or HA1077 inhibited the passage of dextran, suggesting that activation of Rho/Rho-kinase is critical in mediating effects on permeability (Figure 5). It should be noted that some gaps were still evident in monolayers treated with inhibitors plus CA-4-P (Figure 3H), whereas permeability appeared to be blocked under similar conditions. Therefore, the sensitivity of the assay may not fully reflect the absolute extent of changes in permeability. The ability of CA-4-P to increase monolayer permeability correlates with its ability to induce membrane blebbing, and these data are in agreement with previous studies suggesting that thrombin-induced endothelial permeability changes were due to cytoskeletal alterations causing retraction and rounding of cells.38
CA-4-P induces an increase in endothelial monolayer permeability to dextrans.
(A) Confluent endothelial monolayers grown on transwell filters were treated with vehicle control (○), CA-4-P (15 minutes, 1 μM) (●), C3 exoenzyme (24 hours, 10 μg/mL) followed by CA-4-P (15 minutes, 1 μM) (▴), and Y-27632 (30 minutes, 10 μM) followed by CA-4-P (▪). Data for inhibitors alone are not plotted and were not significantly different from inhibitors plus CA-4-P. Medium was replaced with FITC-dextran, and its passage through the filters was monitored at time intervals for 1 hour. In inset, passage of dextran through parallel cultures exposed to EGTA (5 mM, 15 minutes) (▵) is compared with vehicle (○) and CA-4-P (●). (B) Values taken at 60-minute time point in panel A are expressed as the percentage of fluorescent dextran passing through monolayers exposed to EGTA (see inset, 60-minute time point). Preincubation with HA1077 was for 30 minutes at 10 μM. Inhibitors were followed by either 15 minutes of vehicle control or 15 minutes of 1 μM CA-4-P. Mean values ± SEM were obtained from 3 separate filters from one of 3 representative experiments. *P < .05 for CA-4-P compared to vehicle or for inhibitors + CA-4-P compared to CA-4-P vehicle.
CA-4-P induces an increase in endothelial monolayer permeability to dextrans.
(A) Confluent endothelial monolayers grown on transwell filters were treated with vehicle control (○), CA-4-P (15 minutes, 1 μM) (●), C3 exoenzyme (24 hours, 10 μg/mL) followed by CA-4-P (15 minutes, 1 μM) (▴), and Y-27632 (30 minutes, 10 μM) followed by CA-4-P (▪). Data for inhibitors alone are not plotted and were not significantly different from inhibitors plus CA-4-P. Medium was replaced with FITC-dextran, and its passage through the filters was monitored at time intervals for 1 hour. In inset, passage of dextran through parallel cultures exposed to EGTA (5 mM, 15 minutes) (▵) is compared with vehicle (○) and CA-4-P (●). (B) Values taken at 60-minute time point in panel A are expressed as the percentage of fluorescent dextran passing through monolayers exposed to EGTA (see inset, 60-minute time point). Preincubation with HA1077 was for 30 minutes at 10 μM. Inhibitors were followed by either 15 minutes of vehicle control or 15 minutes of 1 μM CA-4-P. Mean values ± SEM were obtained from 3 separate filters from one of 3 representative experiments. *P < .05 for CA-4-P compared to vehicle or for inhibitors + CA-4-P compared to CA-4-P vehicle.
Regulation of membrane blebbing by SAPK2/p38 and ERK-1/2 MAPKs
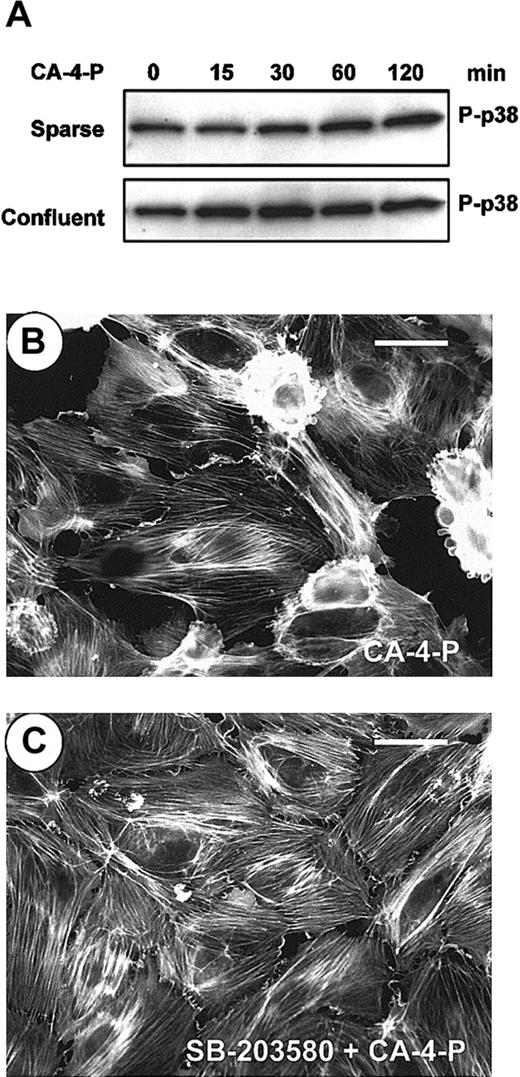
Stress-inducing agents can activate SAPK2/p38, leading to F-actin reorganization and stress fiber formation by HSP27 phosphorylation in endothelial cells.23-25 Under conditions in which ERKs were insufficiently coactivated, SAPK2/p38 led to intense membrane blebbing.25 The possible contribution of SAPK2/p38 and ERK pathways toward stress fiber formation and blebbing was investigated. CA-4-P led to a weak activation of SAPK2/p38, mainly in confluent endothelial cells in which activation peaked at 30 minutes (Figure6A). The SAPK-2/p38 inhibitor SB203580 did not alter the pattern of stress fibers in either sparse (data not shown) or confluent cultures (Figure 6C). However, inhibition of SAPK2/p38 almost abolished CA-4-P–mediated blebbing in confluent cells compared with CA-4-P alone (Figure 6B,C; Figure 4). These data suggest that SAPK2/p38 contributes toward blebbing but does not form stress fibers. The fact that stress fibers are expressed by most cells when SAPK-2/p38 was inhibited suggests that blebbing represents a misassembly of the stress fiber formation.
SAPK2/p38 is activated by CA-4-P and regulates membrane blebbing.
(A) Sparse or confluent endothelial cultures were treated with 1 μM CA-4-P, cell lysates were harvested, and equal amounts of protein were analyzed by Western blotting for phospho–SAPK-2/p38 (P-p38). (B,C) Confluent endothelial cells were preincubated with vehicle control (B) or SB203580 (30 minutes, 10 μM; C) and then were exposed to CA-4-P (60 minutes, 1 μM). Cells were stained for actin with Texas Red–conjugated phalloidin as described in “Materials and methods” section and were examined by fluorescence microscopy. Bar = 35 μm.
SAPK2/p38 is activated by CA-4-P and regulates membrane blebbing.
(A) Sparse or confluent endothelial cultures were treated with 1 μM CA-4-P, cell lysates were harvested, and equal amounts of protein were analyzed by Western blotting for phospho–SAPK-2/p38 (P-p38). (B,C) Confluent endothelial cells were preincubated with vehicle control (B) or SB203580 (30 minutes, 10 μM; C) and then were exposed to CA-4-P (60 minutes, 1 μM). Cells were stained for actin with Texas Red–conjugated phalloidin as described in “Materials and methods” section and were examined by fluorescence microscopy. Bar = 35 μm.
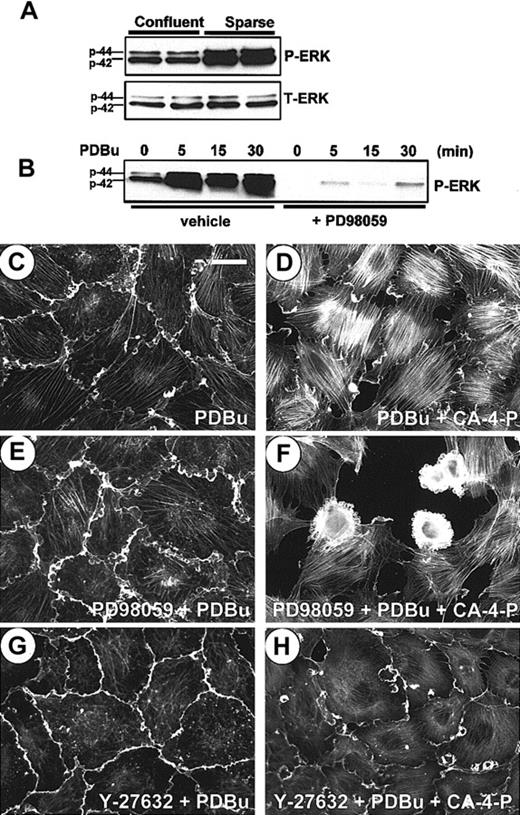
In both sparse and confluent cells CA-4-P failed to significantly alter levels of active ERKs, as deduced by Western blotting of phosphorylated ERK-1/2 (not shown). PD98059, an inhibitor of ERK-1/2 activation, down-regulated basal levels of active ERK-1/2 in sparse and confluent cells (data not shown and Figure 7B, respectively). PD98059 induced membrane blebbing in response to CA-4-P in approximately 7% of sparse cells (not shown) and up-regulated blebbing in confluent cells from 16% to 29% (Figure 4). PD98059 failed to induce any blebbing in response to CA-4-P if Rho-kinase was inhibited, further reinforcing the role of Rho/Rho-kinase in driving the blebbing process (Figure 4). Similarly exposure to PD98059 together with SB203580 did not increase the proportion of blebbing cells in response to CA-4-P compared with SB203580 alone (Figure 4), suggesting that SAPK-2/p38 activity is crucial in driving the blebbing process. Neither PD98059 nor SB203580 caused membrane blebbing in the absence of CA-4-P.
Regulation of membrane blebbing by ERK-1/2.
(A) Cell lysates were harvested 18 hours after the last medium change from 2 independent endothelial cultures grown either sparsely or confluent, and equal amounts of protein (40 μg) were analyzed for phosphorylated ERK-1/2 (P-ERK) and total ERK-1/2 (T-ERK). (B) Confluent endothelial cells were preincubated with either vehicle control or PD98059 (30 minutes, 25 μM) and then exposed to PDBu (200 nM) for the indicated times. Cell lysates were analyzed for P-ERK activity as in panel A. (C-H) Confluent endothelial cells grown on Permanox slides were preincubated with either PDBu (15 minutes, 200 nM; C,D) or PD98059 (30 minutes, 25 μM), followed by PDBu (15 minutes, 200 nM; E,F) or Y-27632 (30 minutes, 10 μM), followed by PDBu (15 minutes, 200 nM; G,H). Cells in panels D, F, and H were then treated with CA-4-P (30 minutes, 1 μM) and stained for actin. Bar = 40 μm.
Regulation of membrane blebbing by ERK-1/2.
(A) Cell lysates were harvested 18 hours after the last medium change from 2 independent endothelial cultures grown either sparsely or confluent, and equal amounts of protein (40 μg) were analyzed for phosphorylated ERK-1/2 (P-ERK) and total ERK-1/2 (T-ERK). (B) Confluent endothelial cells were preincubated with either vehicle control or PD98059 (30 minutes, 25 μM) and then exposed to PDBu (200 nM) for the indicated times. Cell lysates were analyzed for P-ERK activity as in panel A. (C-H) Confluent endothelial cells grown on Permanox slides were preincubated with either PDBu (15 minutes, 200 nM; C,D) or PD98059 (30 minutes, 25 μM), followed by PDBu (15 minutes, 200 nM; E,F) or Y-27632 (30 minutes, 10 μM), followed by PDBu (15 minutes, 200 nM; G,H). Cells in panels D, F, and H were then treated with CA-4-P (30 minutes, 1 μM) and stained for actin. Bar = 40 μm.
We sought to evaluate further a possible correlation between ERK activity and blebbing. As confluence was previously shown to down-regulate ERK activity in murine lung endothelial cells,37 we analyzed extracts from sparse and confluent HUVECs for active ERK-1/2 levels. Higher levels of active ERK-1/2 were present in 2 independent sparse cultures compared with the same cultures grown to confluency (Figure 7A). As phorbol esters can activate ERK-1/2 by a protein kinase C (PKC)–mediated activation of Raf-1/MEK (MAPK/ERK kinase),43 we evaluated their use in elevating ERK activity in confluent cells. Figure 7B demonstrates that PDBu stimulated a significant rapid and persistent increase in ERK activity that was abolished by PD98059 consistent with the requirement for its activation by MEK. PDBu led to a characteristic cortical ruffling (Figure 7C) and a strong expression of actin stress fibers in response to CA-4-P accompanied by a total absence of blebbing cells (Figure 7D). PD98059 added before PDBu allowed blebbing cells to form in response to CA-4-P, indicating that PDBu protected against blebbing through its capacity to activate ERKs (Figure 7F and Figure 4for summary). Stress fiber formation in the presence of PDBu was primarily due to Rho-kinase activation and not because of other cytoskeletal effects because Y-27632 pretreatment prevented stress fiber formation (Figure 7H).
Signal transduction pathways involving ERK1/2 and SAPKs have been implicated in the regulation of cell survival.26 As ERKs and SAPK-2/p38 were found to modulate blebbing, this finding raised the possibility that blebbing may be upstream of a cell death pathway. No apoptosis was detected in confluent cultures exposed to CA-4-P, whereas apoptotic markers were evident in rapidly proliferating cultures44 that do not normally exhibit membrane blebbing (our unpublished data, February 2001). Trypan blue exclusion analysis showed a significant increase in cell necrosis, rising from 5.5% ± 1.2% in control confluent cultures to 16.9% ± 1.6% after a 2-hour exposure to 1 μM CA-4-P. PD98059 led to a further increase in necrosis (24.2% ± 2.1%), whereas SB203580 protected against necrosis (9.8% ± 1.3%). Neither inhibitor significantly affected cell viability in the absence of CA-4-P. Analysis of PI uptake by monolayers exposed continuously to CA-4-P for 0.5 to 4 hours showed that visibly blebbing but still adherent cells remained viable and were PI negative (data not shown). However, as cells started to detach, a process that began after a 2-hour exposure to CA-4-P, uptake of PI was evident by loosely adherent but no longer distinguishable as “blebbing” cells (data not shown). It is, however, likely that it is initially blebbing cells that lose their adherence and become necrotic because continued exposure of monolayers to CA-4-P for up to 6 hours resulted in the disappearance of blebbing cells, whereas the remaining cells continued to express stress fibers. This finding correlated with a decrease in the numbers of adherent cells (data not shown). Blebbing, therefore, is likely to lead to loss of cell adherence and to represent a cytotoxic response to CA-4-P distinct from apoptosis.
Finding that ERKs may confer protective effects during exposure of cells to stressing agents such as CA-4-P by modulating the cytoskeleton may provide clues for the general protective function described for this pathway. Our data are in agreement with previous reports that suggested that early membrane blebbing induced by oxidative stress is a manifestation of cell toxicity distinct from the pathway of apoptosis.25 29
Involvement of MLC, myosin ATPase, and focal adhesions in the response of endothelial cells to CA-4-P
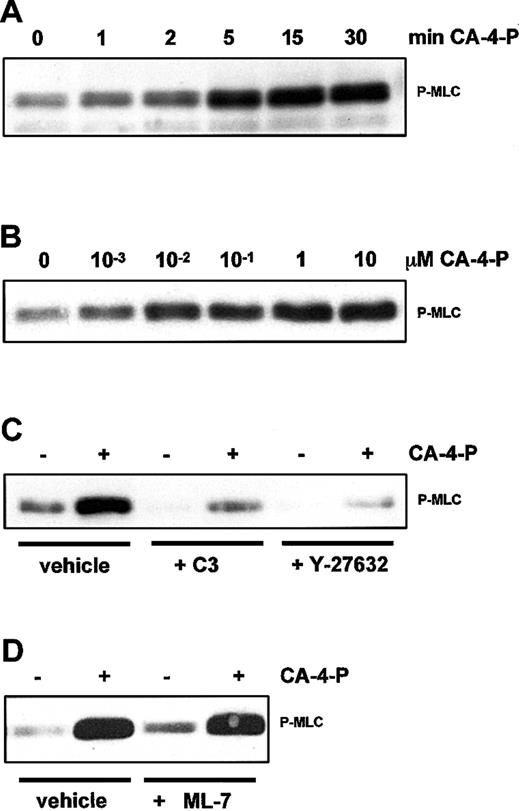
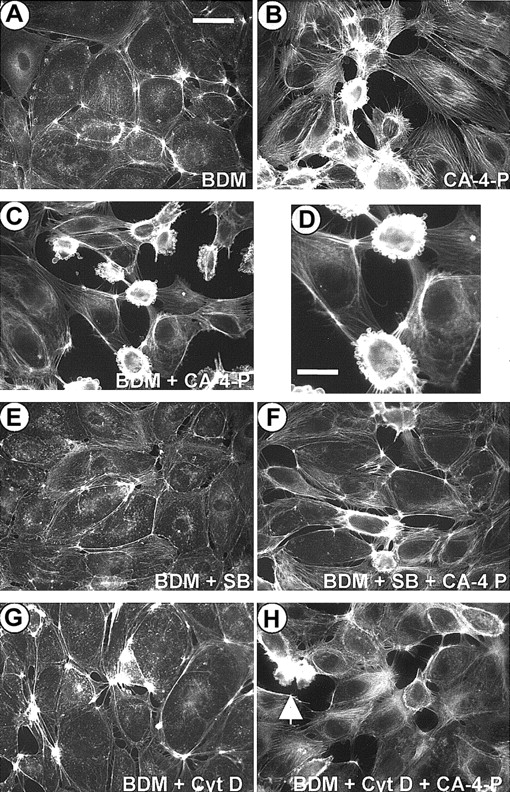
Activation of Rho/Rho-kinase would lead to inhibition of MLC phosphatase, thus shifting the balance toward phosphorylated MLC.11 To determine whether CA-4-P caused an increase in MLC phosphorylation, Western blotting analysis was performed by using an antibody to MLC phosphorylated at Thr-18 and Ser-19, the sites phosphorylated by MLCK9,31 MLC phosphorylation was evident within 1 to 5 minutes of CA-4-P, peaked at 15 minutes, remained elevated for at least 30 minutes, and was optimal at 1 μM CA-4-P (Figure 8A,B). In the presence of C3 exoenzyme or Y-27632, basal and CA-4-P–mediated MLC phosphorylation was significantly attenuated (Figure 8C), suggesting that CA-4-P leads to sequential activation of Rho, Rho-kinase, and MLC phosphorylation. Endothelial cells mainly express high molecular weight MLCK isoforms (around 214 kD) that represent alternatively spliced products of the smooth muscle MLCK.45 MLCK inhibitors such as ML-7 also inhibit endothelial MLCKs by interacting with the catalytic and myosin-binding domain of the enzyme.46 These inhibitors had no effect on CA-4-P–mediated MLC phosphorylation (Figure 8D), suggesting that Rho-kinase and not MLCK is the major kinase upstream of MLC. Additionally, double staining using anti–phospho-MLC antibody and phalloidin showed that phosphorylated MLC continued to be associated with stress fibers in the presence of ML-7, although the level of expression of stress fibers was reduced (data not shown) in accordance with previous reports on the effects of these inhibitors on stress fibers.14 Notably, ML-7 and ML-9 caused a significant increase in the proportion of blebbing cells compared with CA-4-P alone (Figure 4 for summary). Blebbing cells in the presence of ML-7 expressed high levels of phosphorylated MLC, excluding the possibility that MLC phosphorylation was blocked by MLCK inhibitors specifically in this subpopulation of cells (data not shown). Therefore, although MLCK activity appeared to attenuate blebbing, this effect was unlikely to relate to MLC phosphorylation per se. It should also be noted that inhibition of SAPK-2/p38, which abolished blebbing, failed to affect basal or CA-4-P–induced MLC phosphorylation, confirming the absence of correlation between MLC phosphorylation and blebbing (data not shown). Novel functions have been reported for smooth muscle MLCK, as it could activate myosin ATPase activity without phosphorylating MLC.47 We evaluated the contribution of myosin ATPase activity by using the myosin ATPase inhibitor BDM.48 As expected, BDM reduced stress fiber formation in response to CA-4-P but also led to a significant increase in numbers of blebbing cells, indicating the need for myosin activity in maintaining cells in a nonblebbing state (Figure 9 and Figure4). Interestingly, blebbing because of BDM and CA-4-P was markedly inhibited if SAPK-2/p38 was blocked (Figure 9E,F and Figure 4). This observation further supports that SAPK-2/p38 plays an instrumental role in driving the blebbing process. Furthermore, blebbing because of BDM plus CA-4-P was also markedly inhibited by cytochalasin D, suggesting that BDM-induced blebbing relies on increased actin polymerization (Figure 9G,H and Figure 4).
CA-4-P activates MLC phosphorylation via Rho-kinase.
(A,B) Confluent endothelial cells were exposed to 1 μM CA-4-P (A) or 30 minutes CA-4-P (B). Cells in panels C and D were preincubated with vehicle, C3 exoenzyme (24 hours, 10 μg/mL), Y-27632 (30 minutes, 10 μM), or ML-7 (30 minutes, 10 μM) before exposure to CA-4-P (30 minutes, 1 μM). Equal amounts of cell proteins (30 μg) were analyzed by Western blotting for di-phosphorylated MLC (P-MLC).
CA-4-P activates MLC phosphorylation via Rho-kinase.
(A,B) Confluent endothelial cells were exposed to 1 μM CA-4-P (A) or 30 minutes CA-4-P (B). Cells in panels C and D were preincubated with vehicle, C3 exoenzyme (24 hours, 10 μg/mL), Y-27632 (30 minutes, 10 μM), or ML-7 (30 minutes, 10 μM) before exposure to CA-4-P (30 minutes, 1 μM). Equal amounts of cell proteins (30 μg) were analyzed by Western blotting for di-phosphorylated MLC (P-MLC).
Inhibition of myosin ATPase blocks contractility and up-regulates blebbing.
Confluent endothelial cells were preincubated with either vehicle control (DMSO; B) or BDM (5 mM, 30 minutes; A,C-H). In panels E and F, cells were preincubated with SB203580 (10 μM, 30 minutes) together with BDM and in panels G and H with cytochalasin D (10 nM, 30 minutes) together with BDM. Then cells were exposed to CA-4-P (1 μM, 60 minutes; B,C,D,F,H) and fixed and stained with Texas Red phalloidin. Panel D represents a higher magnification of cells in panel C. Bar for panels A-C and panels E-F = 42 μm; bar for panel D = 25 μm.
Inhibition of myosin ATPase blocks contractility and up-regulates blebbing.
Confluent endothelial cells were preincubated with either vehicle control (DMSO; B) or BDM (5 mM, 30 minutes; A,C-H). In panels E and F, cells were preincubated with SB203580 (10 μM, 30 minutes) together with BDM and in panels G and H with cytochalasin D (10 nM, 30 minutes) together with BDM. Then cells were exposed to CA-4-P (1 μM, 60 minutes; B,C,D,F,H) and fixed and stained with Texas Red phalloidin. Panel D represents a higher magnification of cells in panel C. Bar for panels A-C and panels E-F = 42 μm; bar for panel D = 25 μm.
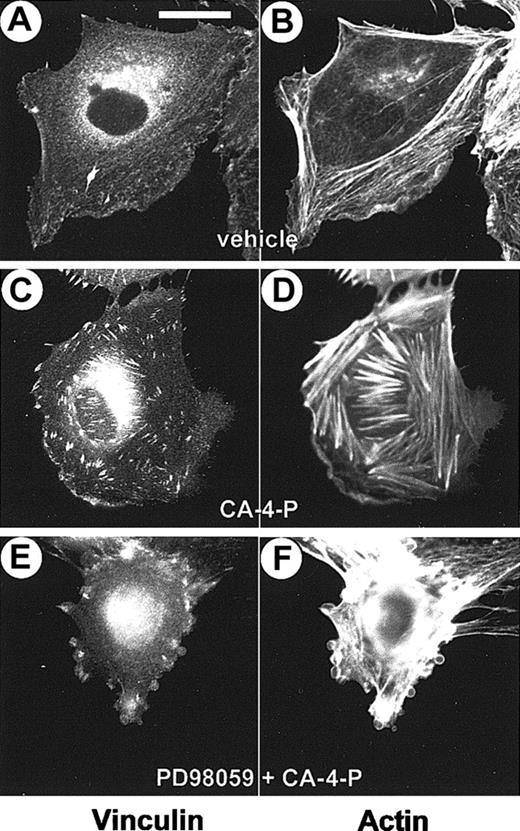
Previous studies have demonstrated that MLCK and myosin activities are necessary for focal adhesion assembly.19 49CA-4-P–mediated stress fiber formation was accompanied by the assembly of focal adhesions, visualized by double staining with phalloidin and an antivinculin antibody, respectively (Figure10). In blebbing cells the distribution and assembly of focal adhesions was found to be altered (Figure 10E,F). In these cells vinculin staining appeared mainly at the periphery of the cells at sites of membrane blebbing. These data indicate that blebbing may correlate with a defective assembly of focal adhesions.
CA-4-P induces the assembly of focal adhesions.
Sparse endothelial cells grown on Permanox slides were exposed to CA-4-P (60 minutes, 1 μM). Cells in panels E and F were pretreated with PD98059 (30 minutes, 25 μM) to induce blebbing. Cells were fixed and double stained with Texas Red phalloidin and antivinculin antibody. Panels A,C,E have vinculin staining; panels B,D,F, actin staining. Bar = 20 μm.
CA-4-P induces the assembly of focal adhesions.
Sparse endothelial cells grown on Permanox slides were exposed to CA-4-P (60 minutes, 1 μM). Cells in panels E and F were pretreated with PD98059 (30 minutes, 25 μM) to induce blebbing. Cells were fixed and double stained with Texas Red phalloidin and antivinculin antibody. Panels A,C,E have vinculin staining; panels B,D,F, actin staining. Bar = 20 μm.
Discussion
This study describes mechanisms controlling the reorganization of F-actin in response to microtubule breakdown by CA-4-P in human endothelial cells. It is generally thought that microtubule-binding agents have few cellular targets other than microtubules.50 Accordingly, trans-CA-4-P that does not interact with tubulin failed to induce tubulin or actin cytoskeletal changes. Furthermore, the extent of MLC phosphorylation and stress fiber formation correlated with the degree of microtubule disruption by CA-4-P, suggesting that microtubule breakdown is the trigger for these events. Rho and Rho-kinase were shown here to be key components of a CA-4-P–mediated signaling pathway leading to actin reorganization. Although microtubule breakdown is known to stimulate an increase in Rho activity in fibroblasts, the mechanisms involved remain poorly understood.16-18 Rho activation requires binding to guanine exchange factors that stimulate guanosine diphosphate exchange for guanosine triphosphate. Some guanine exchange factors were shown to interact with microtubules, suggesting a possible link between microtubule dynamics and Rho signaling.51-53
Rho increases contractility by a Rho-kinase–mediated increase in MLC phosphorylation and activation of actin-myosin interactions, leading to stress fiber formation in fibroblasts.12 Rho-kinase appears to be responsible for the sustained phosphorylation of MLC and stress fiber assembly in endothelial cells in response to microtubule disruption by CA-4-P. MLC phosphorylation is generally regulated by a balance between myosin phosphatase and MLC kinase(s).9,11Rho-kinase is likely to be acting here as a novel endothelial MLCK enzyme by phosphorylating MLC directly as described for other cell types,15 49 as deduced from experiments in which Rho but not MLCK inhibitors reduced basal and CA-4-P–elevated levels of MLC phosphorylation.
Here, we demonstrate for the first time early membrane blebbing that occurred as an immediate response of endothelial cells to microtubule disruption. Despite the fact that early blebbing was not associated with the onset of apoptosis, these cells appeared to share some structural cytoskeletal features described for apoptotic blebbing cells.28 Mechanisms that regulate apoptotic membrane blebbing are rather poorly understood, but actin reorganization and actin-myosin–driven force generation and cell contraction involving MLC phosphorylation are thought to play a central role.27,28 Two recent studies have further demonstrated that apoptotic membrane blebbing results from caspase 3–mediated cleavage and activation of Rho-kinase, leading to increased MLC phosphorylation and myosin force generation.54,55 In common with apoptotic blebbing, significant actin reorganization was evident in early blebbing cells. F-actin accumulated around the perimeter of surface blebs and formed a dense spherical band of fibers. Furthermore, inhibitors of Rho and Rho-kinase as well as cytochalasin D prevented CA-4-P–induced actin reorganization as well as membrane blebbing, confirming the role of actin reorganization in this process. Phosphorylated MLC was found elevated in apoptotic blebbing cells28,55 and was also associated with early blebbing cells, whereas MLC phosphorylation as well as blebbing were abolished by Rho/Rho-kinase inhibitors. The importance of MLCK-dependent MLC phosphorylation in driving apoptotic blebbing was previously suggested.28 Although MLCK inhibitors did not affect the CA-4-P–mediated MLC phosphorylation as such, they nevertheless up-regulated blebbing. This finding would imply that MLCK regulates early endothelial blebbing by a mechanism(s) that is independent of an effect on MLC phosphorylation and early membrane blebbing differs mechanistically from apoptotic blebbing. A plausible explanation for the action of MLCK inhibitors may be offered on the basis of data showing that the myosin-binding domain of MLCK, to which inhibitors such as ML-946 can bind, can activate myosin ATPase without phosphorylating MLC.47 Indeed, inhibiting myosin ATPase with BDM markedly reduced endothelial contractile structures and concurrently stimulated the blebbing response to CA-4-P, reinforcing the importance for myosin ATPase in preventing blebbing. MLCK may indeed have physiologic functions unrelated to its capacity to phosphorylate MLC as shown by recent studies on cell migration that implicate its activity on myosin ATPase.56,57 We, therefore, hypothesize that MLCK activates myosin ATPase in endothelial cells and attenuates blebbing. This hypothesis, however, appears to be in contradiction with models of apoptotic blebbing in which strong myosin activity is required for dynamic protrusion and retraction events in regions in which weak anchor points exist between the membrane and actin.28 It may be argued that other cytoskeletal changes and cleavage of substrates specific to apoptotic cells contribute to these differences. Calpains, for example, are activated by apoptotic stimuli and cleave cytoskeletal components of focal adhesions, and in such systems blebbing was shown to be inhibited by calpain inhibitors.28 Focal adhesions are sites of tight adhesion linking the membrane to the extracellular matrix and the cytoskeleton.19 They are assembled after the recruitment of signaling molecules such as paxillin and structural actin-anchoring proteins such as vinculin at sites of integrin clustering. Contractility mechanisms underlie the assembly of focal adhesions, and, generally, a decrease in myosin-mediated contractility accompanies focal adhesion disassembly.19 Recently, MLCK together with myosin ATPase activity were shown to be necessary for the targeting of active ERKs to focal adhesions, demonstrating for the first time that active ERKs are components of focal adhesions.22 Taken altogether these data may provide some plausible interpretations for our observations. Indeed strong assembly of focal adhesions accompanied the stress fiber formation in response to CA-4-P, in agreement with previous studies on other microtubule disrupting compounds in fibroblasts.16,17 Focal adhesion formation was, however, disrupted in blebbing cells that formed few such structures evident only at the cell periphery. We can, therefore, propose a model in which blebbing results from a defect in an attempt to assemble focal adhesions driven by Rho. Candidate factors that promoted blebbing and would, thus, favor focal adhesion disassembly include inhibited MLCK, myosin ATPase, and ERK activities. The higher levels of basal ERK activity found in subconfluent as opposed to postconfluent cultures may explain why sparse cells exhibited very little blebbing. Our data are in agreement with a previous study showing that insufficient activation of ERKs during HUVEC exposure to oxidative stress promoted early membrane blebbing that was accompanied by focal adhesion redistribution.25 Although PDBu used to activate ERKs could also target cytoskeletal components,58 the fact that PD98059 reversed its effects on blebbing argues in favor of an involvement of an ERK-dependent mechanism(s). PDBu-activated PKC may also target to the membrane, raising the possibility that PKC acts as a local activator of ERKs at sites of membrane contact to the substratum.58 Active ERKs can also phosphorylate and activate MLCK, so their capacity to prevent blebbing could be related to their potential to phosphorylate MLCK.59 However, such potential ERK-mediated MLCK activation is unlikely to relate to MLC phosphorylation because, when ERK activation was inhibited by PD98059 CA-4-P's ability to phosphorylate, MLC was unaffected (data not shown).
Membrane blebbing was also found to be dependent on SAPK-2/p38 activation. SAPK-2/p38 activity was only significantly elevated by CA-4-P in postconfluent cells, which may explain the low level of blebbing in sparse cultures. The primary role of SAPK-2/p38 in regulating blebbing was demonstrated by experiments in which lowering ERK activity did not induce blebbing if SAPK-2/p38 was not coactivated, and blebbing up-regulated by BDM was blocked by SAPK-2/p38 inhibitor. The mechanisms involved in SAPK-2/p38–mediated induction of blebbing remain unclear.25 SAPK-2/p38 may increase actin polymerization and stability via effects of MAPKAP kinase 2-3 on HSP27.24 As inhibition of either Rho or SAPK-2/p38 virtually abolishes blebbing, this finding suggests that both these pathways are important. High levels of HSP27 were previously found localized at the surface of blebbing endothelial cells,25and Rho can mediate the translocation of HSP27 to the membrane of smooth muscle cells.60 On the basis of this finding we may speculate that a Rho-dependent translocation of HSP27 to the membrane promotes localized SAPK-2/p38–dependent actin polymerization. SAPK-2/p38 could also phosphorylate other substrates, including focal adhesion components.61 SB203580, used here to study this pathway, was shown to have a high selectivity and specificity profile for SAPK-2/p38 in cell-based assays, but its effects on other kinases cannot be totally excluded.62
Therefore, we propose that CA-4-P activates Rho/Rho-kinase leading to MLC phosphorylation and increased actin polymerization/filament stabilization driving stress fiber and focal adhesion assembly. Under conditions in which SAPK-2/p38 is coactivated, possibly leading to further surface-localized actin polymerization/stabilization, focal adhesion formation is compromised because of insufficiently activated ERKs or weak actin-myosin interactions, and correct organization of F-actin into transcytoplasmic stress fibers is prevented, resulting in membrane blebbing.
Early membrane blebbing in endothelial cells appears to be a common response to other known Rho/stress fiber activators such as thrombin and colchicine18,63 (data not shown). Furthermore, although prominent in HUVECs and other endothelial cells such as normal and transformed microvascular endothelial cells, blebbing was not observed in either smooth muscle cells or fibroblasts in which CA-4-P induced stress fibers (data not shown). This apparent selectivity possibly reflects differences in composition and regulation of cytoskeletal systems between these cell types.63
Early membrane blebbing appears to be a manifestation of cell toxicity. In vivo endothelial blebbing was described after ischemia, oxidative injury, and inflammation.64,65 Whether CA-4-P induces blebbing in the vasculature remains purely speculative. Interestingly, colchicine was shown to induce the selective rounding and surface blebbing of endothelial cells in arterial but not ventricular valves of chick embryo hearts,66 suggesting that the degree of cell differentiation and specific microenvironment may dictate the nature of endothelial responses to such types of stressing injury in vivo. Endothelial cell contraction, retraction, and surface bleb formation could cause narrowing of the vascular lumen, an increase in vascular resistance and vascular obstruction, and reduction in cell survival, all of which could potentially contribute toward an impairment of the normal function of the microvasculature. In addition, an increase in vascular permeability, which has also been detected in an animal tumor model after CA-4-P treatment67 could contribute to rapid vascular shut down. A remaining challenge is to determine the factors that make the tumor vasculature uniquely sensitive to CA-4-P and other tubulin-binding drugs with potential as tumor vascular-targeting agents.
We thank OXiGENE for supplying the CA-4-P, the Welfide Corporation for the Rho-kinase inhibitor Y-27632, and Drs Bob Pettit, James Staddon, and Michael Seckl for their kind gifts oftrans-CA-4-P, antiphosphospecific MLC antibody, and C3 exoenzyme construct, respectively. We also thank Dr Boris Vojnovic and Ros Locke for their assistance in image acquisition and Drs Omar Benzakour, Olivier Pardo, and Claudia Coralli for critical reading of the manuscript.
Supported by grant no. SP2295/0102 from the Cancer Research Campaign.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chryso Kanthou, Tumour Microcirculation Group, Gray Cancer Institute, Mount Vernon Hospital, PO Box 100, Northwood, Middlesex, HA6 2JR, United Kingdom; e-mail: kanthou@gci.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal