Abstract
Basic fibroblast growth factor (bFGF) and platelet-derived growth factor-BB (PDGF-BB) modulate vascular wall cell function in vitro and angiogenesis in vivo. The aim of the current study was to determine how bovine aorta endothelial cells (BAECs) respond to the simultaneous exposure to PDGF-BB and bFGF. It was found that bFGF-dependent BAEC migration, proliferation, and differentiation into tubelike structures on reconstituted extracellular matrix (Matrigel) were inhibited by PDGF-BB. The role played by PDGF receptor α (PDGF-Rα) was investigated by selective stimulation with PDGF-AA, by blocking PDGF-BB-binding to PDGF-Rα with neomycin, or by transfecting cells with dominant-negative forms of the receptors to selectively impair either PDGF-Rα or PDGF-Rβ function. In all cases, PDGF-Rα impairment abolished the inhibitory effect of PDGF-BB on bFGF-directed BAEC migration. In addition, PDGF-Rα phosphorylation was increased in the presence of bFGF and PDGF, as compared to PDGF alone, whereas mitogen-activated protein kinase phosphorylation was decreased in the presence of PDGF-BB and bFGF compared with bFGF alone. In vivo experiments showed that PDGF-BB and PDGF-AA inhibited bFGF-induced angiogenesis in vivo in the chick embryo chorioallantoic membrane assay and that PDGF-BB inhibited bFGF-induced angiogenesis in Matrigel plugs injected subcutaneously in CD1 mice. Taken together these results show that PDGF inhibits the angiogenic properties of bFGF in vitro and in vivo, likely through PDGF-Rα stimulation.
Introduction
The endothelial layer represents a physical and chemical barrier between the vessel lumen and the underlying tissues. Endothelial cells (ECs) exert a variety of functions and modulate underlying smooth muscle cells by releasing molecules with vasoactive and growth-regulatory properties.1 Endothelial cells present an active replication phenotype in vitro, but in vivo they are quiescent.2 The different expression of membrane-bound receptors3,4 and the in vivo action of specific inhibitors5-7 may account, at least in part, for the different replication pattern observed.
Basic fibroblast growth factor (bFGF) is a potent EC growth factor; it is known to induce a proangiogenic phenotype in ECs and is released under acidosis conditions that induce EC protection from apoptosis.8 bFGF plays a critical role in physiologic and pathologic angiogenesis, including tumor angiogenesis.9,10It exerts its functions by direct action,11 by inducing vascular endothelial growth factor (VEGF) synthesis,12 or by potentiating VEGF activity.13 14
Platelet-derived growth factor (PDGF) is a growth factor known to be active on ECs. Three PDGF isoforms have been identified as disulfide-linked dimers, namely PDGF-AA, PDGF-BB, and PDGF-AB, expressed by ECs under various conditions.15-19 They interact with different affinity with 2 tyrosine-kinase receptors, PDGF-Rα and PDGF-Rβ, which are expressed on ECs in normal4,20-22 and in pathologic conditions.23-26 Recently, PDGF-C and PDGF-D isoforms were also identified.27,28 PDGF isoforms are reported to exert mitogenic and chemotactic action on EC, although PDGF-AA appears to be less potent or inactive.4,21,29-31 In addition, a significant correlation has been found between PDGF-BB mRNA levels and microvessel density in invasive urinary carcinoma,32 and a putative proangiogenic effect of PDGF-BB has been proposed in stroke.33 Both bFGF and PDGF are reported to be increased during neo-angiogenesis observed in tumor34 and in thyroiditis35 and brain abscess.36
We have recently shown that bFGF inhibits PDGF-BB mitogenic and chemotactic activity on primary rat aorta smooth muscle cells in vitro and that this inhibitory action is mediated by PDGF-Rα and bFGF receptors.37 In fact, when either bFGF binding to its receptor or PDGF-Rα signaling was impaired, the inhibitory effect of bFGF was abolished. This finding is in agreement with the known modulating role of PDGF-Rα38-40 and was further confirmed by a recent report indicating that PDGF-Rα activates JNK-1 signaling, counteracting PDGF-Rβ activity.41
Therefore, we have hypothesized that PDGF may inhibit bFGF-induced angiogenesis, and we have, in the current study, determined whether PDGF modulates bFGF activity on ECs in vitro and in vivo. We found that PDGF, through PDGF-Rα stimulation, inhibited bFGF proangiogenic effects in vitro and significantly inhibited bFGF-induced angiogenesis in vivo.
Materials and methods
Cell culture
Primary bovine aorta endothelial cells (BAECs) were obtained as reported previously42 and were cultured at 37°C in a 5% CO2 atmosphere in Dulbecco modified Eagle medium (DMEM; Hyclone, Logan, UT) supplemented with 2 mM l-glutamine, 100 IU/mL penicillin–streptomycin (Gibco, Gaithersburg, MD), and 10% fetal calf serum (FCS; Hyclone). BAEC preparation purity was evaluated by low-density lipoprotein uptake and was consistently greater than 97%. Proliferating BAECs (passages 3 to 7) at 80% confluence were used in all assays.
Migration assay
Migration assays were carried out in modified Boyden chambers (Costar; Corning, Acton, MA) as described.37 BAECs (2 × 105) were placed in the upper chamber of the Boyden apparatus, and polycarbonate filters with 12-μm pores (Costar) were coated by soaking with 5 mg/L solution of porcine skin gelatin (Sigma, Milan, Italy). Human recombinant factors used as chemoattractants were PDGF-BB (R&D Systems, Abingdon, United Kingdom), PDGF-AA (R&D Systems), bFGF (Gibco), and epidermal growth factor (EGF) (Gibco). In addition, FCS was used as a chemoattractant at 0.5% concentration. Chemoattractants were dissolved at the reported concentration in DMEM—0.1% bovine serum albumin (BSA) and were placed in the lower chamber of the Boyden apparatus. Solutions containing bFGF and PDGF-BB or bFGF and PDGF-AA (10 ng/mL each) are here referred to as bFGF/PDGF-BB and bFGF/PDGF-AA, respectively. Neomycin was used to specifically inhibit PDGF-BB binding to PDGF-Rα43 by preincubating cells with 5 mM neomycin for 15 minutes before migration experiments were started.
PDGF-BB heat inactivation was performed by repeating 3 times a cycle consisting of heating at 100°C for 10 minutes followed by fast refrigeration. A polyclonal anti-PDGF-BB neutralizing antibody (AB-220-NA; R&D Systems) was placed in the lower chamber of the Boyden apparatus, at the final concentration of 1 μg/mL, to neutralize PDGF-BB activity. The same concentration was used to block PDGF-BB–dependent receptor phosphorylation. Neutralizing goat anti-human bFGF (Ab-233-NA; R&D Systems) was used at 1 μg/mL concentration.
Migration assays were carried out at 37°C in 5% CO2 for 6 hours, and filters were then removed, fixed with absolute ethanol, and stained with toluidine blue (Sigma). Cells that migrated across the filter were counted at × 400 magnification; 10 fields/filter were evaluated, and the average number of cells/field was reported. All experiments were performed at least 3 times in duplicate.
Proliferation assay
BAECs plated in 6-well plates (1 × 105cells/plate) were grown for 24 hours in DMEM supplemented with 10% FCS, at 37°C in 5% CO2. Medium was then replaced with serum-free DMEM for 24 hours. Subsequently, the medium was replaced with fresh medium containing either 0.1% BSA alone or 0.1% BSA with growth factors. After treatment, cells were harvested and counted with a hemacytometer. All experiments were carried out at least 3 times in duplicate.
Cell transfection with dominant-negative PDGF receptor constructs
BAECs were transfected as previously reported.37Either a dominant-negative PDGF-Rα vector (DN-PDGF-Rα) or an equal amount of dominant-negative PDGF-Rβ vector (DN-PDGF-Rβ) (generous gifts of Dr C. H. Heldin, Ludwig Institute for Cancer Research, Uppsala, Sweden)44 or PcDNA3 (Invitrogen, Groningen, The Netherlands) empty vector was used. Cells were cotransfected with pEGFP-N1 (Clontech, Temecula, CA) reporter vector, with a dominant-negative versus a reporter-vector molar ratio 4:1. BAEC transfection (8 × 105 cells/60-mm diameter dish) was carried out with Lipofectamine Plus reagent (Gibco) for 5.5 hours at 37°C in a 5% CO2 environment. Medium was then replaced with DMEM–10% FCS, and migration and phosphorylation assays were performed. Cotransfection with 2 vectors, at the reported ratio, results in the internalization of both plasmids by the same cell.45 Therefore, migrated cells were counted under a fluorescence microscope to evaluate only GFP-positive cells to overcome the potential limitations of low transfection efficiency. Transfection was carried out according to the manufacturer's instructions, and GFP transfection efficiency was higher than 70%. PDGF receptor phosphorylation in DN-transfected cells was markedly inhibited compared with mock-transfected cells in all experiments, indicating that the transfection process was effective. All transfection experiments were performed at least 3 times in duplicate.
Western blot analysis and receptor phosphorylation
BAECs were grown to 70% confluence and were incubated for 2 days in DMEM containing 1% FCS and for 1 day in serum-free DMEM.46 Transfection with DN-PDGF-Rα was carried out as reported above; then transfected cells were incubated overnight in serum-free conditions. Cells were treated with growth factor or antibodies at the indicated concentrations for 5 minutes, rinsed with ice-cold phosphate-buffered saline (PBS), scraped, and lysed for 15 minutes with 1% triton, 10% glycerol, 100 mM NaCl, 20 mM HEPES pH 7.4, 5 mM EDTA, 1% AEBSF, 1% pepstatin A, 1% E-64, 1% bestatin, 1% leupeptin, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μM NaVO3, and 50 mM NaF (Sigma). Lysates were then centrifuged at 14 000 rpm for 15 minutes. After the determination of protein concentration, 200 μg total proteins were subjected to 7.5% sodium dodecyl fluoride–polyacrylamide electrophoresis (SDS-PAGE). Proteins were electrotransferred to a nitrocellulose membrane (Amersham, Uppsala, Sweden) and were blocked with PBS containing 0.1% Tween 20 and 5% nonfat milk (Bio-Rad, Hercules, CA). Ponceau S (Sigma) staining of the filters was also performed to verify the equal loading. Western blot analysis was carried out by probing the nitrocellulose membrane with the antiphosphotyrosine antibody (1 μg/mL; Upstate Biotechnology, Lake Placid, NY), for 1 hour, followed by washing and incubating with horseradish peroxidase–conjugated secondary antibody (Pierce, Rockford, IL). Bands reported in PDGF receptor phosphorylation figure migrated at the apparent molecular weight corresponding to PDGF receptors. A neutralizing anti-PDGF antibody significantly reduced the phosphorylation, and DN-PDGF-Rα–transfected cells showed marked reduction of the band phosphorylation; therefore, the reported bands were identified as PDGF receptors and PDGF-Rα, respectively. Bands were then revealed by means of the enhanced chemiluminescence (ECL) detection system (Amersham) and were quantified with a calibrated imaging densitometer (GS 710; Bio-Rad). All experiments were performed at least 3 times.
Mitogen-activated protein kinase activation assay
BAECs were seeded onto 150-mm culture plates (1 × 106 cells) incubated in DMEM containing 0.5% FCS for 2 days and in serum-free DMEM for the following day. Medium was then replaced with fresh medium containing either 0.1% BSA alone or 0.1% BSA with growth factors (10 ng/mL bFGF, 10 ng/mL PDGF-BB, or both) for 10 minutes. Cells were washed in ice-cold PBS and scraped in lysis buffer containing 20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% triton, 2.5 mM sodium pyrophosphate, 1 mMβ-glycerol-phosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, and 1 mM PMSF. Cell extracts were sonicated and centrifuged, and supernatants were collected. After protein content determination with the Bio-Rad protein assay system (Bio-Rad), samples (30 μg total protein each), suspended in Laemmli sample buffer and boiled for 5 minutes, were resolved on 8% SDS-PAGE and transferred to nitrocellulose membrane (Amersham). Ponceau S (Sigma) staining of the filters was also performed to verify the equal loading. Filters were then probed with primary antibody antiphosphorylated ERK p44/42 (New England Biolabs, Hitchin, United Kingdom), which specifically recognizes activated forms of ERK1/2, and with horseradish peroxidase–conjugated secondary antibody (Pierce). Detection was carried out using the ECL detection system (Amersham) and was quantified with a calibrated imaging densitometer (GS 710; Bio-Rad). Six experiments were performed.
Differentiation assay
Endothelial cell differentiation into tubular structures (TS) was assessed as previously reported.42 Briefly, 200 μL reconstituted extracellular matrix protein (Matrigel), growth factor–reduced and without phenol red (Collaborative Research, Bedford MA), was applied to 24-well culture plates and was incubated at 37°C for 60 minutes. BAEC (8 × 104 in 1 mL medium) were seeded on Matrigel-coated wells in the presence of 0.1% BSA, 1% FCS, or bFGF (10 ng/mL), or bFGF/PDGF-BB (10 ng/mL each), or PDGF-BB alone (10 ng/mL), and were kept at 37°C. Morphology changes and TS appearance were monitored at 0, 2, 3, 4, 6, 8, and 24 hours after plating by phase-contrast microscopy. Quantification of TS formation was expressed by the mean number of branching points in 10 fields. This experiment was carried out 3 times in duplicate, and similar results were collected.
Angiogenesis on gelatin sponge chick chorioallantoic membrane assay
Chick embryo chorioallantoic membrane (CAM) assay was performed as previously reported.47 Briefly, fertilized White Leghorn chicken eggs (30/group) were incubated at 37°C at constant humidity. On incubation day 3, a square window was opened in the shell, and 2 to 3 mL albumen was removed to allow detachment of the developing CAM. The window was sealed with glass, and the eggs were returned to the incubator. At day 8, 1 μL sterilized gelatin sponges (Gelfoam, Upjohn, Kalamazoo, MI) adsorbed with bFGF alone (500 μg), PDGF-AA alone (500 μg), PDGF-BB alone (500 μg), bFGF/PDGF-AA, or bFGF/PDGF-BB (500 μg each) dissolved in 2 mL PBS were implanted on the top of growing CAMs under sterile conditions. Sponges containing vehicle alone were used as negative control. At day 12, blood vessels entering the sponge within the focal plane of the CAM were recognized macroscopically (at 50×), counted by 2 observers in a double-blind fashion48 under a Zeiss SR stereomicroscope (Zeiss, Oberkochen, Germany), and photographed in vivo with an MC63 Camera System (Zeiss). Percentage inhibition reported in the text was computed after subtracting spontaneous angiogenesis—ie, vessels in the presence of PBS only.
The angiogenic response was also assessed histologically by a planimetric method of point counting.47 Briefly, in every third section within 30 serial slides, an individual specimen was analyzed by a 144-point mesh inserted in the eyepiece of a Leitz-Dialux 20 photomicroscope. Six randomly chosen microscopic fields were evaluated for each section at × 250 magnification. To this purpose, the total number of the intersection points occupied by vessels transversally cut (diameter, 3 to 10 μm) inside the sponge and at the boundary between the sponge and the surrounding CAM mesenchyme were counted. Mean values ± SD were determined for each analysis. Vascular density was indicated by the final mean number of occupied intersection points.
Angiogenesis in Matrigel plugs
Angiogenesis assays in reconstituted basement membrane (Matrigel) plugs were carried out according to a previously reported procedure.49 Matrigel containing bFGF (150 ng/mL) alone or bFGF/PDGF-BB (150 ng/mL each) was injected subcutaneously in CD1 mice (female, 19 weeks of age). Under these conditions bFGF induces a vascular network formation in the Matrigel plug within 8 days; Matrigel plugs were excised 8 days after injection and included in paraffin. Slides were stained with the Trichrome-Masson staining procedure (Bio-Optica, Milan, Italy), and vessel quantification of the portion of histologic sections occupied by Matrigel was obtained by an integrated image analysis system (Quantimet 970; Cambridge Instruments, United Kingdom), according to a published procedure.50 The number of newly formed vessels within the whole Matrigel area was expressed as number of vessels/mm2.
Statistics
Data are expressed as the mean ± SD. Student 2-tailed paired t test was performed, and P ≤ .01 was considered statistically significant.
Results
Effect of PDGF-BB on bFGF-induced BAEC migration, proliferation, and differentiation
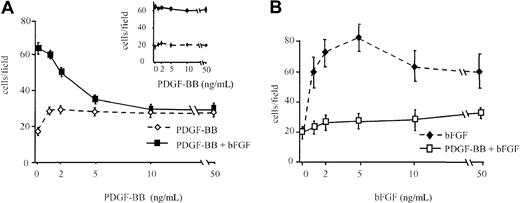
BAEC migration was investigated in response to bFGF alone, PDGF-BB alone, and bFGF/PDGF-BB, in concentration-dependence experiments, with increasing concentrations of one factor and a fixed concentration of the other. PDGF-BB alone (1 to 50 ng/mL) showed a weak chemotactic action, but it inhibited, in a concentration-dependent manner, migration induced by bFGF (10 ng/mL), and at 10 ng/mL it inhibited bFGF-induced migration by approximately 50% (Figure1A). Basic FGF-induced migration was inhibited at all tested concentrations by 10 ng/mL PDGF-BB (Figure 1B). PDGF-BB did not show any inhibitory effect when it was heat-denatured (see inset in Figure 1A) nor when its action was neutralized with a specific antibody (1 μg/mL), whereas a control antibody was ineffective (not shown). Further, EGF did not show any inhibitory activity on bFGF-induced BAEC migration (not shown), indicating that the bFGF chemotactic effect on BAEC is specifically inhibited by PDGF-BB.
BAEC migration in the presence of bFGF and PDGF-BB.
(A) BAEC migration was examined in response to increasing concentrations of PDGF-BB alone (dashed line) or increasing concentrations of PDGF-BB in the presence of 10 ng/mL bFGF (continuous line). The bFGF effect was strongly inhibited by PDGF-BB in a dose-dependent manner. (inset) Denatured PDGF-BB did not show any inhibitory effect. (B) BAEC migration was examined in response to increasing concentrations of bFGF alone (dashed line) or increasing concentrations of bFGF in the presence of 10 ng/mL PDGF-BB (continuous line). The bFGF effect was strongly inhibited by PDGF-BB at all concentrations. Data are expressed as average ± SD of 4 experiments carried out in duplicate.
BAEC migration in the presence of bFGF and PDGF-BB.
(A) BAEC migration was examined in response to increasing concentrations of PDGF-BB alone (dashed line) or increasing concentrations of PDGF-BB in the presence of 10 ng/mL bFGF (continuous line). The bFGF effect was strongly inhibited by PDGF-BB in a dose-dependent manner. (inset) Denatured PDGF-BB did not show any inhibitory effect. (B) BAEC migration was examined in response to increasing concentrations of bFGF alone (dashed line) or increasing concentrations of bFGF in the presence of 10 ng/mL PDGF-BB (continuous line). The bFGF effect was strongly inhibited by PDGF-BB at all concentrations. Data are expressed as average ± SD of 4 experiments carried out in duplicate.
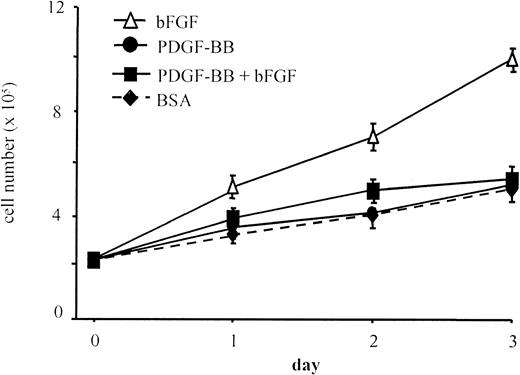
Additional experiments were aimed at evaluating the effect of PDGF-BB on bFGF mitogenic action. PDGF-BB (10 ng/mL) abolished the mitogenic effect of bFGF on BAEC at all time points in a 3-day proliferation assay (Figure 2). As observed in migration experiments, heat-denatured PDGF-BB did not inhibit bFGF mitogenic effect (not shown), PDGF-BB neutralization with a specific antibody abolished its inhibitory effect, and a control antibody was ineffective (not shown). These results show that the native and active forms of PDGF-BB are required to inhibit chemotactic and mitogenic actions of bFGF.
BAEC proliferation in the presence of bFGF and PDGF-BB.
BAEC proliferation was evaluated in response to 0.1% BSA, bFGF alone (10 ng/mL), PDGF-BB alone (10 ng/mL), or bFGF/PDGF-BB (10 ng/mL each). The bFGF mitogenic effect was lowered to control levels in the presence of PDGF-BB. Data are expressed as average ± SD of 4 experiments carried out in duplicate.
BAEC proliferation in the presence of bFGF and PDGF-BB.
BAEC proliferation was evaluated in response to 0.1% BSA, bFGF alone (10 ng/mL), PDGF-BB alone (10 ng/mL), or bFGF/PDGF-BB (10 ng/mL each). The bFGF mitogenic effect was lowered to control levels in the presence of PDGF-BB. Data are expressed as average ± SD of 4 experiments carried out in duplicate.
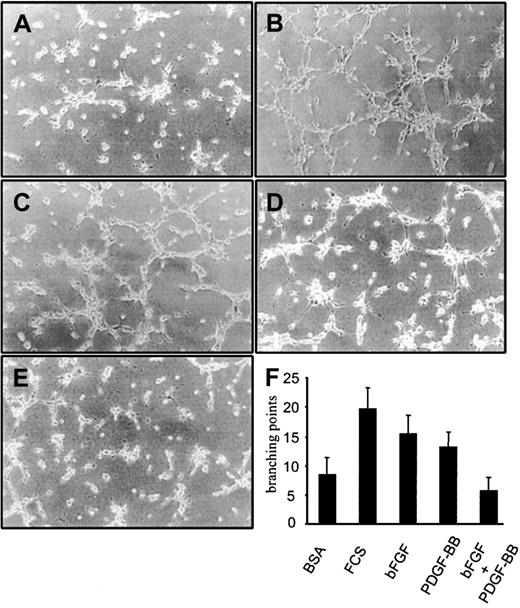
BAECs plated on Matrigel formed TS in a bFGF-dependent way; therefore, we examined whether PDGF-BB modulated bFGF's ability to induce TS formation. Basic FGF alone and PDGF-BB alone (10 ng/mL) induced TS formation, and this effect was less pronounced than in response to 1% FCS (Figure 3). When bFGF was mixed with PDGF-BB (10 ng/mL each), BAECs differentiated significantly less than in response to either factor alone, and the magnitude of the response was comparable to the control treated with BSA alone. PDGF-BB inhibitory effect on bFGF was evident at 3 hours (Figure 3) and at 6 and 24 hours (not shown). These experiments indicated that PDGF-BB, though a weak TS inducer, substantially inhibits BAEC differentiation into TS induced by bFGF.
BAEC differentiation into tubular structures on Matrigel.
BAEC differentiation on Matrigel-coated wells in the presence of (A) 0.1% BSA, (B) 1% FCS, (C) 10 ng/mL bFGF, (D) 10 ng/mL PDGF-BB, or (E) 10 ng/mL bFGF/PDGF-BB. (F) Quantification of branching points was carried out on 10 fields. The differentiation effect of bFGF was lowered to control levels in the presence of PDGF-BB. This effect was evident at 3, 6, 8, and 24 hours. Figures and the bar graph refer to the effect at 3 hours. Experiments were carried out 3 times in duplicate. Panels A to E refer to a representative experiment.
BAEC differentiation into tubular structures on Matrigel.
BAEC differentiation on Matrigel-coated wells in the presence of (A) 0.1% BSA, (B) 1% FCS, (C) 10 ng/mL bFGF, (D) 10 ng/mL PDGF-BB, or (E) 10 ng/mL bFGF/PDGF-BB. (F) Quantification of branching points was carried out on 10 fields. The differentiation effect of bFGF was lowered to control levels in the presence of PDGF-BB. This effect was evident at 3, 6, 8, and 24 hours. Figures and the bar graph refer to the effect at 3 hours. Experiments were carried out 3 times in duplicate. Panels A to E refer to a representative experiment.
In additional experiments, PDGF-BB inhibited FCS-induced BAEC migration (not shown), indicating that PDGF-BB may exert this inhibitory effect in the presence of FCS. Taken together, these results show that PDGF-BB has a marked and specific inhibitory action on bFGF's proangiogenic effect on BAECs in vitro.
Role of PDGF- Rα in the inhibitory activity of PDGF-BB
It has been shown that PDGF-Rα and PDGF-Rβ can initiate positive or negative signals, depending on the cell type,51 and that PDGF-Rα stimulation inhibits smooth muscle cell migration and proliferation.37-40 According to the literature,4,20-22 we found that PDGF and bFGF receptors are expressed on BAECs (data not shown); therefore; we hypothesized that PDGF-BB–dependent inhibition of bFGF-induced BAEC migration, proliferation, and differentiation may be mediated by PDGF receptors. The role of PDGF-Rα was then investigated (1) by testing PDGF-AA, which is a selective PDGF-Rα agonist, (2) by inhibiting PDGF-BB binding to PDGF-Rα with neomycin, which has been shown to specifically inhibit PDGF-BB binding to PDGF-Rα without affecting its binding to PDGF-Rβ,43 52 (3) by determining the effect of PDGF-BB on BAECs transfected with dominant-negative PDGF-Rα or dominant-negative PDGF-Rβ, and (4) by evaluating PDGF-Rα phosphorylation in the presence of PDGF and bFGF.
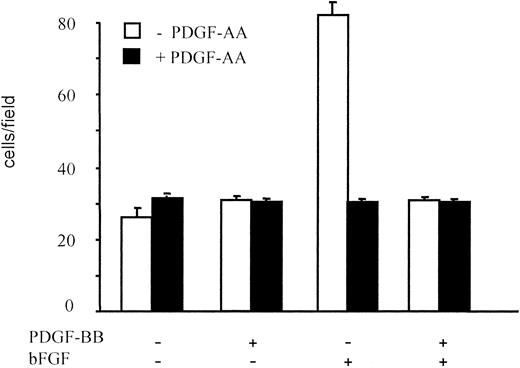
PDGF-AA alone showed no significant chemotactic activity; however, it significantly inhibited migration induced by bFGF. The PDGF-AA effect at 10 ng/mL was comparable to that of PDGF-BB, and it lowered BAEC migration to control levels (Figure 4). Further, PDGF-AA and PDGF-BB, when simultaneously added to bFGF, did not exhibit a synergistic or an additive action. Therefore, PDGF-AA, by selectively binding PDGF-Rα, inhibited BAEC migration mimicking the PDGF-BB effect; this suggested that the inhibitory effect of PDGF-BB, which binds both PDGF-Rα and PDGF-Rβ, may be mediated by PDGF-Rα.
BAEC migration in the presence of PDGF-AA.
BAEC migration in response to PDGF-BB or bFGF, alone or in combination (10 ng/mL), was examined in the absence and in the presence of PDGF-AA (10 ng/mL). PDGF-AA showed no chemotactic effect on BAEC, but it inhibited bFGF-induced migration, and the magnitude of this effect was comparable to that of an equal concentration of PDGF-BB. Data are expressed as average ± SD of 3 experiments carried out in duplicate.
BAEC migration in the presence of PDGF-AA.
BAEC migration in response to PDGF-BB or bFGF, alone or in combination (10 ng/mL), was examined in the absence and in the presence of PDGF-AA (10 ng/mL). PDGF-AA showed no chemotactic effect on BAEC, but it inhibited bFGF-induced migration, and the magnitude of this effect was comparable to that of an equal concentration of PDGF-BB. Data are expressed as average ± SD of 3 experiments carried out in duplicate.
In other experiments, PDGF-BB binding to PDGF-Rα was specifically inhibited by neomycin incubation. Although neomycin did not affect the basal migration or the chemotactic action of PDGF-BB alone and bFGF alone, it reduced the inhibitory effect of PDGF-BB on bFGF-induced migration (not shown). Similar results were obtained in proliferation assays (not shown).
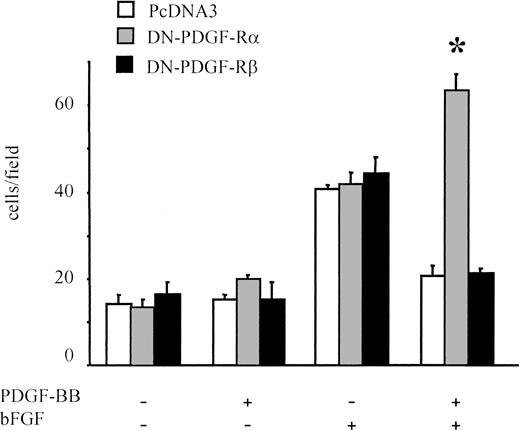
The role of PDGF receptors in PDGF-BB–induced inhibition of bFGF effects was examined further in migration experiments on BAECs transfected either with the dominant-negative form of PDGF-Rα or with PDGF-Rβ (DN-PDGF-Rα or DN-PDGF-Rβ, respectively). In DN-PDGF-Rα–transfected cells, PDGF-BB is expected to act only through ββ receptor dimers because αα and αβ receptor dimers are inactivated.44 Cells transfected with the empty vector (PcDNA3) behaved similarly to untransfected cells, and the chemotactic response to bFGF/PDGF-BB was significantly lower than that induced by bFGF alone (Figure 5). In DN-PDGF-Rα–transfected cells, the inhibitory effect of PDGF-BB was totally abolished; under these conditions, the chemotactic effect of bFGF was potentiated in the presence of PDGF-BB (P < .01 vs bFGF alone), suggesting that PDGF-Rα stimulation may prevent positive synergism between PDGF-BB and bFGF. Conversely, in DN-PDGF-Rβ–transfected cells, the inhibitory effect of PDGF-BB was present, as in control cells. Taken altogether, these results indicate that when PDGF-Rα is inactivated, the inhibitory effect of PDGF-BB is abolished.
Migration of BAECs transfected with dominant-negative receptors.
BAECs were transfected either with a dominant-negative PDGF-Rα vector (DN-PDGF-Rα), with a dominant-negative PDGF-Rβ vector (DN-PDGF-Rβ, or with a PcDNA3 empty vector and were cotransfected with pEGFP-N1 reporter vector. A dominant-negative versus reporter-vector molar ratio of 4:1 was used. Under these conditions both plasmids were internalized in the same cell; when migrated cells were examined, only GFP-positive cells were counted. Neither the empty vector nor the dominant-negative vectors affected bFGF-induced migration. PDGF-BB inhibitory effect on bFGF-induced migration was present in the empty-vector–transfected cells and in the DN-PDGF-Rβ–transfected cells, whereas it was absent in DN-PDGF-Rα–transfected cells. In the latter, BAEC migration was increased in the presence of PDGF-BB and bFGF compared with bFGF alone. *Statistically significant difference (P < .01). Data are expressed as average ± SD of 4 experiments carried out in duplicate.
Migration of BAECs transfected with dominant-negative receptors.
BAECs were transfected either with a dominant-negative PDGF-Rα vector (DN-PDGF-Rα), with a dominant-negative PDGF-Rβ vector (DN-PDGF-Rβ, or with a PcDNA3 empty vector and were cotransfected with pEGFP-N1 reporter vector. A dominant-negative versus reporter-vector molar ratio of 4:1 was used. Under these conditions both plasmids were internalized in the same cell; when migrated cells were examined, only GFP-positive cells were counted. Neither the empty vector nor the dominant-negative vectors affected bFGF-induced migration. PDGF-BB inhibitory effect on bFGF-induced migration was present in the empty-vector–transfected cells and in the DN-PDGF-Rβ–transfected cells, whereas it was absent in DN-PDGF-Rα–transfected cells. In the latter, BAEC migration was increased in the presence of PDGF-BB and bFGF compared with bFGF alone. *Statistically significant difference (P < .01). Data are expressed as average ± SD of 4 experiments carried out in duplicate.
PDGF receptor phosphorylation and mitogen-activated protein kinase activation
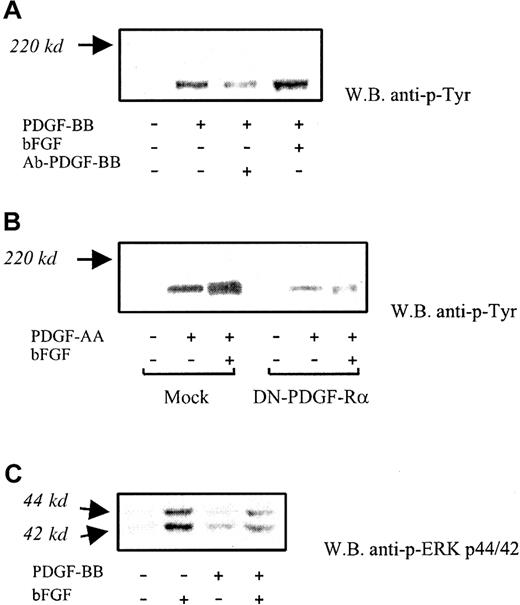
In additional experiments carried out on BAECs, PDGF receptor phosphorylation was found to be increased on bFGF/PDGF-BB treatment (5 minutes) in comparison with PDGF-BB alone (Figure6A). The average increase was 53% ± 15% (n = 3) by densitometric analysis. Receptor phosphorylation was markedly inhibited by anti-PDGF-BB neutralizing antibody (1 μg/mL). Receptor α was then investigated by treating cells with PDGF-AA, which binds only PDGF-Rα in the presence or in the absence of bFGF. Under these conditions, PDGF-Rα phosphorylation was increased in the presence of bFGF/PDGF-AA compared with PDGF-AA alone; phosphorylation of this receptor in DN-PDGF-Rα–transfected cells was almost abolished, as expected. (Figure 6B). No other bands were found to be modulated under these conditions. These results indicate that receptor α-phosphorylation is increased in the presence of bFGF/PDGF compared with PDGF alone.
PDGF receptor phosphorylation and MAP kinase activation.
(A) PDGF receptor phosphorylation was investigated at 5-minute PDGF-BB exposure (10 ng/mL), with or without bFGF. Increased phosphorylation of PDGF-Rα and PDGF-Rβ was found in the presence of bFGF/PDGF-BB compared with PDGF-BB alone (average increase, 52% ± 18%). Anti–PDGF-BB neutralizing antibody (Ab-PDGF-BB) markedly inhibited PDGF receptor phosphorylation. This experiment was carried out 3 times with similar results. One representative experiment is shown. (B) Phosphorylation of PDGF-Rα was investigated in BAECs exposed to PDGF-AA, which is a PDGF-Rα–specific agonist, in the absence and in the presence of bFGF. Under these conditions, bFGF/PDGF-AA–treated cells showed increased phosphorylation compared with PDGF-AA alone. Receptor phosphorylation was markedly reduced in DN-PDGF-Rα–transfected cells. This experiment was carried out 3 times, with similar results. One representative experiment is shown. (C) ERK1/2 phosphorylation induced by bFGF alone, PDGF-BB alone, and bFGF/PDGF-BB was detected with an antibody specifically recognizing the activated forms at 44 kd and 42 kd, respectively. A marked phosphorylation decrease was found in the presence of bFGF/ PDGF-BB compared with bFGF alone. This experiment was carried out 5 times with similar results. One representative experiment is shown.
PDGF receptor phosphorylation and MAP kinase activation.
(A) PDGF receptor phosphorylation was investigated at 5-minute PDGF-BB exposure (10 ng/mL), with or without bFGF. Increased phosphorylation of PDGF-Rα and PDGF-Rβ was found in the presence of bFGF/PDGF-BB compared with PDGF-BB alone (average increase, 52% ± 18%). Anti–PDGF-BB neutralizing antibody (Ab-PDGF-BB) markedly inhibited PDGF receptor phosphorylation. This experiment was carried out 3 times with similar results. One representative experiment is shown. (B) Phosphorylation of PDGF-Rα was investigated in BAECs exposed to PDGF-AA, which is a PDGF-Rα–specific agonist, in the absence and in the presence of bFGF. Under these conditions, bFGF/PDGF-AA–treated cells showed increased phosphorylation compared with PDGF-AA alone. Receptor phosphorylation was markedly reduced in DN-PDGF-Rα–transfected cells. This experiment was carried out 3 times, with similar results. One representative experiment is shown. (C) ERK1/2 phosphorylation induced by bFGF alone, PDGF-BB alone, and bFGF/PDGF-BB was detected with an antibody specifically recognizing the activated forms at 44 kd and 42 kd, respectively. A marked phosphorylation decrease was found in the presence of bFGF/ PDGF-BB compared with bFGF alone. This experiment was carried out 5 times with similar results. One representative experiment is shown.
Additional investigation was carried out on mitogen-activated protein (MAP) kinases, which play a central role in PDGF-BB and bFGF signaling. Therefore, we examined the effect of simultaneous exposure to bFGF and PDGF-BB on the ERK1/2 activation. Western blot analysis indicated that ERK1/2 phosphorylation was markedly inhibited (41% ± 14% and 46% ± 6%, respectively) on treatment with bFGF/PDGF-BB compared with bFGF alone (Figure 6C). This finding suggests that PDGF-BB–mediated inhibition of bFGF activity may involve, at least in part, the inhibition of MAP kinase–dependent signaling.
Inhibitory effect of PDGF-BB on bFGF-induced angiogenesis in vivo
The angiogenic effect of bFGF in the presence of PDGF-BB was tested in 2 angiogenesis assays in vivo.
Chick embryo chorioallantoic membrane assay
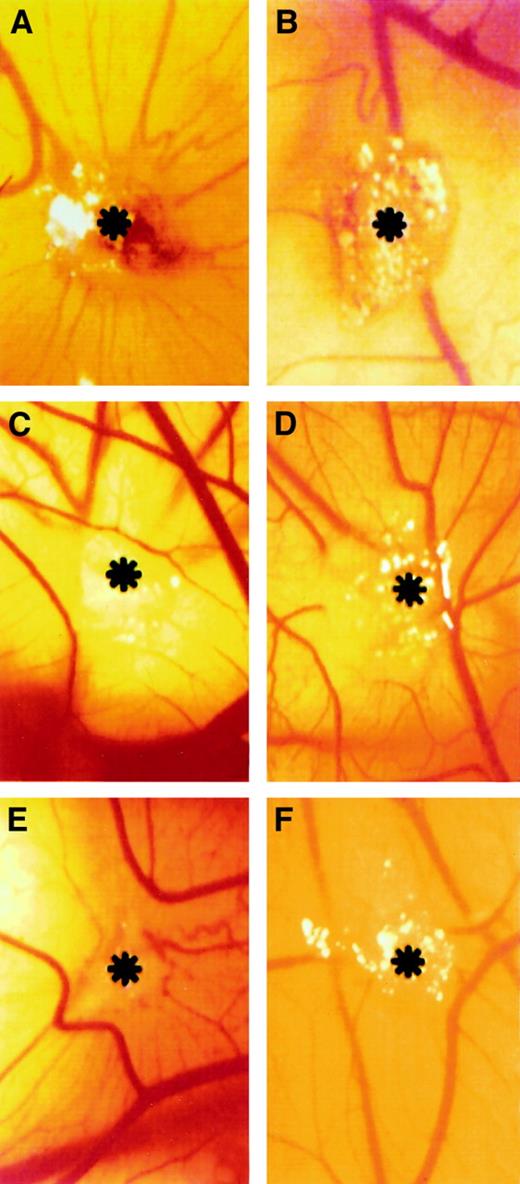
Gelatin sponges adsorbed with bFGF induced an angiogenic response, and allantoic vessels developed radially toward the implant in a spoked-wheel pattern (Figure 7A). Allantoic vessels were less numerous in the specimens treated with bFGF/PDGF-AA and bFGF/PDGF-BB (Figure 7D,F). Almost no vascular reaction was detectable around the sponges treated with PBS only or PDGF-AA (Figure 7B-C), whereas PDGF-BB induced a moderate angiogenic response (Figure 7E). Quantification of vascularization at day 12, in response to bFGF, PDGF-AA, PDGF-BB, bFGF/PDGF-AA, bFGF/PDGF-BB, and PBS, is reported in Table 1. Under these conditions, PDGF-BB and PDGF-AA induced 47% and 61% inhibition of bFGF angiogenic activity, respectively.
Macroscopic observations of the PDGF-AA and PGDF-BB effect on bFGF-induced neovascularization in the CAM assay.
Gelatin sponge adsorbed with bFGF (500 μg) is surrounded by allantoic vessels that develop radially toward the implant in a spoked-wheel pattern (A), whereas no vascular reaction is detectable around PBS-treated (B) and PDGF-AA–treated (C) sponges. Allantoic vessels were less numerous in the specimens treated with bFGF added to PDGF-AA and PDGF-BB (500 μg each) (D and F, respectively), whereas PDGF-BB alone induced a moderate angiogenic response (E). Asterisks indicate sponges. Panels refer to a representative experiment. Original magnification, × 50.
Macroscopic observations of the PDGF-AA and PGDF-BB effect on bFGF-induced neovascularization in the CAM assay.
Gelatin sponge adsorbed with bFGF (500 μg) is surrounded by allantoic vessels that develop radially toward the implant in a spoked-wheel pattern (A), whereas no vascular reaction is detectable around PBS-treated (B) and PDGF-AA–treated (C) sponges. Allantoic vessels were less numerous in the specimens treated with bFGF added to PDGF-AA and PDGF-BB (500 μg each) (D and F, respectively), whereas PDGF-BB alone induced a moderate angiogenic response (E). Asterisks indicate sponges. Panels refer to a representative experiment. Original magnification, × 50.
Chick embryo CAM-sponge assay: macroscopic assessment of vascular density on day 12 of incubation
| Sponge loaded with . | No. of specimens . | No. of blood vessels . |
|---|---|---|
| bFGF | 30 | 40 ± 4 |
| PDGF-AA | 30 | 7 ± 2 |
| PDGF-BB | 30 | 16 ± 3 |
| bFGF + PDGF-AA | 30 | 18 ± 3 |
| bFGF + PDGF-BB | 30 | 23 ± 4 |
| PBS | 30 | 4 ± 2 |
| Sponge loaded with . | No. of specimens . | No. of blood vessels . |
|---|---|---|
| bFGF | 30 | 40 ± 4 |
| PDGF-AA | 30 | 7 ± 2 |
| PDGF-BB | 30 | 16 ± 3 |
| bFGF + PDGF-AA | 30 | 18 ± 3 |
| bFGF + PDGF-BB | 30 | 23 ± 4 |
| PBS | 30 | 4 ± 2 |
At the microscopic level, highly vascularized tissue was present among the trabeculae of the bFGF-treated sponges (not shown). The tissue consisted of newly formed blood vessels, mainly capillaries, with 3- to 10-μm diameters growing perpendicularly to the plane of the CAM and of infiltrating fibroblasts within an abundant network of collagen fibers. Vessels were less numerous in PDGF-BB–, bFGF/PDGF-AA–, and bFGF/PDGF-BB–treated sponges and were absent among the trabeculae of implants treated with PBS and PDGF-AA. These observations were confirmed by quantification of the angiogenic response by a morphometric method of point counting (Table2). Under these conditions, PDGF-BB and PDGF-AA induced 50% and 42% inhibition of bFGF angiogenic activity, respectively. These experiments show that PDGF-BB and PDGF-AA reduce in vivo bFGF-dependent blood vessel formation.
Chick embryo CAM-sponge assay: microscopic assessment of vascular density on day 12 of incubation
| Sponge loaded with . | No. of specimens . | No. of intersection points . | Microvessel density (%) . |
|---|---|---|---|
| bFGF | 30 | 34 ± 4 | 23.6 |
| PDGF-AA | 30 | 0 | 0 |
| PDGF-BB | 30 | 15 ± 2 | 10.4* |
| bFGF + PDGF-AA | 30 | 20 ± 3 | 13.8* |
| bFGF + PDGF-BB | 30 | 17 ± 3 | 11.8* |
| PBS | 30 | 0 | 0 |
| Sponge loaded with . | No. of specimens . | No. of intersection points . | Microvessel density (%) . |
|---|---|---|---|
| bFGF | 30 | 34 ± 4 | 23.6 |
| PDGF-AA | 30 | 0 | 0 |
| PDGF-BB | 30 | 15 ± 2 | 10.4* |
| bFGF + PDGF-AA | 30 | 20 ± 3 | 13.8* |
| bFGF + PDGF-BB | 30 | 17 ± 3 | 11.8* |
| PBS | 30 | 0 | 0 |
Statistically different (P < 0.001) from bFGF.
Subcutaneous Matrigel assay
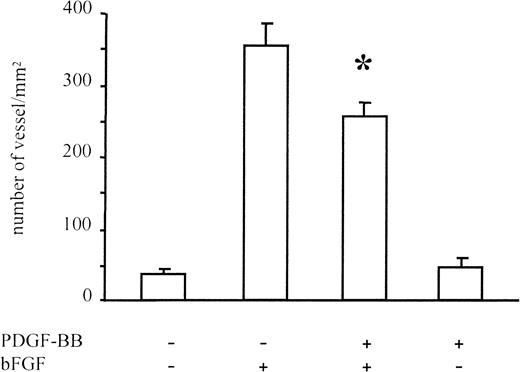
New vessel formation was induced in Matrigel plugs containing bFGF (Figure 8). Under these conditions PDGF-BB alone did not induce the formation of new blood vessels, whereas it inhibited angiogenesis induced by bFGF. Quantification of new vessel formation, carried out by the Quantimet image analyzer, revealed a 30% inhibition of new blood vessel formation in the presence of bFGF/PDGF-BB versus bFGF alone (P < .01). These experiments showed that PDGF-BB reduces in vivo bFGF-dependent blood vessel formation.
Angiogenesis in Matrigel assay.
Angiogenesis was measured in Matrigel plugs injected subcutaneously in CD1 mice. Matrigel was added with bFGF (150 ng/mL), PDGF-BB (150 ng/mL), or both. The bFGF-induced vessel formation was significantly reduced in the presence of PDGF-BB. Data were collected on 8 animals for bFGF alone, 6 animals for PDGF-BB alone, 11 animals for bFGF/PDGF-BB treatment, and 8 animals for negative controls. Data are expressed as average ± SD.
Angiogenesis in Matrigel assay.
Angiogenesis was measured in Matrigel plugs injected subcutaneously in CD1 mice. Matrigel was added with bFGF (150 ng/mL), PDGF-BB (150 ng/mL), or both. The bFGF-induced vessel formation was significantly reduced in the presence of PDGF-BB. Data were collected on 8 animals for bFGF alone, 6 animals for PDGF-BB alone, 11 animals for bFGF/PDGF-BB treatment, and 8 animals for negative controls. Data are expressed as average ± SD.
Discussion
The current study shows that bFGF actions on BAECs are significantly inhibited by PDGF-BB. The native form of the PDGF-BB molecule was required to inhibit bFGF-dependent migration and proliferation; in fact, inhibition was not observed when heat-denatured PDGF-BB was mixed with bFGF. In addition, blocking PDGF-BB with a specific neutralizing antibody abolished the inhibition in migration and proliferation assays, but an aspecific antibody was ineffective. Finally, combining bFGF with EGF did not show any inhibition, confirming that the observed inhibition was a specific event.
We have recently shown37 that PDGF-BB–directed migration and proliferation of smooth muscle cells are inhibited in the presence of bFGF. The inhibition observed was somehow unexpected because PDGF-BB and, to a lesser extent, bFGF are known to be positive regulators of migration and proliferation of these cells. However, there is evidence for opposite effects of PDGF, depending on the receptors expressed on the target cells and, therefore, depending on receptor dimerization events.38,40,53,54 Results presented in other studies show that PDGF-Rα has an intrinsic ability to initiate negative signals,39,41,55 but a direct inhibitory effect of PDGF-Rα on growth factor and FCS activity has never been tested. In this regard it is noteworthy that bFGF is known to up-regulate PDGF-Rα expression in smooth muscle cells56,57 and that patch mice—ie, PDGF-Rα knock-out mice—present severe alterations in the cardiovascular system.58 Therefore, we hypothesized that PDGF-BB may play a negative modulating role in ECs, inhibiting the pro-angiogenic activity of bFGF, through PDGF-Rα signaling.
The PDGF-Rα role was investigated by different approaches. First, PDGF-AA, a specific PDGF-Rα agonist, inhibited bFGF-induced migration with a magnitude comparable to PDGF-BB, which binds both PDGF-Rα and PDGF-Rβ. This indicated that at least 2 members of the PDGF family (AA and BB isoforms) can inhibit bFGF-induced EC migration, and it suggested that the inhibitory effect may require signaling by PDGF-Rα. The putative role of PDGF-Rα was confirmed by impairing PDGF-Rα with neomycin and in DN-PDGF-Rα–transfected cells, whereas impairing PDGF-Rβ did not affect the PDGF-BB inhibitory role. Altogether these observations indicated that the inhibition may be mediated by αα receptor dimers, and not by αβ or ββ dimers. In addition, the phosphorylation of α receptor was greater in the presence of bFGF/PDGF than in the presence of PDGF alone, and the MAP kinase pathway was inhibited in the presence of bFGF/PDGF-BB compared with bFGF alone. The increase of PDGF-Rα phosphorylation and the inhibition of MAP kinases may indicate that the simultaneous presence of bFGF and PDGF might activate PDGF-Rα signaling, targeting specific pathways—for instance, phosphatases—leading to the impairment of MAP kinase–dependent cell functions.
PDGF-BB and bFGF are detected in the serum and within the vascular wall, in normal and pathologic conditions; it has been proposed that altering their balance may underlie, at least in part, vascular wall abnormalities.59 This may help in interpreting the PDGF-BB inhibitory effect on bFGF-induced migration, and it supports the hypothesis that PDGF family members may act as angiogenesis-modulating factors.
The PDGF-BB inhibitory effect was also observed in bFGF-induced angiogenesis in vivo—angiogenesis in CAM and angiogenesis in subcutaneously injected Matrigel plugs. The PDGF-BB effect on the CAM vasculature is still debated. Risau et al31 have shown that PDGF-BB consistently induces an angiogenic response, whereas Oh et al60 demonstrated a network of small α smooth muscle actin-positive vessels in the application area, which may be interpreted as an angiogenic response. On the other hand, in the latter experiments, the intrachorionic capillaries were absent, raising the issue of whether PDGF-BB induced the growth of pre-existing α smooth muscle actin-positive vessels or induced the transformation of capillaries into these vessels. Under our experimental conditions, in in vivo assays, bFGF-induced angiogenesis was significantly inhibited by PDGF-BB, indicating this factor as a possible endogenous inhibitor of bFGF-induced angiogenesis. Interestingly, PDGF-AA, a selective PDGF-Rα agonist, though a poor angiogenic factor in the CAM assay, inhibited bFGF-induced angiogenesis similar to that for PGDF-BB. This result further supports the hypothesis that PDGF-Rα activation may mediate the observed inhibition of bFGF-induced angiogenesis.
The imbalance of positive and negative regulators of vascular cells may underlie many vascular abnormalities.61,62 Modulating factors include the expression of known angiogenesis inhibitors5,63 and the altered expression of PDGF receptors64-66 or bFGF-receptors.67 As reviewed by Battegay et al,18 PDGF is considered a pro-angiogenic factor through an indirect effect, according to in vitro and in vivo experiments. Although PDGF-Rβ expression has been found in several angiogenic processes, conclusive data are still lacking on the role PDGF-Rα plays in angiogenesis. Kanda et al68showed that brain capillary ECs proliferate in response to PDGF-BB, whereas they do not respond to PDGF-AA. In addition, PDGF-AA, while inducing PDGF-Rα autophosphorylation, has no mitogenic activity on microvascular ECs.4 Finally, the expression levels of PDGF-AA have shown an inverse correlation with the proliferation index in aorta, measured in vivo by PCNA staining. In fact, normal adult aorta, which shows a low proliferation index, also shows a high expression level of PDGF-AA compared with fetal aorta, which shows a much higher proliferation index and a significantly lower PDGF-AA level.69 Taken together, these data support the hypothesis of an opposing role of PDGF-Rα and PDGF-Rβ in angiogenesis and are in agreement with a recent report indicating that PDGF-Rα antagonizes PDGF-Rβ.41 The results of the current study may explain, at least in part, the EC quiescent state in vivo and may suggest a dual role of PDGF-BB on the angiogenesis process. In fact, through binding to either β or α receptors, PDGF-BB can act as a pro-angiogenic molecule or as an endogenous inhibitor of angiogenesis, respectively. Given that endothelial cells express PDGF-Rα and PDGF-Rβ, the inhibitory effect reported in this study and the opposite pro- and antiangiogenic effects reported in the literature may depend on the simultaneous presence of different cross-talking stimuli or on the expression levels of PDGF-Rα and PDGF-Rβ. This may also explain, at least in part, different effects of PDGF-Rα signaling in different cell types.
Recent evidence shows that PDGF-BB and bFGF directly interact with high affinity,70 and this may, at least in part, explain the observed inhibitory effect.
In conclusion, the results of the current study show that activating PDGF-Rα triggers negative signals inhibiting the activity of bFGF on EC. They also suggest that these mechanisms may play a role in vascular remodeling when bFGF and PDGF expression are modulated.
We thank Francesco Facchiano for useful discussions. We also thank Gabriella Ricci and Cinzia Carloni for secretarial assistance.
Supported in part by project grants from the European Union (contracts BMH4-CT95-1160 and BMH4-CT97-2270) (F.D.M., M.S., M.C.C., A.F.); from FIRC (Italian Foundation for Cancer Research) and ASI grant ASI I/R/31/00 (A.F.); and from Associazione Italiana per la lotta al Neuroblastoma, Genoa, and Ministero dell' Università e della Ricerca Scientifica, Rome (D.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Antonio Facchiano, Laboratorio di Patologia Vascolare, Istituto Dermopatico dell'Immacolata, Via dei Monti di Creta 104, 00167 Rome, Italy; e-mail: a.facchiano@idi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal