Abstract
Follicular lymphoma is a rare lymphoid malignancy in pediatric patients and consequently remains poorly characterized, particularly with respect to its immunophenotype and molecular pathogenesis. A total of 23 pediatric patients with follicular lymphoma were identified, with a median age of 11 years and a male-to-female ratio of 2.3:1. Of the 19 patients for whom presenting clinical features were available, 15 patients had stage I, 1 had stage II, and 3 had stage III or IV disease. All tumors had a follicular architecture, and 74% of cases had grade 2 or 3 histologic features. All patients expressed CD20 and bcl-6, and 80% were positive for CD10. Bcl-2 expression was detected in only 5 of 16 cases. Consistent with this finding, bcl-2 gene rearrangements were detected in only 2 of 16 cases by polymerase chain reaction. These patients were treated primarily with cyclophosphamide, doxorubicin, vincristine, and prednisone–based chemotherapy; 4 patients also received involved-field irradiation. Of the 13 patients with available clinical follow-up, all but 2 achieved durable clinical remission. Importantly, all 4 patients with tumors diffusely positive for bcl-2 either presented with stage III/IV disease or had disease refractory to therapy, whereas patients with bcl-2–negative tumors uniformly had stage I disease, achieved complete remission, and experienced no relapses. These findings indicate that, in contrast to adult follicular lymphomas, dysregulated bcl-2 expression does not play a significant pathogenetic role in most pediatric follicular lymphomas. However, bcl-2 expression in pediatric follicular lymphoma identifies a subset of patients in whom disease is often disseminated at clinical presentation and is more refractory to combination chemotherapy.
Introduction
Lymphomas in children are typically aggressive malignancies, such as lymphoblastic lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma.1 Follicular lymphoma is quite rare in children, in contrast to the adult population. In several series, follicular lymphoma comprised approximately 1% to 2% of all cases of pediatric lymphomas.2-5 Few reports of follicular lymphoma of childhood have included detailed immunophenotypic and molecular findings. Analyses of a limited number of cases suggest that pediatric follicular lymphoma may have histopathologic features that differ from those typically observed in follicular lymphoma in adults.6-9 Additionally, nearly all cases analyzed to date lack expression of bcl-2. Thus, pediatric follicular lymphoma may differ from its adult counterpart in its pathogenesis. Follicular lymphoma in adults typically pursues an indolent yet progressive clinical course; however, the biologic behavior of pediatric follicular lymphoma has not been well defined. Therefore, we investigated a large series of pediatric follicular lymphomas to better characterize the histopathologic, immunophenotypic, and molecular features of this uncommon childhood malignancy.
Materials and methods
Biopsy material from lymphoma patients
Biopsy material from cases of pediatric follicular lymphoma, with or without a diffuse component, in patients younger than 18 years of age was retrieved from the surgical pathology and consultation files at St Jude Children's Research Hospital (Memphis, TN), Carolinas Medical Center (Charlotte, NC), and the Hammersmith Hospital NHS Trust (London, United Kingdom). The morphologic features of some of the cases have been previously reported.10 The tissue was fixed in either B5 or 10% neutral buffered formalin, embedded in paraffin, and processed routinely. Then, 4-μm–thick sections were prepared and stained with hematoxylin and eosin or used for immunohistochemistry as described below. The histopathology of all cases was reviewed by one of the authors (R.B.L.). Cases were evaluated and graded according to World Health Organization criteria.11 This study was approved by the St Jude Children's Research Hospital institutional review board.
Immunohistochemistry
Immunoperoxidase staining was performed on paraffin sections of formalin-fixed tissue. Briefly, sections were used for immunoperoxidase analysis after heating for 1 hour at 60°C, deparaffinization, and rehydration. Following antigen retrieval, immunohistochemistry was then performed by means of an avidin-biotin peroxidase complex technique on an automated immunostaining module (Ventana, Tucson, AZ). The antibodies and dilutions employed were as follows: CD20, clone L-26, prediluted (Ventana); CD3, 1:50 dilution (DAKO, Carpenteria, CA); CD43, clone DF-T1, 1:25 dilution (DAKO); CD10, clone 56C6, 1:50 (Vector Laboratories, Burlingame, CA); bcl-2, clone 124, 1:10 dilution (DAKO); bcl-6, clone PG-B6p, 1:10 dilution (DAKO); and, CD21, clone 1F8, 1:25 dilution (DAKO).
Polymerase chain reaction to detectbcl-2 gene rearrangements
DNA was extracted from paraffin-embedded tissue as described.12 Polymerase chain reaction (PCR) for n-ras was first performed to confirm the integrity of the extracted DNA (primers: 5′-ACCTGTAGAGGTTAATATC CGCAAATGACT-3′; 5′-AGGAATTCTTACAGAAAACAAGTGGT TATA-3′). To detect bcl-2 translocations occurring within the major breakpoint region, PCR was performed with primers for the mbr (5′-GAGTTGCTTTACGTGGCCTG-3′) and immunoglobulin JH(5′-ACCTGAGGAGACGGTGACC-3′). The PCR was performed under the following conditions: 95°C for 5 minutes (1 cycle); 94°C for 30 seconds and 58.3°C for 1 minute (45 cycles); and 72°C for 3 minutes (1 cycle). PCR products were resolved on a 2% agarose gel containing ethidium bromide. To confirm the specificity of the amplified products, the gels were blotted to nylon membranes (GeneScreen) (NEN, Boston, MA), probed with a32P–end labeled oligonucleotide internal to the mbr primer (5′-TTTCAACACAGACCCACCCAGA-3′), and detected by phosphorimaging (Molecular Dynamics, Sunnyvale, CA). PCR was independently performed twice for each patient to confirm the results, and identical results were obtained in both assays for every patient.
Results
Clinical features
The surgical pathology and consultation files were searched for cases of malignant lymphoma occurring in children that manifested at least a partial follicular growth pattern. A total of 30 cases were identified. Upon review, 7 of these cases were excluded from this study either because (1) they represented high-grade lymphomas, arising primarily in the small bowel, that manifested a follicular growth pattern only focally and that were therefore thought to represent colonization of pre-existing germinal centers by neoplastic cells (6 cases),13 14 or (2) neither the slides nor tissue blocks were available for analysis (1 case). The clinical features of the remaining 23 cases are summarized in Table1. The mean and median ages at presentation were 10.5 and 11 years, respectively; one patient who was included in the study had disseminated lymphadenopathy of at least 3 years' duration prior to her diagnosis at age 20. Males were more commonly affected than females, with a male-to-female ratio of 2.3:1. Disease stage at presentation was available for 19 patients. Strikingly, 15 of 19 (79%) patients presented with isolated lymphadenopathy and stage I disease; the remainder of the patients consisted of 1 patient with stage II, 2 patients with stage III, and 1 patient with stage IV disease. The lymphomas were nodal or tonsillar in all but 2 patients. There was marked predilection for involvement of head and neck sites, particularly the tonsil, with 13 of 16 (81%) stage I or II patients having disease in the head and neck region. The 2 visceral tumors involved kidney and testicular epididymis. Clinical follow-up was obtained on 13 patients, with mean and median follow-ups of 11.4 and 9.1 years, respectively. Most patients were treated on a cyclophosphamide, doxorubicin, vincristine, and prednisone–based chemotherapy regimen; 4 patients also received involved-field irradiation. All but 1 patient (patient 11; Table 1) achieved complete clinical remission. This patient presented with stage III disease, had a poor response to therapy, and eventually succumbed to his disease nearly 8 years after initial diagnosis. One patient has developed multiple recurrences (patient 5; Table 1); he underwent stem cell transplantation and has not experienced disease recurrence to date.
Clinical features of pediatric patients with follicular lymphoma
| Patient . | Age at presentation . | Sex . | Involved sites . | Disease stage . | Tumor grade . | Clinical follow-up . |
|---|---|---|---|---|---|---|
| 1 | 13 | F | T | NA | 3 | NA |
| 2 | 4 | M | Hn | NA | 1 | NA |
| 3 | 8 | M | T | I | 1 | NED |
| 4 | 11 | F | C | I | 3 | NA |
| 5 | 13 | M | T | I | 3 | MR; NED after PBSCT |
| 6 | 17 | F | E | IV | 1 | NA |
| 7 | 4 | M | T | I | 3 | NED |
| 8 | 14 | M | I | I | 2 | NED |
| 9 | 15 | M | T | I | 2 | NED |
| 10 | 17 | M | C | I | 3 | NED |
| 11 | 17 | M | I, C | III | 1 | DOD |
| 12 | 20 | F | A, I | III | 1 | NA |
| 13 | 7 | M | T | NA | 3 | NA |
| 14 | 12 | M | C | I | 2 | NA |
| 15 | 14 | F | Hn | I | 2 | NA |
| 16 | 4 | M | E | I | 3 | NED |
| 17 | 5 | M | I | I | 2 | NED |
| 18 | 3 | M | Hn | I | 2 | NED |
| 19 | 4 | F | T | I | 2 | NED |
| 20 | 10 | M | Hn | I | 2 | NED |
| 21 | 13 | M | C | NA | 1 | NA |
| 22 | 5 | F | T, C | II | 3 | NA |
| 23 | 11 | M | He | I | 3 | NED |
| Patient . | Age at presentation . | Sex . | Involved sites . | Disease stage . | Tumor grade . | Clinical follow-up . |
|---|---|---|---|---|---|---|
| 1 | 13 | F | T | NA | 3 | NA |
| 2 | 4 | M | Hn | NA | 1 | NA |
| 3 | 8 | M | T | I | 1 | NED |
| 4 | 11 | F | C | I | 3 | NA |
| 5 | 13 | M | T | I | 3 | MR; NED after PBSCT |
| 6 | 17 | F | E | IV | 1 | NA |
| 7 | 4 | M | T | I | 3 | NED |
| 8 | 14 | M | I | I | 2 | NED |
| 9 | 15 | M | T | I | 2 | NED |
| 10 | 17 | M | C | I | 3 | NED |
| 11 | 17 | M | I, C | III | 1 | DOD |
| 12 | 20 | F | A, I | III | 1 | NA |
| 13 | 7 | M | T | NA | 3 | NA |
| 14 | 12 | M | C | I | 2 | NA |
| 15 | 14 | F | Hn | I | 2 | NA |
| 16 | 4 | M | E | I | 3 | NED |
| 17 | 5 | M | I | I | 2 | NED |
| 18 | 3 | M | Hn | I | 2 | NED |
| 19 | 4 | F | T | I | 2 | NED |
| 20 | 10 | M | Hn | I | 2 | NED |
| 21 | 13 | M | C | NA | 1 | NA |
| 22 | 5 | F | T, C | II | 3 | NA |
| 23 | 11 | M | He | I | 3 | NED |
T indicates tonsil; NA, not available; Hn, other head and neck nodal site; NED, no evidence of disease; C, cervical lymph node; MR, multiple relapses; PBSCT, peripheral blood stem cell transplant; E, extranodal (kidney, 1 case; epididymis, 1 case); I, inguinal lymph node; DOD, dead of disease; A, axillary lymph node; He, other head and neck extranodal site.
Histopathology
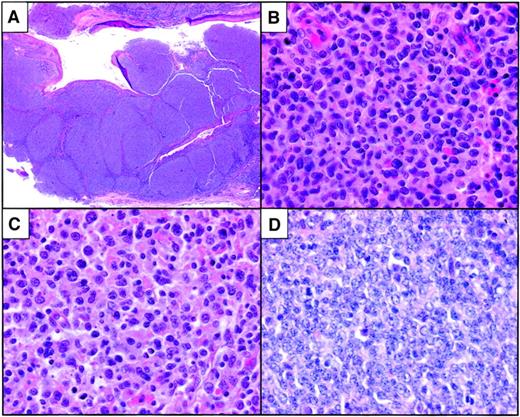
In most cases, there was complete or near-total effacement of the nodal architecture by a nodular proliferation of malignant cells. Well-formed mantle zones were generally absent. In several cases, the nodules were quite expansive and irregular and were coalescent in some areas (Figure 1A). One case showed the “floral” growth pattern.15 16Cytologically, most tumors contained an admixture of centroblasts with prominent nucleoli and variably abundant cytoplasm together with a variable component of centrocytic cells that contained irregular, clefted nuclei; condensed chromatin; and scant cytoplasm (Figure 1B-C). Centroblasts with irregular nuclei, ie, large cleaved cells, were predominant in some cases. Overall, 74% of the tumors were of intermediate or high histologic grade (Table 1). In some cases, the mitotic rate was quite high and frequent apoptotic cells were present (Figure 1D). Geographic necrosis was not a prominent feature of any of the tumors. Occasional tumors contained admixed tingible-body macrophages, imparting a “starry sky” appearance to the neoplasm.
Histopathological features of pediatric follicular lymphoma.
(A) Low-grade tumor with large, focally coalescent nodular architecture. (B) Higher-power magnification of a case with predominantly intermediate-grade cytologic features. A grade 3 diffuse component was focally present in the tumor. (C) One case with floral variant morphology was composed predominantly of centroblasts, some of which had cleaved-cell morphology. (D) High-grade tumor with brisk mitotic rate and frequent apoptotic cells. Original magnification A, × 10; B-D, × 400. All images were stained with hematoxylin and eosin.
Histopathological features of pediatric follicular lymphoma.
(A) Low-grade tumor with large, focally coalescent nodular architecture. (B) Higher-power magnification of a case with predominantly intermediate-grade cytologic features. A grade 3 diffuse component was focally present in the tumor. (C) One case with floral variant morphology was composed predominantly of centroblasts, some of which had cleaved-cell morphology. (D) High-grade tumor with brisk mitotic rate and frequent apoptotic cells. Original magnification A, × 10; B-D, × 400. All images were stained with hematoxylin and eosin.
Immunohistochemistry
Paraffin-embedded tissue was available on 17 cases for immunohistochemical analysis. The results of immunophenotyping studies are summarized in Table 2; the case numbers are identical with the patient numbers in Table 1. The neoplastic cells were strongly positive for CD20 in all cases (Figure2A). Nearly all tumors were positive for bcl-6 in most cells (Figure 2B). CD10 was expressed in 12 of 15 (80%) cases, and CD43 was positive in 4 of 17 (24%) tumors. Immunostaining for CD21 revealed prominent, expanded networks of follicular dendritic cells in all tumors, further supporting the follicular nature of the malignant cells (Figure 2C). Immunostaining for bcl-2 revealed diffuse positivity in 4 of 16 (25%) cases (Figure 2D). One tumor showed expression of bcl-2 focally within a subset of the neoplastic nodules (data not shown). The remaining 11 cases did not express bcl-2.
Immunophenotypic and molecular characteristics of pediatric follicular lymphoma
| Case no.* . | CD20 . | CD43 . | CD10 . | bcl-6 . | bcl-2 . | CD21 + FDC present . | |
|---|---|---|---|---|---|---|---|
| IHC . | PCR . | ||||||
| 1 | + | + | + | + | − | − | Y |
| 2 | + | − | − | + | − | − | Y |
| 3 | + | − | − | + | − | − | Y |
| 4 | + | − | +, F | +, F | − | − | Y |
| 5 | + | − | ND | + | + | + | Y |
| 6 | + | − | + | + | + | − | Y |
| 7 | + | + | + | + | − | − | ND |
| 8 | + | − | − | + | − | − | ND |
| 9 | + | − | ND | ND | ND | − | ND |
| 10 | + | + | + | + | − | − | Y |
| 11 | + | − | + | + | + | + | Y |
| 12 | + | − | + | + | + | − | Y |
| 13 | + | + | + | + | − | − | Y |
| 14 | + | − | + | + | +, F | − | Y |
| 15 | + | − | + | + | − | − | Y |
| 16 | + | − | + | + | − | − | Y |
| 17 | + | − | + | + | − | − | Y |
| Case no.* . | CD20 . | CD43 . | CD10 . | bcl-6 . | bcl-2 . | CD21 + FDC present . | |
|---|---|---|---|---|---|---|---|
| IHC . | PCR . | ||||||
| 1 | + | + | + | + | − | − | Y |
| 2 | + | − | − | + | − | − | Y |
| 3 | + | − | − | + | − | − | Y |
| 4 | + | − | +, F | +, F | − | − | Y |
| 5 | + | − | ND | + | + | + | Y |
| 6 | + | − | + | + | + | − | Y |
| 7 | + | + | + | + | − | − | ND |
| 8 | + | − | − | + | − | − | ND |
| 9 | + | − | ND | ND | ND | − | ND |
| 10 | + | + | + | + | − | − | Y |
| 11 | + | − | + | + | + | + | Y |
| 12 | + | − | + | + | + | − | Y |
| 13 | + | + | + | + | − | − | Y |
| 14 | + | − | + | + | +, F | − | Y |
| 15 | + | − | + | + | − | − | Y |
| 16 | + | − | + | + | − | − | Y |
| 17 | + | − | + | + | − | − | Y |
IHC indicates immunohistochemistry; PCR, polymerase chain reaction; FDC, follicular dendritic cell; Y, yes; F, immunoreactivity for the marker being present only focally; ND, not done or not interpretable.
Case numbers are identical with the patient numbers used in Table 1.
Immunophenotypic features of pediatric follicular lymphoma.
(A) Immunohistochemistry for CD20 highlights the follicular architecture of this tumor. (B) The neoplastic cells of nearly all cases were positive for bcl-6. (C) Most of the cases manifested expanded networks of follicular dendritic cells as highlighted in this immunostain for CD21. (D) Immunostaining for bcl-2 reveals moderately intense expression of bcl-2 by neoplastic lymphoma cells within the nodules. Original magnification A, × 10; B, × 200; C-D, × 100; insets, × 400.
Immunophenotypic features of pediatric follicular lymphoma.
(A) Immunohistochemistry for CD20 highlights the follicular architecture of this tumor. (B) The neoplastic cells of nearly all cases were positive for bcl-6. (C) Most of the cases manifested expanded networks of follicular dendritic cells as highlighted in this immunostain for CD21. (D) Immunostaining for bcl-2 reveals moderately intense expression of bcl-2 by neoplastic lymphoma cells within the nodules. Original magnification A, × 10; B, × 200; C-D, × 100; insets, × 400.
Molecular analysis of bcl-2 gene rearrangements
None of the cases had frozen material available for Southern blot analysis. Therefore, to detect the t(14;18) involving the major breakpoint region of the bcl-2 gene, PCR was performed on DNA extracted from paraffin-embedded tissue. The amplified products were blotted and probed with a radiolabeled oligonucleotide probe to confirm the specificity of the amplified products. The quality of the extracted DNA was first assessed by performing control PCR reactions for ras; amplifiable DNA was obtained for 16 cases. The bcl-2 PCR results are shown in Figure3 and summarized in Table 2; the lane numbers in the figure correspond to the case numbers in Tables 1 and 2. Two of 16 cases were positive for bcl-2 gene rearrangements (Figure 3). The case shown in lane 12 had only a weakly detectable bcl-2 signal; however, the quality of DNA obtained in this case was suboptimal, as indicated by the weak signal obtained from the ras PCR (data not shown). Both of the bcl-2 PCR-positive cases also expressed bcl-2 protein as determined by immunohistochemistry (Table 2).
PCR to detect t(14;18) at the mbr.
PCR was performed as described in “Materials and methods” and subsequently subjected to Southern analysis by means of a radiolabeled internal oligonucleotide probe. − indicates negative water control; +, positive control. Lanes 1 through 16 are pediatric follicular lymphoma cases. A faint band was present in lane 11; this was confirmed on longer exposure of the autoradiogram. The lane numbers correspond directly to the patient and case numbers in Tables 1 and 2.
PCR to detect t(14;18) at the mbr.
PCR was performed as described in “Materials and methods” and subsequently subjected to Southern analysis by means of a radiolabeled internal oligonucleotide probe. − indicates negative water control; +, positive control. Lanes 1 through 16 are pediatric follicular lymphoma cases. A faint band was present in lane 11; this was confirmed on longer exposure of the autoradiogram. The lane numbers correspond directly to the patient and case numbers in Tables 1 and 2.
Discussion
Follicular lymphoma is a rare lymphoid malignancy in children; consequently, the pathogenesis and biologic behavior of this uncommon entity are poorly understood. Here we present the first analysis of a large series of pediatric follicular lymphoma that includes morphologic, immunophenotypic, and molecular characterization. Additionally, we have long-term clinical follow-up on a significant number of patients to permit a more complete assessment of the clinical behavior of follicular lymphoma in this patient population.
In some recent reports of pediatric follicular lymphoma, the majority of cases were extranodal with a striking propensity for testicular involvement.8,9,17 In the current study and one other, however, the majority of patients had nodal- or tonsillar-based disease, with a marked predilection for head and neck sites.7 We encountered only 1 case of a paratesticular tumor. The reason for this difference in clinical presentation between the various studies is unclear but may be attributable to the small numbers of patients included in some of the studies.
The majority of these cases had typical histologic features of follicular lymphoma. Involved lymph nodes generally showed complete effacement of normal architecture. In contrast to follicular lymphoma in adults, these pediatric tumors commonly had high histologic grades, with only 24% of cases having grade 1 features. Others have similarly shown that follicular lymphoma in the pediatric setting tends to manifest high-grade histology.7,8,10 All of the tumors expressed bcl-6 and most expressed CD10 as well; immunophenotypic features are compatible with a follicular center cell origin for these neoplasms.18-20 Interestingly, 24% of cases were positive for CD43, a marker infrequently expressed in follicular lymphoma in adults. Immunostaining for CD21 demonstrated expanded networks of follicular dendritic cells within the nodules of most tumors, including the extranodal cases, further supporting their follicular origin. Importantly, we found that bcl-2 expression in pediatric follicular lymphoma is relatively infrequent, in contrast to its adult counterpart.
Because fresh or frozen material was not available for any of the cases in this study, we were unable to assess these tumors for clonal immunoglobulin gene rearrangements by Southern analysis. At diagnosis, however, 2 of the bcl-2–negative tumors were clonal as determined by either flow cytometry to assess immunoglobulin light chain restriction or PCR to detect immunoglobulin heavy chain rearrangements (data not shown). Further, the neoplastic nature of these tumors is supported by the fact that 9 of the 11 bcl-2–negative tumors had grade 2 or 3 histologic features.
Bcl-2 is a mitochondrial protein that plays a central role in resistance to apoptosis.21 The bcl-2 gene was first identified by virtue of its involvement in the t(14;18) reciprocal translocation with the immunoglobulin heavy chain locus that is characteristically present in the majority of follicular lymphomas in adults.22-24 Bcl-2 is physiologically expressed in certain lymphoid subsets, including T cells and mantle B cells. However, germinal-center B cells do not normally express bcl-2. Aberrant expression of bcl-2 by a centrocytic B cell, either as a result of the t(14;18) or through other mechanisms, is thought to play an important role in the pathogenesis of most cases of follicular lymphoma. The frequency of bcl-2 expression in follicular lymphoma does vary somewhat according to the histologic grade.25-27 Most large studies have shown that 83% to 100% of grade 1 and 2 tumors and approximately 75% of grade 3 tumors are bcl-2 positive by immunohistochemistry.
There are limited data regarding the expression of bcl-2 in pediatric follicular lymphoma.6-9 Finn et al8 recently reported the immunophenotypic and molecular features of 4 cases of follicular lymphoma, all of which were primary testicular tumors in young boys. They found that the tumors in their series were uniformly negative for bcl-2 protein expression by immunohistochemistry and were negative for rearrangements of the bcl-2 locus. In an analysis of 7 pediatric follicular lymphoma patients, Atra et al7 found that 3 of 4 of cases investigated were negative for bcl-2 expression by immunohistochemistry. Case reports of testicular follicular lymphomas in children have yielded similar findings.9 17
In contrast to these studies, our analysis revealed that although less common than in its adult counterpart, bcl-2 expression is not infrequently seen in follicular lymphoma occurring in the pediatric population. Approximately 30% of cases were positive for bcl-2 as determined by immunohistochemistry. Only 2 of these cases were also positive for the t(14;18) by PCR, suggesting that the aberrant bcl-2 expression either was due to a t(14;18) involving the mcr (which would not be detected by our PCR assay) or occurred through an alternative mechanism. Interestingly, all 4 of the patients with bcl-2–positive tumors were 13 years of age or older at the time of diagnosis. With the exception of a single case,7 all pediatric patients under 12 years of age with follicular lymphoma have had tumors that were negative for bcl-2.6-10 Collectively, these findings suggest that bcl-2–positive follicular lymphoma in young children and preadolescents is rare; however, tumors arising in older children not infrequently express bcl-2. Most importantly, our findings suggest that the expression of bcl-2 in pediatric follicular lymphoma may have prognostic significance. Three of 4 patients with bcl-2–positive tumors presented with either stage III or stage IV disease, whereas all patients with tumors negative for bcl-2 had low-stage disease. The 2 patients with long-term follow-up who had refractory or recurrent disease both had bcl-2–positive tumors. One of these patients eventually succumbed to his disease, and the other patient developed multiple relapses. This second patient ultimately underwent stem cell transplantation and is now disease free, with limited posttransplantation follow-up. In their analysis of 7 patients with pediatric follicular lymphoma, Atra et al7 found that 6 achieved clinical remission with limited follow-up (median, 1.5 years). One patient in this study died of disease; however, it is not certain whether this patient's tumor was positive for bcl-2. Taken together, the results of our study suggest that bcl-2 expression is less frequent in pediatric follicular lymphoma than in its adult counterpart and that pediatric patients whose tumors lack bcl-2 expression appear to have limited-stage disease and excellent disease-free and overall survival when treated with multiagent chemotherapy.
Our results suggest that in most instances follicular lymphoma in children has a pathogenesis that is distinct from its counterpart in the adult population. This is not altogether surprising given the differences in the clinical course of follicular lymphoma in these 2 populations. Adults with follicular lymphoma most commonly present with high-stage disease and an indolent yet progressive clinical course that is refractory to current chemotherapeutic approaches, including bone marrow transplantation. In contrast, our findings and those of others7 8 have shown that follicular lymphoma in pediatric patients usually presents with localized, low-stage disease that can be successfully treated with conventional multiagent chemotherapy regimens with durable remissions.
Finally, our results also have relevance for the surgical pathologist confronted with a lymph node biopsy from a child containing a nodular lymphoid proliferation. Bcl-2 expression is not diagnostic of any particular type of lymphoma.25,28 However, immunohistochemistry to detect bcl-2 is often helpful in evaluating difficult lymphoproliferative processes in which the differential diagnosis includes follicular lymphoma and a florid follicular hyperplasia, since reactive follicular center cells are negative for bcl-2.29-31 We now show that although bcl-2 expression is lacking in the majority of pediatric follicular lymphomas, its expression is of use in diagnosing a malignant follicular lymphoma in up to 30% of these cases. The absence of its expression in the majority of these pediatric tumors suggests a fundamental difference in the molecular pathogenesis of pediatric and adult follicular lymphomas.
We would like to thank Ms Charlene Henry for expert assistance with the immunohistochemistry, Drs Sheila Shurtleff and Geoff Neale for advice on the PCR, and Dr Jim Downing for critical review of the manuscript. We also thank the following physicians and clinical personnel for contributing clinical information: Dr C. Abramowsky, Dr Frank R. Eldridge, Dr John P. Hanson Jr, Dr Allen Korenblit, Dr Ihkoo Park, and Nita Raidy.
Supported in part by National Institutes of Health Cancer Center CORE grant CA21765 and by the American Lebanese and Syrian Associated Charities of St Jude Children's Research Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert Lorsbach, Department of Pathology, St Jude Children's Research Hospital, 332 N Lauderdale St, Memphis, TN 38105; e-mail: robert.lorsbach@stjude.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal