Abstract
This study explored whether repeated infusions of intravenous anti-D could allow adults with recently diagnosed immune thrombocytopenic purpura (ITP) who had failed an initial steroid course to postpone and ultimately avoid splenectomy. Twenty-eight Rh+, nonsplenectomized adults with ITP diagnosed within 1 to 11 months and platelet counts 30 × 109/L (30 000/μL) or below were enrolled. Anti-D was infused whenever the platelet count decreased to 30 × 109/L (30 000/μL) or below. “Response” was defined as a platelet increase of more than 20 × 109/L (20 000/μL) to more than 30 × 109/L (30 000/μL) within 7 days of treatment. Patients were a median 3.5 months from ITP diagnosis at enrollment and had received a median of 2 previous therapies, including prednisone in 26 of 28 cases. They were followed for a median 26 months. A total of 93% responded to their initial infusion of anti-D, and 68% repeatedly responded with counts maintained above 30 × 109/L (30 000/μL) using anti-D alone. Currently, 12 (43%) of 28 patients have been off all treatment for more than 6 months without undergoing splenectomy, 6 maintaining counts above 100 × 109/L (100 000/μL). Seven continue on treatment, 8 underwent splenectomy, and 1 was lost to follow-up at 10 months. One patient discontinued anti-D because of toxicity. Patients with platelet counts at least 14 × 109/L (14 000/μL) at enrollment were more likely to discontinue treatment (P < .05). Anti-D was an effective maintenance treatment for two thirds of Rh+, nonsplenectomized adults with ITP who had failed an initial steroid course. Intermittent infusions of intravenous anti-D allowed more than 40% of these adults to avoid splenectomy and to achieve stable platelet counts off all therapy, even after many months of treatment. Platelet count at study entry was the primary predictor of outcome.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune bleeding disorder in which antiplatelet antibodies cause accelerated platelet destruction by the mononuclear phagocytic system. Patients suffering from persistent, severe thrombocytopenia are at risk for hemorrhage and have an increased mortality rate.1 2Many patients, however, maintain a normal quality of life at below normal platelet counts with or without treatment.
Unlike children, less than one third of adults with this disease will go into sustained remission after an initial short course of steroids.2-8 The American Society of Hematology practice guidelines for ITP in 1996 show that there is considerable variation in the recommendations of expert hematologists on the treatment of those patients who do not go into remission.2 Generally, the standard of practice for patients who cannot maintain an adequate platelet count on tapering steroids is to undergo splenectomy. In 60% to 70% of patients this restores a normal platelet count for at least 5 to 10 years.9-11 It allows those patients who respond to this procedure to avoid both the risks of low platelet counts and the toxicity of daily steroids. However, although both the site of platelet destruction and the response to intravenous immunoglobulin (IVIG) have been suggested to predict response to splenectomy, there is no universally acknowledged way to predict which patients will respond.12-16 Patients are often reluctant to be exposed to the inherent risks of surgery and the small but significant risk of overwhelming sepsis after splenectomy without a guarantee of success.17 18
Because the standard of practice has been to perform early splenectomy in steroid nonresponders, little is known of the natural history of those patients who do not go into early remission. This pilot study was designed to assess how many patients would improve their platelet counts to a level sufficient to avoid splenectomy if they were supported with intermittent infusions of IV anti-D to maintain their platelet count above 30 × 109/L (30 000/μL). This platelet count was chosen as the threshold for treatment so that patients would be protected from the risks of serious hemorrhage while minimizing unnecessary treatment. IV anti-D was chosen in preference to IVIG because it is substantially less expensive, has a much shorter infusion time, and is made from a smaller donor pool. It was selected in preference to prednisone because it does not have the toxicities associated with the prolonged use of daily steroids.
A secondary aim of this study was to assess whether any clinical or biologic variables could predict which patients would respond to anti-D treatment and, in particular, which patients would improve their counts sufficiently to discontinue therapy and avoid splenectomy.
Patients and methods
Twenty-eight patients, including 7 men and 21 women, were enrolled between May 1996 and March 1999 from the 31 eligible patients referred for this institutional review board–approved study at the Platelet Disorders Center at the New York Presbyterian Hospital; 3 patients declined to participate. Patients were eligible if they had been diagnosed within the last year (but not within the last month) with primary ITP; had no history or findings suggestive of human immunodeficiency virus; had platelet counts 30 × 109/L (30 000/μL) or below at study entry; were Rh+; and had not undergone splenectomy. All eligible patients were invited to participate, including patients with profound thrombocytopenia and those with active bleeding. Patients were not excluded on the basis of previous treatment, and if on prednisone at the time of study this was quickly tapered. Patients also had to be appropriate candidates for splenectomy. After their initial treatment and 1-week follow-up at the New York Presbyterian Hospital, most patients were managed by their referring hematologists.
Patients were randomized for their initial treatment to receive either 50 μg/kg or 75 μg/kg of anti-D (WinRho SDF; Cangene, Winnipeg, MB). The data describing the acute platelet increases and hemoglobin decreases of these 2 doses have been reported separately.19 There was no distinction in the dose effect on the outcome of this study, and it is not therefore used or analyzed here. Subsequent to the initial infusion, patients received 50 to 75 μg/kg for each treatment, rounded off to the nearest multiple of 300 μg.
Treatment was administered whenever the platelet count was 30 × 109/L (30 000/μL) or below. If patients maintained a stable platelet count above 30 × 109/L (30 000/μL) for more than 4 weeks and then had a single count between 20 × 109/L to 30 × 109/L (20 000-30 000/μL) in the absence of bleeding symptoms, treatment might not be administered at the discretion of the investigator provided that a subsequent count was 30 × 109/L (30 000/μL) or more.
Complete blood counts with platelet counts were obtained on days 0, 1, 7, 14, and 21 of therapy for the first infusion and weekly thereafter. The interval between these counts was extended to every 2 to 4 weeks in patients maintaining stable platelet counts. Plasma levels of glycocalicin20 and soluble CD16 (sCD16) levels21 were measured using an ELISA technique as previously described, prior to the first infusion in 17 and 22 patients, respectively. Twenty-five patients were assessed for their CDE rhesus phenotype to estimate the number of D sites on their red cells.22
Patients could receive IV anti-D for up to 1 year as part of the study. They were seen as clinically indicated and at least monthly for the first 12 months and at 3- to 6-month intervals until March 2001, until they underwent splenectomy, or until they were lost to follow-up.
Patients were assessed for signs of bleeding at their clinic visits and completed weekly diaries of their bleeding symptoms during the study period. In addition, they answered a series of specific questions at baseline, 6, 12, and 18 months aimed at determining their change in quality of life during the period of the study.
The questions analyzed were as follows: (1) I am as healthy as anybody I know. (2) Compared to 1 year ago, how would you rate your health in general now? (3) During the past 4 weeks, how much of the time did you have a lot of energy? (4) During the past 4 weeks, how much of the time did you feel tired? (5) During the past 4 weeks, to what extent has your physical health or emotional problems interfered with your normal social activities with family and friends? (6) During the past 4 weeks, how much of the time did you feel full of pep?
Response criteria
Acute response to anti-D was defined as a platelet increment of 20 × 109/L (20 000/μL) or more to a count above 30 × 109/L (30 000/μL) within 7 days of treatment. Responders were continued on intermittent IV anti-D as needed but were given the option of alternative therapy including splenectomy throughout the study. Patients who did not respond were offered alternative therapy including splenectomy. Patients were considered “off treatment” if they maintained a platelet count above 30 × 109/L (30 000/μL) without treatment for at least 6 months.
Patients were classified depending on their treatment status as of their last clinic visit. Group A includes patients who were considered off treatment. Group B includes patients who received treatment within 6 months of their last visit. This group included patients who (1) continued to receive anti-D; (2) received medication for their ITP other than anti-D, such as IVIG, prednisone, or danazol; and (3) received medication for other medical conditions that could have affected their platelet count. Group C includes patients who underwent splenectomy.
Cost analysis
Cost analysis for the period of this study was performed assuming the average cost of anti-D as $80 per 300-μg vial and the average cost of splenectomy as $16 000.23 The actual number of vials used per patient was used in the calculations.
Statistical analysis
The mean, median, SD, and range were used to describe baseline variables, including age at study entry, months of ITP diagnosis, platelet counts before and after treatment, duration of response to treatment, posttreatment hemoglobin change, and initial mean corpuscular volume (MCV). The differences between the 3 outcome groups for these parameters were analyzed using the Kruskal-Wallis test and Mann-Whitney U test. The paired t test was used to assess the difference between the number of infusions of anti-D received before and after 12 months on study and to compare the health survey questions at 6, 12, and 18 months when compared with baseline. Relationships between the biologic variables were assessed using the Spearman rank correlation coefficient.
Results
Patients were a median age of 28.5 years (range, 18-63 years) and a median of 3.5 months (range, 1-11 months) from their diagnosis of ITP at study initiation. As of March 2001, there was a median 26 months (range, 6-54 months) follow-up on study. Prior to study entry, 26 of the 28 patients had received a course of steroids; 15 had received IVIG; 4, danazol; 3, high-dose dexamethasone; and 6, IV anti-D. Fourteen patients had previously received both IVIG and steroids. Only 1 patient had received no previous ITP therapy.
Twelve-month interim outcome
Patients were classified into the outcome groups described in “Patients and methods.” The percentage of patients in each outcome group for the first 12 months of the study is shown in Figure1. In summary, 26 (93%) of 28 patients responded to their initial infusion of IV anti-D (increase 20 × 109/L [20 000/μL] or more to a level above 30 × 109/L [30 000/μL]). Nineteen (68%) repeatedly responded with their platelet counts being maintained above 30 × 109/L (30 000/μL) on intermittent infusions of IV anti-D alone. Three of these 19 discontinued anti-D before the 12-month follow-up point for reasons unrelated to their platelet count. One developed breast cancer and received chemotherapy (patient no. 11, Figure 2), 1 elected to undergo splenectomy after 9 months despite continuing to respond to anti-D (no. 24), and 1 was lost to follow-up at 10 months (no. 16). Five of the 19 improved sufficiently to discontinue treatment, and 11 were still receiving intermittent doses of IV anti-D at 12 months. Of the 9 patients who could not be maintained on IV anti-D alone, 1 could not tolerate IV anti-D due to persistent anemia and repeated chill reactions despite premedication (no. 10), 2 did not respond to their first infusion, and 6 stopped responding within the first 4 months. Four of the 8 nonresponders underwent splenectomy, and 4 chose alternative therapy for their ITP (described below).
A bar chart representing the percentage of patients in each outcome group for each month up to and including month 12.
The x-axis is time in months. The y-axis is the percentage of patients in each outcome group. Outcome groups are identified as follows: Patients who underwent splenectomy are represented in yellow; patients who received other medical therapy such as chemotherapy for breast cancer are shown in dark blue; patients who received other ITP therapy such as IVIG, prednisone, or danazol are shown in intermediate blue; patients who were still on intermittent anti-D are shown in light blue; and patients who came off treatment are shown in pink.
A bar chart representing the percentage of patients in each outcome group for each month up to and including month 12.
The x-axis is time in months. The y-axis is the percentage of patients in each outcome group. Outcome groups are identified as follows: Patients who underwent splenectomy are represented in yellow; patients who received other medical therapy such as chemotherapy for breast cancer are shown in dark blue; patients who received other ITP therapy such as IVIG, prednisone, or danazol are shown in intermediate blue; patients who were still on intermittent anti-D are shown in light blue; and patients who came off treatment are shown in pink.
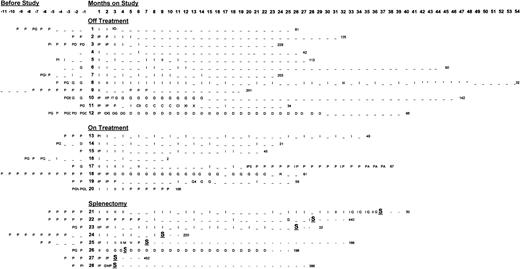
All 28 patients from diagnosis to last platelet count and their month-by-month treatment for their ITP from diagnosis to last known platelet count.
The x-axis represents time in months; the y-axis is composed of individual patients divided according to outcome group into which they fall: group A, “off treatment”; group B, “receiving treatment (anti-D or other)”; and group C, those undergoing “splenectomy.” The letters represent ITP treatments (explained in the key). The number at the end of each line is the last known platelet count for each patient. I indicates anti-D treatment; –, no treatment; S, splenectomy; D, danazol; G, IVIG; P, prednisone; M, IV methylprednisolone; A, azathioprine; R, rituxan; E, dexamethasone; 1, IVIG for surgery; 2, lost to follow-up; 3, prednisone for SLE (not low platelets); 4, IVIG for obstetric reasons; 5, chemotherapy for breast cancer; T, toxicity; C, chemotherapy; X, radiotherapy; and *, intermittent counts less than 30 000/uL, no treatment given.
All 28 patients from diagnosis to last platelet count and their month-by-month treatment for their ITP from diagnosis to last known platelet count.
The x-axis represents time in months; the y-axis is composed of individual patients divided according to outcome group into which they fall: group A, “off treatment”; group B, “receiving treatment (anti-D or other)”; and group C, those undergoing “splenectomy.” The letters represent ITP treatments (explained in the key). The number at the end of each line is the last known platelet count for each patient. I indicates anti-D treatment; –, no treatment; S, splenectomy; D, danazol; G, IVIG; P, prednisone; M, IV methylprednisolone; A, azathioprine; R, rituxan; E, dexamethasone; 1, IVIG for surgery; 2, lost to follow-up; 3, prednisone for SLE (not low platelets); 4, IVIG for obstetric reasons; 5, chemotherapy for breast cancer; T, toxicity; C, chemotherapy; X, radiotherapy; and *, intermittent counts less than 30 000/uL, no treatment given.
Long-term outcome
The long-term outcome (to March 2001) of all patients, including their month-by-month treatment from diagnosis until their last month of follow-up, is illustrated in Figure 2 and discussed by groups below. As of their last follow-up, 12 (43%) of the initial 28 patients have been off all treatment for at least 6 months with a stable platelet count above 30 × 109/L (30 000/μL) (group A). Seven (25%) continue to receive treatment (group B): 3 with IV anti-D, 1 with IVIG and then rituximab, and 1 with low-dose prednisone. One patient received prednisone for lupus, 1 received 3 infusions of IVIG, and 1 IV anti-D during pregnancy. Eight (29%) underwent splenectomy (group C). The patient lost to follow-up at 10 months, who had not received anti-D for 5 months as of his last visit, is not included in the analyses of long-term outcome. The background demographics for each outcome group, including months from diagnosis to study entry, age, gender, and previous treatments are summarized in Table1.
Summary of patient characteristics at enrollment
| Outcome group . | Median no. of months from ITP diagnosis to enrollment (range) . | Median age at enrollment, y (range) . | Gender (F, M) . | Steroids* . | IVIG* . | Danazol* . |
|---|---|---|---|---|---|---|
| Off treatment (n = 12) | 4 (1-11) | 29.5 (21-63) | 8, 4 | 10 | 9 | 2 |
| On treatment (n = 7) | 2 (1-11) | 29 (18-56) | 6, 1 | 7 | 4 | 1 |
| Splenectomy (n = 8) | 2 (2-10) | 25 (20-61) | 7, 1 | 8 | 3 | 1 |
| Outcome group . | Median no. of months from ITP diagnosis to enrollment (range) . | Median age at enrollment, y (range) . | Gender (F, M) . | Steroids* . | IVIG* . | Danazol* . |
|---|---|---|---|---|---|---|
| Off treatment (n = 12) | 4 (1-11) | 29.5 (21-63) | 8, 4 | 10 | 9 | 2 |
| On treatment (n = 7) | 2 (1-11) | 29 (18-56) | 6, 1 | 7 | 4 | 1 |
| Splenectomy (n = 8) | 2 (2-10) | 25 (20-61) | 7, 1 | 8 | 3 | 1 |
Number of patients in each outcome group receiving these treatments prior to enrollment.
Group A.
The 12 patients who are now off all treatment were enrolled a median of 4 months (range, 1-11 months) from diagnosis. They received a median of 11.5 months (range, 3-41 months) of treatment on study. The median time from diagnosis to their last treatment was 16 months (range, 7-45 months). Eight of the 12 continuously responded to intermittent anti-D and received it alone for a median of 8.5 months (range, 3-41 months) before stopping treatment altogether. Two of the 12 stopped responding to anti-D within 5 months of study initiation and transiently used either prednisone or danazol (patient nos. 9 and 12). One received chemotherapy for breast cancer and then temporarily resumed IV anti-D (no. 11), and 1 discontinued anti-D because of toxicity (no. 10) and received 12 months of IVIG before coming off all treatment.
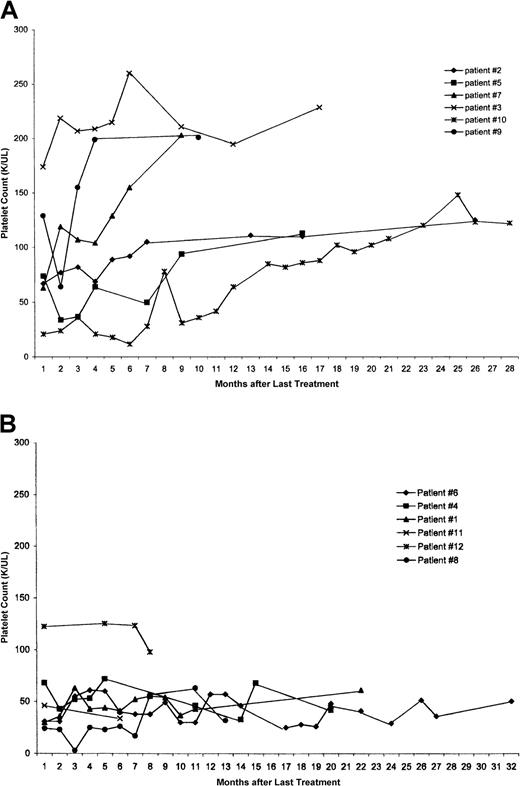
As of the last follow-up visit, 6 of the 12 have maintained platelet counts above 100 × 109/L (100 000/μL), with 3 of these maintaining normal counts (> 150 × 109/L [150 000/μL]) for more than 4 months. Strikingly, these 6 patients were on study for a median 15 months (range 8-35 months) and had been diagnosed with ITP for a median of 22.5 months (range 13-38 months) before achieving platelet counts above 100 × 109/L (100 000/μL). They were treated for a median of 16 months (range 7-20 months) from diagnosis to last treatment, including a median of 9 months (range 5-14 months) on study. The platelet counts of these 6 patients, beginning the month after they stopped treatment, are shown in Figure 3A. These patients continued to improve their counts even after stopping treatment. The platelet counts for those off treatment with counts below 100 × 109/L (100 000/μL) are shown in Figure 3B (also beginning the month after stopping treatment); their counts appear to be stable.
Platelet counts.
(A) The platelet counts for the 6 patients who have been off treatment for more than 6 months and have platelet counts at least 100 × 109/L (100 000/μL) at their last recorded count. The x-axis is time in months, and the y-axis is the platelet count. The counts are plotted beginning with the month after their last treatment. (B) The platelet count for the 6 patients who have been off treatment for more than 6 months with platelet counts below 100 × 109/L (100 000/μL) at their last follow-up count. The x-axis is time in months, and the y-axis is the platelet count. The counts are plotted beginning with the month after their last treatment.
Platelet counts.
(A) The platelet counts for the 6 patients who have been off treatment for more than 6 months and have platelet counts at least 100 × 109/L (100 000/μL) at their last recorded count. The x-axis is time in months, and the y-axis is the platelet count. The counts are plotted beginning with the month after their last treatment. (B) The platelet count for the 6 patients who have been off treatment for more than 6 months with platelet counts below 100 × 109/L (100 000/μL) at their last follow-up count. The x-axis is time in months, and the y-axis is the platelet count. The counts are plotted beginning with the month after their last treatment.
Group B.
The 7 patients still receiving treatment were enrolled a median of 2 months (range 1-11 months) from diagnosis and have been followed since study initiation for a median of 25 months (range 10-37 months). Three patients still receive IV anti-D. These 3 received a median of 0.42 infusions (range 0.42-0.58 infusions) per month during the first 12 months of the study and significantly less frequent (median 0.27 infusions [range 0.11-0.32]) infusions per month during the 9- to 22-month additional follow-up period (P = .037). Two patients stopped responding to IV anti-D within the first 4 months of the study. One received IVIG for 20 months and is now maintaining counts above 60 × 109/L (60 000/μL) following a course of rituximab (patient no. 18). The other has a count of 105 × 109/L (105 000/μL) on 7.5 mg prednisone a day (no. 20). Two patients received medication for other medical conditions. One developed systemic lupus erythematosus 20 months after starting the study and received prednisone for symptoms other than her platelet count (no. 17). The other was infused 3 times with 400 mg/kg IVIG while pregnant to prevent fetal loss (no. 19) and then received IV anti-D once immediately prior to delivery.
Group C.
The 8 patients who underwent splenectomy were enrolled a median of 4 months (range 2-10 months) from diagnosis and have been followed for a median of 27.5 months (range 6-39 months). Four were early nonresponders to anti-D and underwent splenectomy a median of 3.5 month (range 2-7 months) from starting the study. Four were continually responsive to anti-D but decided to undergo splenectomy a median of 27 months (range 9-36 months) from starting the study. Follow-up platelet counts are available from 2 to 10 months after splenectomy (median 3 months) in these patients. Six (75%) of the 8 had counts above 150 × 109/L (150 000/μL) after splenectomy without other therapy.
Biologic variables and acute response to IV anti-D
There was no correlation between glycocalicin levels, sCD16 levels, initial MCV or initial hemoglobin level, and platelet response to treatment or hemoglobin change after treatment. There was also no relationship between the number of D sites on the red cell as estimated by CDE typing and the day 7 platelet response or hemoglobin decrease following anti-D.
Comparisons between the outcome groups
Comparisons between the groups are shown in Table2. The 12 patients in the “off treatment” category had significantly higher platelet counts at study entry than the 8 patients who underwent splenectomy (P < .05). Eleven of the 12 “off treatment” patients had platelet counts at least 14 × 109/L (14 000/μL) at study entry, compared with only 2 of the 8 patients who underwent splenectomy. Furthermore, all of the 8 patients who were unresponsive to anti-D within the first few months had platelet counts below 14 × 109/L (14 000/μL) at study entry.
Comparison of pretreatment and peritreatment varables in each outcome group
| Outcome group . | No. of patients . | Median initial platelet count (range) . | Median platelet increment after first infusion* (range) . | Median duration of first infusion† (range) . |
|---|---|---|---|---|
| Off treatment | 12 | 19.5‡ (4-29) | 106.5 (6-366) | 28 (10-45.62) |
| On treatment | 7 | 12 (5-26) | 126 (8-440) | 21 (11.15-56.75) |
| Splenectomy | 8 | 9‡ (4-21) | 47 (3-273) | 19.5 (15.5-42.5) |
| Outcome group . | No. of patients . | Median initial platelet count (range) . | Median platelet increment after first infusion* (range) . | Median duration of first infusion† (range) . |
|---|---|---|---|---|
| Off treatment | 12 | 19.5‡ (4-29) | 106.5 (6-366) | 28 (10-45.62) |
| On treatment | 7 | 12 (5-26) | 126 (8-440) | 21 (11.15-56.75) |
| Splenectomy | 8 | 9‡ (4-21) | 47 (3-273) | 19.5 (15.5-42.5) |
Platelet count day 7 − day 0 × 109/L.
Days between infusions no. 1 and 2.
P < .05 MWU.
There was no significant difference in pretreatment glycocalicin or sCD16 levels between the long-term outcome groups, possibly because the numbers in each group were small. There was no significant predictive value of initial MCV, initial hemoglobin, or platelet increment or duration of response to the first infusion on the long-term outcome. There was no significant difference between the outcome groups for either rhesus phenotype (CDE) or ABO blood group.
Bleeding diaries
No patient had a severe bleeding episode throughout the 64 years of patient follow-up following study enrollment. Twenty-four of the patients complained of nonsevere bleeding, including 7 nose bleeds, 7 mouth bleeds, 10 petechiae, and 11 increased bruising. Six of the 15 females described menorrhagia. Bleeding symptoms tended to occur at lower platelet counts; however, occasional patients described petechiae, bruising, and nose bleeds at normal platelet counts.
Quality of life survey
There was no change from baseline to 6, 12, or 18 months in the scores of questions 3, 4, and 6. There was an increased score (greater well being) for question 5 at 12 months (P < .05) and at 18 months (P < .01) when compared with baseline. There was also an increased score at 6 (P < .05), 12 (P < .01), and 18 (P < .01) months in question 2 when compared with baseline. Question 1 showed a decreased score (decreased feeling of well being) at 6 (P < .05) and 18 (P < .05) months when compared with baseline.
Cost analysis
The cost of treating patients with IV anti-D for each outcome group is estimated in Table 3 using the number of vials of anti-D actually used by each patient and the costs listed in “Patients and methods.” Using these estimates, the cost of the anti-D used in all 28 patients plus the 8 splenectomies would be $370 400. In comparison, the cost of performing splenectomy in all 28 patients would have been $448 000. The cost of treating the 12 patients who were able to discontinue treatment for the study period was $87 280, or $229 per patient per month. The cost of treating those patients still on treatment was $68 480, or $389 per patient per month. The cost of treating the patients who underwent splenectomy, including the costs of both anti-D and splenectomy but not treatment after splenectomy, was $214 640, or $1,850 per patient per month. These estimates are an oversimplification because they do not include many other costs, such as the cost of other therapies, blood tests, outpatient visits, nursing time, or the cost of time lost from work.
Cost comparisons of anti-D and splenectomy
| Outcome group . | Number of anti-D infusions on study . | Other ITP treatment on study3-150 . | Months of patient follow-up . | Total cost of anti-D and splenectomy per group . | Cost per patient . | Cost per patient per month . |
|---|---|---|---|---|---|---|
| Off treatment (n = 12) | 79 | 16 G, 13 mo P, 28 mo D | 381 | $87 280 | $7 273 | $229 |
| On treatment (n = 7) | 54 | 22 G, 31 mo P, 3 mo A | 176 | $68 480 | $9 978 | $389 |
| Splenectomy (n = 8) | 64 | 8 G, 1 V, 2 M, 18 mo D, 14 P | 1163-151 | $214 640 ($86 6403-152) | $26 830 ($10 8303-152) | $1 850 ($7473-152) |
| Sum | 197 | 673 | $370 400 ($242 4003-152) |
| Outcome group . | Number of anti-D infusions on study . | Other ITP treatment on study3-150 . | Months of patient follow-up . | Total cost of anti-D and splenectomy per group . | Cost per patient . | Cost per patient per month . |
|---|---|---|---|---|---|---|
| Off treatment (n = 12) | 79 | 16 G, 13 mo P, 28 mo D | 381 | $87 280 | $7 273 | $229 |
| On treatment (n = 7) | 54 | 22 G, 31 mo P, 3 mo A | 176 | $68 480 | $9 978 | $389 |
| Splenectomy (n = 8) | 64 | 8 G, 1 V, 2 M, 18 mo D, 14 P | 1163-151 | $214 640 ($86 6403-152) | $26 830 ($10 8303-152) | $1 850 ($7473-152) |
| Sum | 197 | 673 | $370 400 ($242 4003-152) |
G indicates IVIG; P, prednisone; D, danazol; V, vincristine; M, methylprednisolone; and A, azathioprine.
The number of IVIG, vincristine, or methylprednisolone treatments and the number of months of prednisone, danazol, or azathioprine per group.
Follow-up until month of splenectomy.
Cost of anti-D alone.
Discussion
Studies assessing the long-term outcome of adults with ITP (Table4) suggest that between 9% and 32% of newly identified adults will go into remission either spontaneously or following a short course of steroids.3-8 Those patients who do not go into early remission tend to undergo splenectomy.
Summary of studies assessing the long-term outcome of patients with ITP
| . | Year . | Country . | No. of patients treated with steroids . | Initial CR, steroids alone (%) . | CR maintained after discontinuing treatment (%) . | Late CR . | Total CR (%) . |
|---|---|---|---|---|---|---|---|
| Thompson et al3 | 1972 | US | 57 | 13 (23) | 13 (23) | 3 | 16 (28) |
| DiFino et al4 | 1980 | US | 59 | 25 (43) | 13 (22) | 3 | 16 (27) |
| Pizzuto et al5 | 1984 | South America | 818 | 386 (47) | 262 (32) | — | 262 (32) |
| JiJi et al6 | 1984 | US | 91 | 22 (24) | 21 (23) | 5 | 26 (29) |
| Stasi et al7 | 1995 | Italy | 121 | 52 (39) | 11 (9) | — | 11 (9) |
| Ikkala et al8 | 1978 | Finland | 40 | 16 (40) | 10 (25) | — | 10 (25) |
| . | Year . | Country . | No. of patients treated with steroids . | Initial CR, steroids alone (%) . | CR maintained after discontinuing treatment (%) . | Late CR . | Total CR (%) . |
|---|---|---|---|---|---|---|---|
| Thompson et al3 | 1972 | US | 57 | 13 (23) | 13 (23) | 3 | 16 (28) |
| DiFino et al4 | 1980 | US | 59 | 25 (43) | 13 (22) | 3 | 16 (27) |
| Pizzuto et al5 | 1984 | South America | 818 | 386 (47) | 262 (32) | — | 262 (32) |
| JiJi et al6 | 1984 | US | 91 | 22 (24) | 21 (23) | 5 | 26 (29) |
| Stasi et al7 | 1995 | Italy | 121 | 52 (39) | 11 (9) | — | 11 (9) |
| Ikkala et al8 | 1978 | Finland | 40 | 16 (40) | 10 (25) | — | 10 (25) |
CR indicates complete response.
Several studies have reported anecdotal patients who achieve late remission after multiple courses of steroids.3,4 6 In general, however, the natural history of those adults who do not improve after an initial course of steroids and do not undergo splenectomy is unknown. This pilot study was designed to assess the 1- to 5-year outcome of patients with recently diagnosed ITP who did not improve after their initial treatment. The hemorrhagic complications associated with persistently low platelet counts were avoided by using palliative treatment with repeated infusions of IV anti-D.
The results were encouraging. Twelve (43%) of the 28 adults who participated in this study have been off all treatment and have maintained platelet counts above 30 × 109/L (30 000/μL) for more than 6 months. Six (21%) of these continued to improve after discontinuing therapy and now have counts above 100 × 109/L (100 000/μL) with 3 (11%) in complete remission. Furthermore, 71% of the 28 patients have so far avoided splenectomy, and those still receiving IV anti-D are receiving it significantly less frequently. Of particular note, those patients with counts above 100 × 109/L (100 000/μL) (Figure 3A) achieved this a median of 22.5 months (range 13-38 months) from diagnosis and received a median of 16 months (range 7-20 months) of treatment. Given that the 28 patients enrolled in this study had completed a median of 2 courses of treatment and were a median 3.5 months from diagnosis at study entry, it was surprising that almost half the patients were able to discontinue treatment and that some patients were still improving and achieving near normal platelet counts up to 38 months after diagnosis.
It is possible that a referral bias resulted in a disproportionate number of patients with less severe ITP entering the trial. However, 28 of 31 patients eligible for the study were enrolled; 15 had platelet counts below 15 × 109/L (15 000/μL) at study entry, and 14 had previously received both steroids and IVIG.
One of the disadvantages of this approach is that patients were seen more frequently for outpatient visits and often had lower platelet counts than if they had undergone successful splenectomy. In addition, patients with marginal platelet counts in the range of 30 × 109/L to 50 × 109/L (30 000-50 000/μL), although off treatment, could potentially have periodic crises. These crises might require additional treatment, including hospitalization, all of which could have been relieved by early splenectomy, if it was successful. However, no ITP-related hospitalizations occurred during the course of the study, and there were no episodes of serious bleeding symptoms in the 64 patient years of follow-up in this study; nor were there significant adverse events or decrease in quality of life. Furthermore, many patients avoided both surgery and the toxicity of long-term steroids by following this protocol. The rudimentary cost analysis in Table 3 shows that if a substantial proportion of patients can eventually discontinue treatment without undergoing splenectomy, estimated previously to be 47%,23 the use of intermittent IV anti-D, even for many months, may be cost-effective in comparison to all patients undergoing splenectomy.
Predicting which patients will improve over time would further improve the cost-effectiveness and give patients a better idea of what they could expect if they do not undergo early splenectomy. Patients with higher platelet counts at study entry, in particular those with platelet counts above 14 × 109/L (14 000/μL), appear to be more likely to improve. No other variables—ie, glycocalicin levels, sCD16 levels, or blood type—were predictive, although the sample sizes were small.
Overall, this study proposes that splenectomy, which is only successful in 60% to 70% of patients,9-11 may not be immediately necessary in all adults with recently diagnosed ITP who do not go into remission with initial steroids. It suggests that most adults with ITP have a tendency for their platelet counts to improve over time. It also shows that IV anti-D is a safe and effective treatment in approximately two thirds of Rh+, nonsplenectomized adults with ITP and can be used as a steroid-sparing agent. There is no reason to believe that anti-D is curative or that it affected the natural history of the ITP in these adults. Rather, it appeared to give patients time to improve on their own.
ITP is clearly a heterogeneous disease. Better understanding of the pathology of this disease and identification of good predictors of response to splenectomy and other therapies would help to define treatment strategies that would be most appropriate for individual patients.
We thank Howard Fleit at the State University of New York at Stony Brook for performing the sCD16 assays, Ginette Lanoix for technical assistance, and all the physicians and their staff whose patients participated in this study: Drs Bernard Bernhardt, Edward Amorosi, Roy Berger, Ellin Berman, Laurence Bilsky, Terry Davies, Richard Furman, Gerard Hellman, Gary Horbar, Alexandra Ikeguchi, Robert Jacobson, Shirley Levine, Neal Lewin, Stuart Lewis, Eva Levitan, William Lipera, Taibor Moskowitz, Vijay Roy, Eduardo Saponara, William Solomon, Richard Taubman, Zella Zeigler, and Robert Zielinski.
Supported in part by a clinical research grant from Cangene, Winnipeg, Canada; the ITP Society of the Children's Blood Foundation, New York, NY; and the Swiss National Foundation of Science, Baden, Switzerland.
B.M.R.W. is an employee of Cangene Corporation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nichola Cooper/James Bussel, Division of Hematology/Oncology, P-695, Dept of Pediatrics, New York Presbyterian Hospital—Weill Medical College of Cornell University, 525 East 68th St, New York, NY 10021; e-mail: nicholacooper@yahoo.com;jbussel@med.cornell.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal