The clinical significance of minimal residual disease (MRD) after treatment for chronic lymphocytic leukemia (CLL) has recently been addressed by Rawstron et al1 in an analysis of 25 patients in complete remission (CR) after autologous stem cell transplantation or treatment with alemtuzumab (CAMPATH-1H). In this study the authors emphasized the applicability of a multiparametric flow cytometry strategy for a sensitive detection of CLL residual population using a 2-step acquisition protocol and a 4-color combination, and they observed that the persistence of MRD after treatment correlated with a shorter duration of the response and survival compared to patients achieving MRD− CR.
We would like to comment on this important issue on the basis of updated results in 32 patients with CLL who received stem cell transplants at our institution.2 The results show that the predictive value of MRD depends on the type of stem cell transplantation (autologous or allogeneic).
MRD was detected by the cytofluorometric analysis of peripheral blood and bone marrow samples using a triple combination strategy (CD20/CD5/CD19, CD22/CD23/CD19, and kappa/lambda/CD19), with a level of sensitivity of 5 × 10−5 and performed in parallel with nonspecific allele CDR3 polymerase chain reaction (PCR) analysis (data not shown).
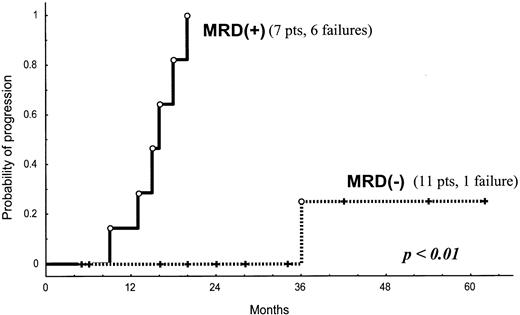
Results are summarized in Table 1. In the group of patients autografted (n = 18) MRD persisted in 7 patients (39%) at first assessment, performed 3 months after transplantation, and a clinical relapse was observed in 6 patients after a median follow-up of 15.5 months (range, 9-20 months). Of note, a steady increase in the MRD level was observed in all MRD+patients, although the rate of increase varied among patients. In contrast, only 1 of the 11 patients achieving a MRD−status after transplantation has progressed (median follow-up, 28 months; range, 5-62 months), thus translating into a significantly lower risk of relapse (P < .01; Figure1). In addition, reappearance of MRD has been observed in 3 patients (at 11, 31, and 43 months after transplantation), although only 1 of these patients has relapsed (at 36 months from transplantation). Therefore, MRD status after autotransplantation anticipates clinical progression in most patients, with an 87% (SE ± 12%) actuarial risk of relapse at 25 months after MRD detection.
Clinical outcome according to MRD status after autologous and allogenic stem cell transplantation
| . | Autologous stem cell transplantation (n = 18) . | Allogeneic stem cell transplantation (n = 14) . | ||
|---|---|---|---|---|
| MRD+ . | MRD− . | MRD+ . | MRD− . | |
| MRD response | 7 | 11 | 4 | 6 |
| MRD relapse | NA | 3 | 0 | 0 |
| Clinical progression | 6 | 1 | 0 | 0 |
| . | Autologous stem cell transplantation (n = 18) . | Allogeneic stem cell transplantation (n = 14) . | ||
|---|---|---|---|---|
| MRD+ . | MRD− . | MRD+ . | MRD− . | |
| MRD response | 7 | 11 | 4 | 6 |
| MRD relapse | NA | 3 | 0 | 0 |
| Clinical progression | 6 | 1 | 0 | 0 |
NA indicates not applicable.
Clinical outcome and MRD response.
Patients in CR but are MRD+ have a higher risk of progression than those in CR with no detectable MRD (log-rank test;P < .01).
Clinical outcome and MRD response.
Patients in CR but are MRD+ have a higher risk of progression than those in CR with no detectable MRD (log-rank test;P < .01).
In the allografted group of patients (n = 14), MRD was observed in 4 of 10 responding patients surviving for at least 3 months. Remarkably, disease progression has not been observed in any of these patients after a median follow-up of 38 months (range, 6-79 months). Among these patients, a delayed clearance of MRD was observed in 1 patient, up to 12 months after transplantation, whereas in 2 patients an intermittent detection of MRD has been observed during follow-up (47 and 79 months, respectively) and the remaining patient died due to a transplantation-related cause more than 6 months afterward without evidence of disease at the necropsy. Quantification of MRD positive samples after allotransplantation showed a low level of detectable CLL in all 4 patients, below 1 cell/μL in peripheral blood and less than 0.5% of total bone marrow cellularity. On the other hand, none of 6 patients achieving a MRD− status after allogeneic transplantation have presented MRD relapse (median follow-up, 59 months; range, 8-120 months).
In summary, the persistence of MRD in CLL patients appears to have different implications depending on the type of transplantation, autologous or allogeneic. Thus, while persistence of MRD after autologous transplantations is highly predictive of clinical progression, the detection of MRD after allogeneic transplantations does not necessarily predict clinical relapse. Moreover, delayed responses or persistently low-level MRD can be observed in some patients, thus indicating a possible graft-versus-leukemia effect.3-6 Therefore, sequential quantitative strategies such as real-time PCR7 or multiparametric flow cytometry analysis are required to define more accurately the clinical significance of MRD status in CLL patients undergoing intensive treatments, particularly stem cell transplantations. Finally, this type of study might be useful for designing posttransplantation therapy based on MRD status.
Interpretation of sequential minimal residual disease assessment in chronic lymphocytic leukemia
Esteve et al have presented interesting and informative observations regarding the assessment of minimal residual disease (MRD) following intensive therapy for chronic lymphocytic leukemia (CLL). Their report is consistent with our own experience in that the eradication of detectable disease in CLL is associated with prolonged progression-free survival. We also agree that CLL patients who have detectable MRD at any time following monoclonal antibody therapy or autologous stem cell transplantation will experience progressively increasing levels of CLL in their peripheral blood and will eventually relapse clinically. To determine whether it is possible to predict the time at which further therapy will be required, we have assessed sequential samples in 45 patients for up to 5 years following treatment with Alemtuzumab (MabCampath) for refractory CLL (manuscript in preparation). In summary, following Alemtuzumab therapy the kinetics of disease progression is biphasic in most (24 of 34) patients: there is a rapid increase in peripheral blood CLL cells within 2 months of stopping therapy, followed by a second phase characterized by more gradual increase in CLL cells. It appears that the first phase, with rapidly increasing peripheral CLL cells, is due to redistribution of CLL cells from bone marrow or lymphoid tissue to peripheral blood, while the second phase represents expansion of absolute numbers of CLL cells. During this second phase it is possible to calculate a doubling time for the CLL cells (in this series it was a median of 30 days [range, 5-289 days]), which can be used to predict when an individual with CLL is likely to progress clinically.
It is of considerable importance that Esteve et al have demonstrated persistent but stable levels of CLL in patients after allogeneic transplantation. This indicates that there must be continuing suppression of the CLL clone following an allograft, and therefore that graft-versus-leukemia is a clinically significant phenomenon in this setting. We therefore agree that to understand the clinical implication of detectable MRD after allograft in an individual patient requires sequential studies and should not in itself be used to define therapy. But we believe that both our own data and the data of Esteve et al indicate that after Alemtuzumab and/or after autograft, the presence of detectable MRD is always indicative of progressive disease and that the tumor burden at the end of therapy is predictive of outcome.
This work was partially supported by a grant from Fondo de Investigaciones Sanitarias, Madrid, Spain (FIS 01/1581).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal