We previously reported that chronic stimulation with low, noncytotoxic doses of extracellular adenosine triphosphate (ATP) induced a distorted maturation of dendritic cells (DCs) and impaired their capacity to initiate T-helper (Th) 1 responses in vitro. Here, we examined the effects of ATP on chemokine-receptor expression and chemokine production by DCs. ATP strongly induced expression of CXC chemokine receptor 4 on both immature and lipopolysaccharide (LPS)–stimulated DCs and slightly up-regulated CC chemokine receptor (CCR) 7 on both DC types. In contrast, ATP reduced CCR5 expression on immature DCs. These effects were confirmed at both the messenger RNA and protein levels and were not produced by uridine triphosphate (UTP). Consistent with the changed receptor expression, ATP increased migration and intracellular calcium of immature and mature DCs to stromal-derived factor 1 (CXC ligand [CXCL] 12) and macrophage inflammatory protein [MIP] 3β (CC ligand [CCL] 19), whereas responses to MIP-1β (CCL4) were reduced. DCs are an important source of chemokines influencing recruitment of distinct T-lymphocyte subsets. ATP, but not UTP, significantly reduced LPS-induced production of interferon-inducible protein 10 (CXCL10) and regulated upon activation, normal T-cell expressed and secreted chemokine (CCL5); increased secretion of macrophage-derived chemokine (CCL22); and did not change production of thymus and activation-regulated chemokine (CCL17). Consistent with these findings, supernatants from ATP-treated mature DCs attracted Th1 and T-cytotoxic 1 cells less efficiently, whereas migration of Th2 and T cytotoxic 2 cells was not affected. Our data suggest that ATP provides a signal for enhanced lymph node localization of DCs but that it may, at the same time, diminish the capacity of DCs to amplify type 1 immune responses.
Introduction
Migration of dendritic cells (DCs) from the site of antigen capture to secondary lymphoid organs is a crucial event in the initiation and amplification of immune responses. DCs reside in an immature state in peripheral tissues, where they are highly efficient in capturing antigens. On exposure to danger signals such as pathogens, dying cells, or inflammatory cytokines, DCs differentiate into mature DCs. Maturing DCs migrate to draining lymph nodes, where they are very effective at activating naive and central memory T cells.1,2 In turn, inflammatory signals recruit circulating DCs and their precursors from the blood to sites of inflammation, replacing DCs that have migrated to lymph nodes.2 Trafficking of DCs through tissues is regulated by the pattern of chemokine receptors expressed on DCs and the local availability of chemokines. Immature DCs and monocytes express receptors for inflammatory chemokines (CXC chemokine receptor [CXCR] 1, CC chemokine receptor [CCR] 1, CCR2, and CCR5), which account for the capacity of these cells to migrate to inflamed tissues where cognate ligands are produced. Maturation of DCs is associated with the coordinated down-regulation of receptors for inflammatory chemokines and the induction of lymphoid chemokine receptors such as CXCR4, CCR4, and CCR7.2-4 Thus, maturing DCs become responsive to lymphoid chemokines such as CXC ligand (CXCL) 12 (stromal-derived factor [SDF] 1), CC ligand (CCL) 2 (macrophage-derived chemokine [MDC]), and CCL19 (macrophage inflammatory protein [MIP] 3 β) and they colocalize with naive T cells in secondary lymphoid organs.
DCs are also a relevant source of chemokines. Immature DCs constitutively release MDC and CCL17 (thymus- and activation-regulated chemokine [TARC]).5 At early stages of maturation, DCs produce high levels of inflammatory chemokines, such as CCL2 (monocyte chemoattractant protein 1 [MCP-1]), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (regulated upon activation, normal T-cell expressed and secreted [RANTES] chemokine), CXCL8 (interleukin-8 [IL-8]), and CXCL10 (interferon-inducible protein 10 [IP-10]), that sustain the recruitment of circulating immature DCs, DC precursors, and T cells to inflamed tissue.3,6 Lymphoid chemokines, including MIP-3β, TARC, and MDC, are produced or up-regulated later during DC maturation,6,7 providing chemotactic signals for mature DCs and for T cells in secondary lymphoid organs. MIP-3β attracts naive T cells that express CCR7, whereas TARC and MDC act on naive and recently activated type 2 T cells expressing CCR4.8 9
Extracellular nucleotides are important regulators of inflammatory and immune responses.10 Nucleotides are present at high concentrations in the cytoplasm and can be released by regulated exocytosis or by passive leakage after cell damage. In the extracellular compartment, nucleotides bind P2 purinergic receptors, which are expressed by both human and murine DCs.11-14 We previously reported that low, noncytotoxic doses of extracellular adenosine triphosphate (ATP), but not uridine triphosphate (UTP), suppressed IL-12 production by maturing DCs, thereby impairing their ability to initiate T-helper (Th) 1 responses.15 In the current study, we found that extracellular ATP, but not UTP, changes chemokine-receptor expression and chemokine production by DCs. In particular, ATP induced functional expression of CXCR4 and CCR7, setting DCs for lymph node localization. Moreover, ATP up-regulated MDC while inhibiting RANTES and IP-10 production by maturing DCs, thus reducing their ability to attract type 1 T lymphocytes specifically.
Materials and methods
Reagents and antibodies
Lipopolysaccharide (LPS) from Escherichia coli(055:B5) was purchased from Sigma-Aldrich (Milan, Italy). ATP and UTP were obtained from Roche Molecular Biochemicals (Mannheim, Germany). The mouse antihuman monoclonal antibody (mAb) CCR4 (328B, IgG) was provided by ICOS (Bothell, WA). Anti-CCR5 (45531.111, IgG2B), anti-CXCR3 (49801.111, IgG1), and neutralizing anti–IP-10 (33036.211, IgG1) and anti-MDC (57226.11, IgG2B) mAbs were from R&D Systems (Minneapolis, MN). The mAbs anti-CCR7 (2H4, IgM), anti-CXCR4 (12G5, IgG2A), fluorescein isothiocyanate (FITC)–conjugated anti-CD14, anti-CD1a, anti-CD45RO, anti-CD45RA, anti-CD4 and anti-CD8, and control mouse immunoglobulin (Ig) were supplied by BD PharMingen (San Diego, CA). Anti-CD83 mAb was from Immunotech (Marseille, France). The rabbit polyclonal anti-TARC antibody (Ab) was from PeproTech (London, United Kingdom). Recombinant human (rhu) SDF-1α, MIP-1 β, and MIP-3 β, neutralizing Ab to IL-12 and IL-4, and rhu IL-12 were provided by R&D Systems. Nucleotides had undetectable endotoxin levels (< 10 pg/mg) onLimulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
Preparation and stimulation of DCs
DCs were prepared from purified peripheral blood monocytes from healthy donors, as described previously.16 Briefly, more than 90% pure CD14+ cells were cultured at a concentration of 1 × 106 cells/mL in RPMI 1640 supplemented with 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM L-glutamine, 25 mM HEPES, 0.05 mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen Italia, San Giuliano Milanese, Italy; complete RPMI) and complemented with 10% fetal-calf serum (FCS; HyClone, Logan, UT), 100 ng/mL rhu granulocyte-macrophage colony-stimulating factor, and 200 U/mL rhu IL-4 (R&D Systems) at 37°C with 5% carbon dioxide. The medium was changed after 3 days, and on day 6 of culture, cells were recovered and depleted of CD2+ and CD19+ cells by means of immunomagnetic beads coated with specific mAbs (Dynal, Oslo, Norway). This procedure yielded more than 97% pure CD1a+ and CD14− DC preparations. DCs were left untreated, incubated for 2 hours at 37°C with nucleotides, or induced to mature with 10 μg/mL LPS in the presence or absence of nucleotides. The cells were then washed and incubated for 24 hours at 37°C.
Flow cytometry analysis of DCs
DCs that were either untreated or stimulated for 24 hours with LPS in the presence or absence of nucleotides were washed and then incubated in phosphate-buffered saline (2% FCS and 0.01% sodium azide) with FITC-conjugated mAbs for 40 minutes at 4°C. When pure mAbs were used, cells were washed 3 times and a second incubation with an FITC-coupled goat (Fab′)2 antimouse IgG (Southern Biotechnology Associates, Birmingham, AL) was done. For CCR7 staining, the second incubation was done with biotin-coupled rat antimouse IgM and was followed by extensive washing and additional incubation with FITC-conjugated streptavidin (BD PharMingen). Isotype-matched mouse Ig was used in control samples. Cells were analyzed with a fluorescence-activated cell-sorter scanner (FACScan; Becton Dickinson) and Cell Quest software. Data analysis used WinMDI software (http://facs.scripps.edu). Results are shown as the net mean fluorescence intensity (MFI), which is the mean fluorescence obtained with mAbs subtracted from the mean fluorescence measured with isotype-matched control Ig.
Release of chemokines from DCs
Cell-free supernatants from DCs were tested for chemokine content by enzyme-linked immunosorbent assay (ELISA). RANTES was assessed by using the Ab pair, rabbit polyclonal 20581D (for coating) and 20582D (for detection; BD PharMingen). IP-10 was assayed with the purified 4D5/A7/C5 and the biotinylated 6D4/D6/G2 antihuman IP-10 mAbs (BD PharMingen). MDC and TARC were measured by using ELISA kits from R&D Systems. SDF-1α was measured by using purified 79018.111 and biotinylated antihuman SDF-1α goat IgG (AQL02; R&D Systems). The plates were analyzed in an ELISA reader (3550 UV; Bio-Rad, Hercules, CA). Samples for each condition were assayed in triplicate.
Ribonuclease protection assay
Total RNA was extracted from purified DCs after 24 hours of incubation with the selected stimuli by using Trizol (Invitrogen Italia) according to the manufacturer's instructions. Two multiprobe template sets, hCR5 and hCR6 (RiboQuant; BD PharMingen), were used for in vitro transcription reactions in the presence of a GACU pool and a T7 RNA polymerase to synthesize phosphorus 32-UTP–labeled antisense probes. Ribonuclease (RNase) protection analysis of 5 μg total RNA was done after overnight hybridization at 60°C with 2.5 × 106 cpm hCR5 or hCR6, followed by digestion with RNase A and T1 according to standard protocols. Protected fragments were treated with proteinase K, extracted with phenol-chloroform and isoamyl alcohol (50:1), and precipitated in ethanol in the presence of ammonium acetate. The samples were electrophoresed on 5% denaturing sequencing gels and exposed to Kodak films.
Calcium-flux analysis
DCs (106 cells/mL) were loaded with 8 μM fluo-3–acetoxymethyl ester in the presence of 1 μM pluronic F-127 (Molecular Probes, Eugene, OR) in complete RPMI with 1% FCS for 40 minutes at 37°C and subjected to frequent gentle agitation. Cells were then washed twice; stimulated with SDF-1α, MIP-1β, or MIP-3β (all at 100 ng/mL; R&D Systems); and analyzed on a FACScan device. Cells and chemokines were kept at 37°C during the assay.
Migration assay
Chemotaxis of DCs and T cells was evaluated by measuring their migration through 5-μm-pore polycarbonate filters in 24-well transwell chambers (Corning Costar, Cambridge, MA), as described previously.17 DC migration was studied by adding different concentrations of chemokines to the lower wells and 105 DCs suspended in complete RPMI with 0.5% bovine serum albumin (BSA) in the transwell insert. The chemotactic property of DC supernatants was evaluated by adding 105 T cells to the top chamber and various dilutions of the supernatants (0.6 mL) to the bottom chamber of the transwell. After 1 hour of incubation at 37°C with 5% carbon dioxide, cells that transmigrated into the lower chamber were recovered and acquired with a FACScan device for 60 seconds at a flow rate of 60 μL/min. Data acquisition and analysis were restricted to events with the forward and side scatter properties of cells and not cell debris. In some experiments, DC supernatants were used after 30 minutes of incubation with a neutralizing anti–IP-10 mAb, anti-MDC Ab, and anti-TARC Abs or mouse Ig. Results are shown as net migration, which is the number of cells in the lower chamber containing the agonistic chemokine or DC supernatant subtracted from the number of cells in chambers containing medium alone.
T-cell lines and clones
T-cell lines were generated by stimulating peripheral blood mononuclear cells (PBMCs) from healthy donors with 1 μg/mL phytohemagglutinin (PHA) in complete RPMI complemented with 5% human serum in the presence of 2 ng/mL rhu IL-12 and 10 μg/mL anti–IL-4 mAb (for type 1 cell polarization) or the presence of 250 U/mL rhu IL-4 and 10 μg/mL anti–IL-12 mAb (for type 2 cell polarization). After 10 to 14 days of culture in the presence of 30 U/mL IL-2, T cells were restimulated with plate-coated anti-CD3 and soluble anti-CD28 mAbs (both at 1 μg/mL; BD PharMingen) and examined for intracellular interferon (IFN-γ) and IL-4 after 6 hours. For 2-color intracellular staining, monensin (10 μM; Sigma-Aldrich) and brefeldin A (10 μg/mL; Sigma-Aldrich) were added to the cultures before staining to prevent cytokine secretion. T cells were then fixed with 2% paraformaldehyde, permeabilized with 0.5% saponin, stained with FITC-conjugated mouse anti–IFN-γ and phycoerythrin-conjugated rat anti–IL-4 (BD PharMingen), and analyzed with a FACScan device. In control samples, staining was done with isotype-matched control Ab. T cells that were not restimulated did not show any lymphokine production (data not shown).
T-cell clones were generated from CD4+ or CD8+purified and polarized T-cell lines by limiting dilution (0.4 cells/well) in the presence of irradiated 2 × 105 PBMCs, 30 U/mL IL-2, and 1% PHA in complete RPMI plus 10% FCS. Clones were grown in the presence of IL-2 and periodically stimulated with 1% PHA and feeder cells or plate-coated anti-CD3 and soluble anti-CD28. Phenotype was assessed by flow cytometry. Release of IL-4 and IFN-γ in the supernatant was measured 48 hours after activation with anti-CD3 and anti-CD28 mAbs by ELISA using OptEia kits (BD PharMingen).
Statistical analysis
The Mann-Whitney test was used to compare differences in chemokine release and cell migration. P values of .05 or less were considered to indicate significance.
Results
Extracellular ATP, but not UTP, induces CCR7 and CXCR4 and down-regulates CCR5 expression
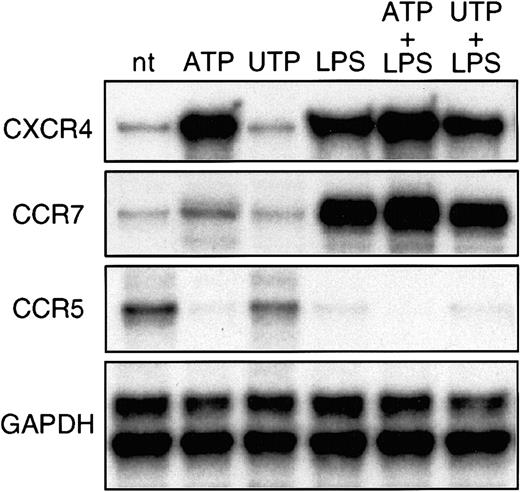
RNase protection assays were done to study the pattern of expression of chemokine receptors in DCs stimulated with ATP or UTP (Figure 1). Immature DCs expressed high levels of CCR5 and low levels of CXCR4 and CCR7 messenger RNA (mRNA). Incubation with 250 μM ATP strongly up-regulated CXCR4 and, to a lesser extent, CCR7 and completely down-regulated CCR5 mRNA. In addition, ATP further increased CXCR4 and CCR7 mRNA levels induced by LPS. In contrast, UTP did not affect expression of receptors in either immature or LPS-stimulated DCs (Figure 1).
Extracellular ATP induces CCR7 and CXCR4 and down-regulates CCR5 mRNA.
DCs were generated from purified peripheral blood CD14+monocytes by culture with granulocyte-macrophage colony-stimulating factor and IL-4. On day 6, CD2+ and CD19+ cells were removed; resulting cells were more than 97% CD1a+ and CD14−. Immature DCs were not treated (nt), stimulated with 250 μM ATP or UTP, or induced to mature with 10 μg/mL LPS in the absence or presence of nucleotides. After 16 hours, total RNA was extracted and RNase protection assay done to visualize chemokine-receptor mRNA transcripts. Shown is one representative experiment of 3 done.
Extracellular ATP induces CCR7 and CXCR4 and down-regulates CCR5 mRNA.
DCs were generated from purified peripheral blood CD14+monocytes by culture with granulocyte-macrophage colony-stimulating factor and IL-4. On day 6, CD2+ and CD19+ cells were removed; resulting cells were more than 97% CD1a+ and CD14−. Immature DCs were not treated (nt), stimulated with 250 μM ATP or UTP, or induced to mature with 10 μg/mL LPS in the absence or presence of nucleotides. After 16 hours, total RNA was extracted and RNase protection assay done to visualize chemokine-receptor mRNA transcripts. Shown is one representative experiment of 3 done.
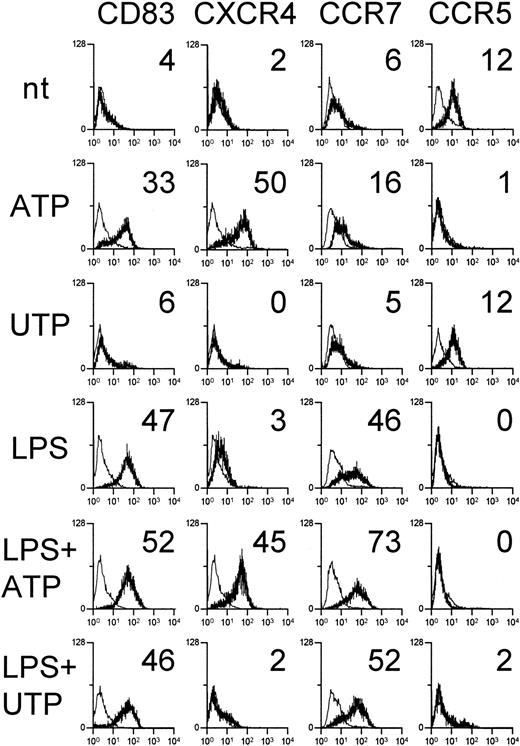
Expression of chemokine receptors on the membranes of immature or mature DCs stimulated with ATP was also assessed by flow cytometry (Figure 2). ATP, but not UTP, acted as a maturation stimulus for DCs inducing up-regulation of CD83, as described previously.15 DCs treated with ATP had high membrane expression of CXCR4. In contrast, CXCR4 was not detected on the surface of LPS-stimulated DCs despite the high mRNA expression, a finding described previously.18 19 The presence of CCR7 and CCR5 molecules on the DC surface was consistent with the respective mRNA expression: CCR7 was detected at low levels on ATP-treated DCs and more abundantly on LPS-stimulated DCs, with DCs exposed to both ATP and LPS expressing the highest amount. CCR5 was expressed by immature and UTP-stimulated DCs but completely disappeared on DCs treated with ATP, LPS, or both.
ATP up-regulates CXCR4, CCR7, and CD83 and reduces CCR5 on DC membranes.
Immature DCs were untreated, stimulated with 250 μM ATP or UTP, or induced to mature with 10 μg/mL LPS in the absence or presence of nucleotides. After 24 hours, DCs were stained with mAbs against the indicated surface molecules (bold histograms) or with isotype-matched control Ig (thin histograms). Numbers indicate the net mean MFI. Results are representative of 5 independent experiments.
ATP up-regulates CXCR4, CCR7, and CD83 and reduces CCR5 on DC membranes.
Immature DCs were untreated, stimulated with 250 μM ATP or UTP, or induced to mature with 10 μg/mL LPS in the absence or presence of nucleotides. After 24 hours, DCs were stained with mAbs against the indicated surface molecules (bold histograms) or with isotype-matched control Ig (thin histograms). Numbers indicate the net mean MFI. Results are representative of 5 independent experiments.
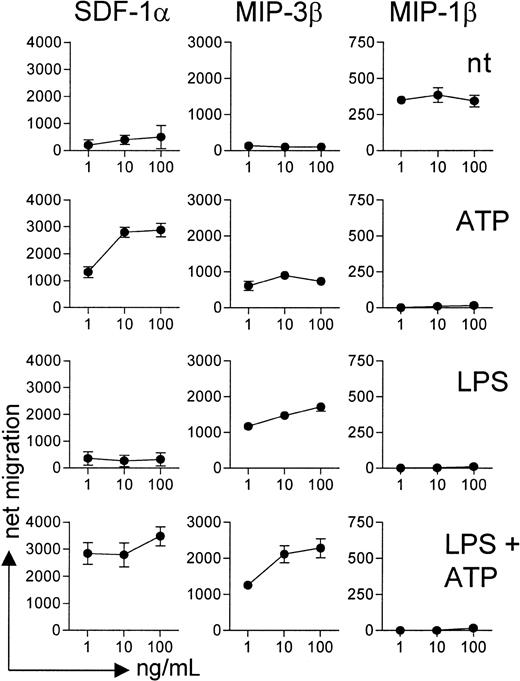
The functional activity of chemokine receptors was then examined by measuring intracellular calcium flux (Figure3) and cell migration (Figure4) in response to agonistic chemokines. Immature DCs showed intracellular calcium mobilization and migrated in response to the CCR5 ligand MIP-1β but not to MIP-3β (CCR7 agonist) or SDF-1α (CXCR4 agonist). ATP-stimulated DCs mobilized calcium and migrated in response to SDF-1α and, to a lesser extent, MIP-3β, whereas the responsiveness to MIP-1β was completely lost. On LPS activation, DCs became insensitive to MIP-1β and acquired responsiveness to MIP-3β but not to SDF-1α, a finding consistent with the lack of membrane expression of CXCR4. Finally, DCs matured in the presence of ATP showed calcium flux and chemotaxis to SDF-1α and MIP-3β but not MIP-1β.
Functional expression of CXCR4, CCR7, and CCR5 in immature or maturing DCs exposed to ATP.
DCs were incubated for 24 hours with ATP alone, LPS alone, LPS and ATP, or none of these agents; loaded with 8 μM fluo-3–acetoxymethyl ester in the presence of 1 μM pluronic F-127; and tested for intracellular calcium mobilization in response to SDF-1α (CXCR4 ligand), MIP-3β (CCR7 ligand), and MIP-1β (CCR5 ligand) (all at a concentration of 100 ng/mL). Calcium flux was measured with a FACScan device. Results are the kinetics of MFI and are representative of 4 independent experiments.
Functional expression of CXCR4, CCR7, and CCR5 in immature or maturing DCs exposed to ATP.
DCs were incubated for 24 hours with ATP alone, LPS alone, LPS and ATP, or none of these agents; loaded with 8 μM fluo-3–acetoxymethyl ester in the presence of 1 μM pluronic F-127; and tested for intracellular calcium mobilization in response to SDF-1α (CXCR4 ligand), MIP-3β (CCR7 ligand), and MIP-1β (CCR5 ligand) (all at a concentration of 100 ng/mL). Calcium flux was measured with a FACScan device. Results are the kinetics of MFI and are representative of 4 independent experiments.
ATP-treated DCs migrate in response to SDF-1α and MIP-3β but not MIP-1β.
DCs preincubated for 24 hours with ATP alone, LPS alone, LPS and ATP, or none of these agents were tested for their ability to migrate in response to increasing concentrations of SDF-1α, MIP-3β, and MIP-1β. Data are mean ± SD net migration results from 4 independent experiments using DCs from 3 different donors.
ATP-treated DCs migrate in response to SDF-1α and MIP-3β but not MIP-1β.
DCs preincubated for 24 hours with ATP alone, LPS alone, LPS and ATP, or none of these agents were tested for their ability to migrate in response to increasing concentrations of SDF-1α, MIP-3β, and MIP-1β. Data are mean ± SD net migration results from 4 independent experiments using DCs from 3 different donors.
ATP up-regulates MDC and inhibits IP-10 and RANTES release by DCs and reduces the capacity of DCs to attract type 1 lymphocytes
In the next series of experiments, we investigated the effect of ATP on chemokine production by DCs. Consistent with previous observations, immature DCs constitutively produced MDC and TARC but not IP-10 or RANTES.5 16 In these cells, treatment with ATP significantly increased release of MDC but not of TARC, IP-10, or RANTES (Table 1). Moreover, ATP increased the production of MDC and strongly inhibited secretion of IP-10 and RANTES induced by LPS (Table 1 and Figure5). SDF-1α was not detected in the supernatants of immature or mature DCs, regardless of whether nucleotides were present (data not shown). Suppression of IP-10 and enhancement of MDC were already evident at an ATP dose of 2.5 μM, whereas the minimal ATP concentration inhibiting RANTES secretion was 100 μM. UTP did not affect chemokine production by DCs.
Effects of ATP, UTP, and LPS on release of 4 chemokines from dendritic cells
| Treatment . | IP-10 (ng/mL) . | RANTES (ng/mL) . | MDC (ng/mL) . | TARC (ng/mL) . |
|---|---|---|---|---|
| None | ND | ND | 65.2 ± 4.9 | 122 ± 16.2 |
| ATP | ND | ND | 165 ± 15.2* | 172 ± 28.3 |
| UTP | ND | ND | 72.1 ± 9.0 | 134 ± 12.7 |
| LPS | 11.3 ± 1.2 | 18.1 ± 1.7 | 315 ± 32.4 | 283 ± 27.1 |
| LPS + ATP | ND | 4.3 ± 0.6† | 512 ± 37.2† | 316 ± 29.6 |
| LPS + UTP | 10.8 ± 0.8 | 17.5 ± 1.9 | 307 ± 35.4 | 266 ± 21.9 |
| Treatment . | IP-10 (ng/mL) . | RANTES (ng/mL) . | MDC (ng/mL) . | TARC (ng/mL) . |
|---|---|---|---|---|
| None | ND | ND | 65.2 ± 4.9 | 122 ± 16.2 |
| ATP | ND | ND | 165 ± 15.2* | 172 ± 28.3 |
| UTP | ND | ND | 72.1 ± 9.0 | 134 ± 12.7 |
| LPS | 11.3 ± 1.2 | 18.1 ± 1.7 | 315 ± 32.4 | 283 ± 27.1 |
| LPS + ATP | ND | 4.3 ± 0.6† | 512 ± 37.2† | 316 ± 29.6 |
| LPS + UTP | 10.8 ± 0.8 | 17.5 ± 1.9 | 307 ± 35.4 | 266 ± 21.9 |
Dendritic cells were left untreated or stimulated with either 250 μM ATP, 250 μM UTP, 10 μg/mL LPS, LPS and ATP, or LPS and UTP. After 24 hours, chemokine levels in the supernatants were measured by enzyme-linked immunosorbent assay. Results are mean ± SD nanograms per milliliter/106 cells from 5 independent experiments.
ATP indicates adenosine triphosphate; UTP, uridine triphosphate; LPS, lipopolysaccharide; IP-10, interferon-inducible protein 10; RANTES, regulated upon activation, normal T-cell expressed and secreted; MDC, macrophage-derived chemokine; TARC, thymus- and activation-regulated chemokine; and ND, not detectable.
P < .03 compared with results with untreated or UTP-treated DCs.
P < .03 compared with results with DCs treated with LPS alone or with LPS and UTP.
ATP inhibits IP-10 and RANTES release and augments MDC secretion in a dose-dependent fashion.
Immature DCs were stimulated with 10 μg/mL LPS and a graded concentration of ATP (▪) or UTP (▴). After 24 hours of incubation, IP-10 (A), RANTES (B), MDC (C), and TARC (D) were measured in culture supernatants by ELISA. Results are mean ± SD nanograms per milliliter/106 cells from triplicate cultures. Differences in IP-10, RANTES, and MDC secretion of DCs stimulated with LPS alone and those stimulated with both LPS and ATP were significant (P < .05) when ATP amounts were at least 2.5 μM, at least 100 μM, and at least 2.5 μM, respectively, for the 3 chemokine assessments.
ATP inhibits IP-10 and RANTES release and augments MDC secretion in a dose-dependent fashion.
Immature DCs were stimulated with 10 μg/mL LPS and a graded concentration of ATP (▪) or UTP (▴). After 24 hours of incubation, IP-10 (A), RANTES (B), MDC (C), and TARC (D) were measured in culture supernatants by ELISA. Results are mean ± SD nanograms per milliliter/106 cells from triplicate cultures. Differences in IP-10, RANTES, and MDC secretion of DCs stimulated with LPS alone and those stimulated with both LPS and ATP were significant (P < .05) when ATP amounts were at least 2.5 μM, at least 100 μM, and at least 2.5 μM, respectively, for the 3 chemokine assessments.
Depending on their polarization, T lymphocytes differentially express receptors for IP-10, RANTES, MDC, and TARC, with type 1 cells preferentially expressing CXCR3 and CCR5 and type 2 cells expressing CCR3, CCR4, and CCR8.18 20-22 We thus investigated whether ATP could affect the capacity of DCs to attract type 1 and type 2 T lymphocytes. First, polarized T-cell lines were generated by stimulating PBMCs with PHA in the presence of IL-12 and neutralizing anti–IL-4 mAb or IL-4 and neutralizing anti–IL-12 mAb. T-cell lines were about 60% CD8+ and 40% CD4+ (data not shown). As expected, type 1 lymphocytes showed high levels of IFN-γ and low levels of IL-4, whereas type 2 cells had high IL-4 and low IFN-γ production (Figure 6A-B). ELISA of cell-culture supernatants 48 hours after restimulation with plate-bound anti-CD3 and soluble anti-CD28 mAbs confirmed that type 1 cells released up to 2 ng/mL IFN-γ and less than 0.2 ng/mL IL-4, whereas type 2 cells produced 1.3 ng/mL IL-4 and less than 0.02 ng/mL IFN-γ. CCR5 and CXCR3 were expressed mostly by type 1 cells, whereas CCR4 was present on most type 2 cells (Figure 6C-D).
ATP inhibits the capacity of mature DCs to attract type 1 but not type 2 polarized T-cell lines.
Polarized type 1 and type 2 T-cell lines were obtained from PBMCs stimulated with PHA in the presence of rhu IL-12 and anti–IL-4 mAb or rhu IL-4 and anti–IL-12 mAb, respectively. Cells were cultured for 10 to 14 days in the presence of 30 U/mL IL-2 and then examined for intracellular IFN-γ and IL-4 by flow cytometry after 6 hours of incubation with plate-bound anti-CD3 and soluble anti-CD28 mAbs to assess polarization (A,B). Expression of the chemokine receptors CCR4, CCR5, and CXCR3 was studied by flow cytometry on resting polarized type 1 (C) and type 2 (D) cells. Type 1 (E,G) and type 2 (F,H) T lymphocytes were tested for their migratory capacity to DC-derived supernatants diluted in complete medium and 0.5% BSA. Shown are results with supernatants from immature DCs (E,F) that were either untreated (○) or stimulated with 250 μM ATP (■) or 250 μM UTP (▵) and results with supernatants from DCs treated with 10 μg/mL LPS alone (●), LPS and ATP (▪), or LPS and UTP (▴) (G,H). Data from the migration assays are mean ± SD net migration results from 4 independent experiments using T-cell lines from 2 different donors. In panel G, differences in T-cell migration induced by supernatants from DCs stimulated with LPS alone and from DCs stimulated with LPS and ATP were significant (P < .03) at supernatants dilutions of 1:500 or less.
ATP inhibits the capacity of mature DCs to attract type 1 but not type 2 polarized T-cell lines.
Polarized type 1 and type 2 T-cell lines were obtained from PBMCs stimulated with PHA in the presence of rhu IL-12 and anti–IL-4 mAb or rhu IL-4 and anti–IL-12 mAb, respectively. Cells were cultured for 10 to 14 days in the presence of 30 U/mL IL-2 and then examined for intracellular IFN-γ and IL-4 by flow cytometry after 6 hours of incubation with plate-bound anti-CD3 and soluble anti-CD28 mAbs to assess polarization (A,B). Expression of the chemokine receptors CCR4, CCR5, and CXCR3 was studied by flow cytometry on resting polarized type 1 (C) and type 2 (D) cells. Type 1 (E,G) and type 2 (F,H) T lymphocytes were tested for their migratory capacity to DC-derived supernatants diluted in complete medium and 0.5% BSA. Shown are results with supernatants from immature DCs (E,F) that were either untreated (○) or stimulated with 250 μM ATP (■) or 250 μM UTP (▵) and results with supernatants from DCs treated with 10 μg/mL LPS alone (●), LPS and ATP (▪), or LPS and UTP (▴) (G,H). Data from the migration assays are mean ± SD net migration results from 4 independent experiments using T-cell lines from 2 different donors. In panel G, differences in T-cell migration induced by supernatants from DCs stimulated with LPS alone and from DCs stimulated with LPS and ATP were significant (P < .03) at supernatants dilutions of 1:500 or less.
Consistent with the high production of MDC and TARC by immature DCs, type 2 cells (Figure 6F) migrated more efficiently (P < .05) than type 1 cells (Figure 6E) to supernatants from immature DCs, regardless of the treatment with nucleotides. Type 1 and type 2 cells showed a similar, higher level of migration to supernatants from LPS-stimulated DCs compared with supernatants from immature DCs (Figure 6G-H). However, supernatants from DCs matured in the presence of ATP, but not UTP, showed a strongly reduced capacity to attract type 1 lymphocytes, whereas attraction of type 2 cells was not affected (Figure 6G-H). Preincubation of supernatants from immature or mature DCs with anti-MDC and anti-TARC Abs markedly reduced (70% inhibition) migration of type 2 cells but not type 1 cells. In contrast, IP-10 neutralization suppressed (60% inhibition) the migratory response of type 1 cells but only slightly inhibited (20% inhibition) the response of type 2 cells induced by supernatants from mature DCs (data not shown).
We next assessed the migratory activity of DC supernatants on type 1 and type 2 T-cell clones (Table2). As shown in Figure7, Th2 clones migrated more vigorously than Th1 and T- cytotoxic (Tc) clones to supernatants from immature DCs, a function that was not affected by treatment with ATP or UTP. Supernatants from mature DCs were more potent than those from immature DCs in inducing migration of Th1 and Tc1 clones. In contrast, migration of Th2 and Tc2 clones was not influenced by the maturation stage of donor DCs. Interestingly, supernatants from mature DCs that had been treated with ATP attracted Th1 and Tc1 cells less efficiently, whereas migration of Th2 and Tc2 cells was not affected.
T-cell clones used in the study
| Clone . | Phenotype . | IFN-γ (pg/mL) . | IL-4 (pg/mL) . | T-cell type . |
|---|---|---|---|---|
| AL 1.16 | CD4+ | 1812 ± 109 | 15 ± 2 | Th1 |
| CS 2.43 | CD4+ | 2234 ± 102 | 13 ± 4 | Th1 |
| EF 1.3 | CD4+ | 4031 ± 156 | 130 ± 21 | Th1 |
| FA 1.42 | CD8+ | 2078 ± 167 | 52 ± 9 | Tc1 |
| AL 1.19 | CD8+ | 1974 ± 172 | 68 ± 7 | Tc1 |
| MB 2.9 | CD8+ | 2343 ± 188 | 15 ± 4 | Tc1 |
| AL 1.15 | CD4+ | ND | 3105 ± 265 | Th2 |
| CS 2.3 | CD4+ | 151 ± 23 | 6775 ± 436 | Th2 |
| EF 2.4 | CD4+ | ND | 6540 ± 541 | Th2 |
| FA 2.26 | CD8+ | ND | 7462 ± 854 | Tc2 |
| CS 2.12 | CD8+ | 51 ± 4 | 2437 ± 484 | Tc2 |
| MB 2.27 | CD8+ | 27 ± 4 | 1585 ± 123 | Tc2 |
| Clone . | Phenotype . | IFN-γ (pg/mL) . | IL-4 (pg/mL) . | T-cell type . |
|---|---|---|---|---|
| AL 1.16 | CD4+ | 1812 ± 109 | 15 ± 2 | Th1 |
| CS 2.43 | CD4+ | 2234 ± 102 | 13 ± 4 | Th1 |
| EF 1.3 | CD4+ | 4031 ± 156 | 130 ± 21 | Th1 |
| FA 1.42 | CD8+ | 2078 ± 167 | 52 ± 9 | Tc1 |
| AL 1.19 | CD8+ | 1974 ± 172 | 68 ± 7 | Tc1 |
| MB 2.9 | CD8+ | 2343 ± 188 | 15 ± 4 | Tc1 |
| AL 1.15 | CD4+ | ND | 3105 ± 265 | Th2 |
| CS 2.3 | CD4+ | 151 ± 23 | 6775 ± 436 | Th2 |
| EF 2.4 | CD4+ | ND | 6540 ± 541 | Th2 |
| FA 2.26 | CD8+ | ND | 7462 ± 854 | Tc2 |
| CS 2.12 | CD8+ | 51 ± 4 | 2437 ± 484 | Tc2 |
| MB 2.27 | CD8+ | 27 ± 4 | 1585 ± 123 | Tc2 |
Resting T-cell clones were stimulated with plate-bound anti-CD3 and soluble anti-CD28 monoclonal antibodies. After 48 hours, cytokine concentrations in the supernatants were measured by enzyme-linked immunosorbent assay. Unstimulated T cells did not produce detectable amounts of cytokines.
IFN-γ indicates interferon γ; IL-4, interleukin 4; Th, T helper; Tc, T cytotoxic; and ND, not detectable.
ATP-treated mature DCs attract Th1 and Tc1 clones less efficiently but not Th2 or Tc2 clones.
Supernatants from untreated DCs (○) or DCs stimulated with either 250 μM ATP (■), 250 μM UTP (▵), 10 μg/mL LPS (●), LPS and ATP (▪), or LPS and UTP (▴) were diluted with 0.5% BSA RPMI to stimulate resting type 1 or type 2 T-cell clones in a migration assay. Data are mean ± SD net migration results from 3 different T-cell clones of each type. Differences in T-cell migration induced by supernatants from immature (A,C,E,G) compared with mature (B,D,F,H) DCs, as well as differences between DCs stimulated with LPS alone and DCs stimulated with LPS and ATP (B,F), were significant (P < .03) at all supernatant dilutions.
ATP-treated mature DCs attract Th1 and Tc1 clones less efficiently but not Th2 or Tc2 clones.
Supernatants from untreated DCs (○) or DCs stimulated with either 250 μM ATP (■), 250 μM UTP (▵), 10 μg/mL LPS (●), LPS and ATP (▪), or LPS and UTP (▴) were diluted with 0.5% BSA RPMI to stimulate resting type 1 or type 2 T-cell clones in a migration assay. Data are mean ± SD net migration results from 3 different T-cell clones of each type. Differences in T-cell migration induced by supernatants from immature (A,C,E,G) compared with mature (B,D,F,H) DCs, as well as differences between DCs stimulated with LPS alone and DCs stimulated with LPS and ATP (B,F), were significant (P < .03) at all supernatant dilutions.
Discussion
DCs express functional purinergic receptors of both the P2X and P2Y families, including P2X1, P2X2, P2X4, P2X5, P2X7, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11.11-14 P2X receptors are membrane channels, whereas P2Y are G protein–coupled 7-membrane spanning receptors that can, on activation, mobilize calcium from the intracellular stores by means of generation of inositol triphosphate.10 Extracellular ATP can thus affect many biologic aspects of DCs through activation of distinct P2 purinergic receptors.10-14,23,24 In particular, extracellular ATP showed the ability to suppress production of proinflammatory cytokines and IL-12 by mature DCs.15 In addition, patch-clamp studies found that DCs reoriented their dendrites in the vicinity of a patch pipette containing ATP, thus suggesting sensitivity to an ATP-based chemotactic gradient.11 This preliminary finding was confirmed by a study showing that ATP can modulate DC chemotaxis by means of activation of pertussis toxin–sensitive P2Y receptors (M.I., S. Dichmann, D.F., et al, unpublished data, July 2001). These observations are consistent with data showing that extracellular nucleotides are powerful chemoattractants for inflammatory cells,25-27 and they support the hypothesis that these nucleotides have an important role in alerting the immune system after cell injury or tissue damage.
The current study showed that ATP, but not UTP, modifies the pattern of chemokine-receptor expression on DCs leading to up-regulation of the lymphoid chemokine receptors CCR7 and CXCR4 and the coordinated down-regulation of CCR5. Functionally, DCs exposed to ATP migrated vigorously to MIP-3β and SDF-1, whereas chemotaxis to MIP-1β was reduced. Thus, ATP appears to set DCs for enhanced migration to lymphoid organs.2-4 ATP also induced functional CXCR4 expression on DC membranes, a property that was not shared by LPS, which stimulated mRNA but not membrane expression of CXCR4.18,19 In a previous study, cholera toxin, like ATP, induced DC maturation associated with membrane CXCR4 expression.19 Wilkin et al24 suggested that ATP could induce DC maturation through stimulation of the P2Y11 purinoreceptor, which is the only P2 receptor coupled to phospholipase C and adenylyl cyclase activation.28Thus, ATP or cholera toxin might lead to CXCR4 expression on DC membranes by elevating the intracellular levels of cyclic adenosine monophosphate (cAMP). Consistent with this hypothesis, Cole et al29 reported that cAMP up-regulates membrane CXCR4 expression on lymphocytes by decreasing receptor internalization without affecting the level of gene expression. Finally, UTP, an agonist at P2Y2, P2Y4, and P2Y6 but not P2Y1 and P2Y11 receptors, failed to modify chemokine-receptor expression by DCs.
ATP also affected the pattern of chemokine release from DCs by up-regulating the constitutive production of MDC and inhibiting the LPS-induced secretion of IP-10 and RANTES. ATP was found to block the production of IP-10 by astrocytes stimulated with IL-1β.30 Prostaglandin E2 and other cAMP-elevating agents were reported to have similar modulatory effects on DCs, by inhibiting release of IP-10 and RANTES but not MDC.5,19,31 The pattern of chemokine release from DCs matured in the presence of ATP suggested an altered capacity of DCs to attract T-cell subsets, since CCR5 and CXCR3 (which can be activated by RANTES and IP-10, respectively) are preferentially expressed by Th1 cells, whereas CCR4 (which is activated by MDC and TARC) is prominent on Th2 cells.9,20-22 32 Therefore, we assessed the capacity of supernatants from DC cultures to induce migration of type 1 or type 2 polarized T cells. We found that DCs matured in the presence of ATP attracted type 1 polarized T-cell lines and Th1 and Tc1 clones less efficiently, whereas migration of type 2 T cells was not affected. These results suggest that DCs exposed to ATP have a diminished capacity to amplify type 1 immune responses.
ATP and other nucleotides are present in the cell cytoplasm at millimolar concentrations and can be released by exocytosis, transport through unidentified plasma-membrane pathways, or (more easily), passive leakage after cell damage.10 ATP is thus likely to be present at high concentrations in the extracellular milieu at sites of inflammation associated with tissue damage. ATP concentration in the pericellular space after cell stimulation has been measured in a few experimental systems and found to vary from the high nanomolar to the low micromolar range.33,34 However, ATP acts as a short-range extracellular messenger that can reach higher levels in the space between adjacent cells. Extracellular ATP appears to have a dual effect on maturing DCs. On the one hand, it can by itself stimulate secretion of IL-1β and tumor necrosis factor (TNF-α), especially if added rapidly at concentrations higher than 500 μM.14 On the other hand, when added during a longer period and at low doses (< 500 μM), ATP up-regulates surface expression of molecules that may be involved in lymphocyte activation.12,15 At the same time, however, ATP suppresses the production of cytokines such as TNF-α and IL-12 stimulated by endotoxin. This graded action of ATP suggests that in the presence of sudden and extensive tissue damage, ATP acts as a wide-ranging, nonspecific emergency signal to alert body defenses. In contrast, after less dramatic insults or under conditions that cause a prolonged release of low amounts of ATP, this nucleotide has a more subtle modulatory function, inducing a distorted DC maturation and diminishing the ability of mature DCs to initiate type 1 immune responses.15
In summary, we found that low doses of ATP change the chemokine-receptor aspect of DCs, conferring on them an enhanced capacity to localize to lymph nodes. We also observed an additional mechanism by which extracellular ATP can promote immune deviation toward type 2 responses, that is, by preventing DC recruitment of type 1 but not type 2 T lymphocytes. Our findings support the hypothesis that ATP released by damaged cells in inflamed tissues can act as a potent regulatory signal for DCs and for the polarization of the immune response.
We thank Brian Kelsall, Warren Strober, and Michael Braun for helpful discussions.
Supported by the Italian Ministry of Health, the Italian Ministry for Education, the Italian Association for Cancer Research, the Italian Space Agency, the National Research Council of Italy (Target Project on Biotechnology), and Telethon of Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giampiero Girolomoni, Istituto Dermopatico dell'Immacolata, IRCCS, Via Monti di Creta 104, 00167 Rome, Italy; e-mail: giro@idi.it.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal