Hermansky-Pudlak syndrome (HPS) is an inherited hemorrhagic disease affecting the related subcellular organelles platelet dense granules, lysosomes, and melanosomes. The mouse genes for HPS, pale ear and pearl, orthologous to the human HPS1 and HPS2 (ADTB3A) genes, encode a novel protein of unknown function and the β3A subunit of the AP-3 adaptor complex, respectively. To test for in vivo interactions between these genes in the production and function of intracellular organelles, mice doubly homozygous for the 2 mutant genes were produced by appropriate breeding. Cooperation between the 2 genes in melanosome production was evident in increased hypopigmentation of the coat together with dramatic quantitative and qualitative alterations of melanosomes of the retinal pigment epithelium and choroid of double mutant mice. Lysosomal and platelet dense granule abnormalities, including hyposecretion of lysosomal enzymes from kidneys and depression of serotonin concentrations of platelet dense granules were likewise more severe in double than single mutants. Also, lysosomal enzyme concentrations were significantly increased in lungs of double mutant mice. Interaction between the 2 genes was specific in that effects on organelles were confined to melanosomes, lysosomes, and platelet dense granules. Together, the evidence indicates these 2 HPS genes function largely independently at the whole organism level to affect the production and function of all 3 organelles. Further, the increased lysosomal enzyme levels in lung of double mutant mice suggest a cause of a major clinical problem of HPS, lung fibrosis. Finally, doubly mutant HPS mice are a useful laboratory model for analysis of severe HPS phenotypes.
Introduction
Hermansky-Pudlak syndrome (HPS) is an inherited human disease affecting several intracellular organelles including melanosomes, platelet dense granules, and lysosomes.1-3Abnormalities in these 3 organelles cause hypopigmentation, prolonged bleeding times, and in some patients ceroid deposition in several tissues including lung. Associated clinical problems include severe visual deficiencies, hemorrhaging requiring repeated platelet transfusions, and fibrotic lung disease often leading to premature death in midlife. Only symptomatic treatment is available.
The syndrome has been described in animal models such as the mouse where at least 15 genetically distinct mutants occur.4,5The disease is likewise genetically heterogeneous in humans where 3 distinct forms of HPS (HPS1, HPS2, and HPS3) have been described.1,2,6,7 HPS1 comprises the majority of HPS patients and is caused by a mutation in a chromosome 10 gene, which produces a novel 79-kd protein product.8 This protein is found predominantly in a cytosolic complex; its function remains unknown.9,10 Mutations in the β3A subunit gene (ADTB3A) of the AP-3 adaptor complex produce HPS2.7 Such an alteration is entirely consistent with the vesicle abnormalities of HPS because the AP-3 complex is well documented1 to function in the sorting of lysosomal membrane proteins. The recently described HPS3gene6 is a novel gene of unknown molecular function and is mutated in a group of central Puerto Rican patients with HPS.
The corresponding or orthologous mouse models for HPS1 and HPS2 are the pale ear11,12 and pearl13-15 pigment mutants, respectively. The phenotypes of the 2 mouse mutants share significant features in hypopigmentation, lysosomal secretion abnormalities, and platelet physiology, possibly consistent with action on a common organelle regulatory pathway. On the other hand, the mutant mice differ somewhat in pigmentation, lysosomal secretion in certain cell types, and in quantity of the contents of platelet dense granules.4 Although it is certain that the HPS2(ADTB3A) gene affects vesicle trafficking, and it is highly likely from phenotypes of pale ear mice and patients with HPS1 that this gene does likewise, it is less certain whether the 2 genes act independently or by common pathways. This is especially true in regard to whole animal phenotypes and patient clinical characteristics. The fact that each mouse model is maintained as a congenic mutant on the C57BL/6J background enables genetically controlled in vivo tests for interaction of these 2 important genes. A classical method of testing for interaction of genes in mutant mice is to analyze double mutant offspring that contain mutant versions of both genes.16
The pale ear and pearl genes are important not only because of their critical roles in organelle production, function, and trafficking but also because they are the corresponding mouse models for 2 forms of HPS thus far identified in humans, including the most common form, HPS1. HPS1 is the most common form as a result of a founder mutation in Puerto Rico. We report that the HPS1 and ADTB3Agenes cooperate in independent pathways in the formation/function of platelet dense granules, melanosomes, and lysosomes at the level of the whole organism. Further, these double mutant mice are experimentally useful in the study of HPS because they exhibit a particularly severe form of the disease.
Materials and methods
Mice
Pale ear (ep/ep) and pearl (pe/pe) mutant mice together with control normal C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were subsequently bred and maintained in the animal facilities of Roswell Park Cancer Institute. Both pearl and pale ear mutants originally arose on the C3H inbred strain background and have subsequently been transferred to and maintained as congenic mutants on the C57BL/6J inbred strain background. All mice used in these experiments were 2 to 4 months old.
Genotyping
Polymerase chain reaction (PCR) primers (epF: 5′-ACTGTGGGGTGGACATTGG-3′ and epR: 5′-AGAAGCCTGCAAGACAGACG-3′) derived from GenBank sequence AF00435312 were used to amplify the wild-type (264-base pair [bp] product) pale ear allele specifically. The pale ear mutation allele (ep) was detected as a 348-bp product produced by the same epF primer and a primer epR-mu (5′-AGTGATGCGCCCTAGGCAAT-3′) derived from GenBank sequences AF004353and U7911912. The above 3 primers were generously provided by Dr Margit Burmeister at the University of Michigan.
PCR primers (23F9: 5′-GAAATGGGGCTGCACATAG-3′ and 17R3: 5′-GAACCCTCACACAGGACTCG-3′) that specifically amplify the genomic junction point (678-bp product) in the pe genomic duplication17 were used to detect the pearl allele. However, this test cannot differentiate heterozygous pe/+from homozygous pe/pe mice. Therefore, microsatellite markers D13Mit145 and D13Mit159, which are 0.3 cM distal and 0.5 cM proximal, respectively, to the pelocus,13 were used to type polymorphisms between C3H and C57BL/6J backgrounds to confirm the homozygosity of the pearl mutation allele (pe) in double mutants. Primers for amplification of microsatellite markers D13Mit145 andD13Mit159 were purchased from Research Genetics (Huntsville, AL).
The PCR products of microsatellite markers were separated by 8% polyacrylamide gel electrophoresis and visualized with ethidium bromide. Other PCR products were separated by 1% agarose gel electrophoresis and visualized with ethidium bromide.
Bleeding times and platelet collection
Platelet serotonin assays
Platelets were lysed in 1 mL distilled water and assayed fluorometrically for serotonin.18
Mepacrine uptake
Platelets were incubated with mepacrine and analyzed with a Leitz MPV-2 fluorescent microscope.19
Thrombin-stimulated platelet secretion
Platelets at 109 cells/mL were washed 2 times in phosphate-buffered saline containing 2% bovine serum albumin (BSA). For measurements of secretion of lysosomal enzymes and α-granule contents, platelets were treated with 1.6 U/mL thrombin (Sigma Chemical, St Louis, MO) for 3 minutes with constant shaking.20 The reaction was stopped with 2.5 nmol/mL of Thromstop (American Diagnostics, Greenwich, CT).
Urine and tissue collection
To amplify lysosomal enzyme concentrations in kidney and urine, female mice were treated for 20 days with testosterone. Testosterone greatly amplifies both the synthesis and secretion of lysosomal enzymes in kidney proximal tubule cells.21 At days 20 to 24, mice were placed in metabolism cages at 2 to 4 per cage and urine was collected at 24-hour intervals. After day 24, mice were killed by anoxia with CO2 and tissues were homogenized and stored frozen.
Enzyme assays
β-Glucuronidase and β-galactosidase were assayed with fluorescent methylumbelliferyl substrates.18 Protein was determined with the Bio-Rad protein assay system (Bio-Rad Laboratories, Hercules, CA).
Immunoblotting
Fibrinogen and platelet factor 4 (PF4) were measured in platelets and platelet secretions by immunoblotting using rabbit antiserum to human fibrinogen (Diagnostica Stago, Asnieres, France) and a rabbit polyclonal antibody to rat PF4.22 All blots were exposed to film for several different lengths of time to ensure that the density of bands was within the linear range. Equivalent loading and transfer were verified by India ink staining of blots.
Electron microscopy
Eyes from 2 animals per each genetic group were immersion fixed with 4% formaldehyde, 2% glutaraldehyde, and 3% sucrose in 100 mM Hepes buffer at pH 7.4 for 10 minutes. Eyes were then cut, exposing the internal surface of the retina to the fixative. After fixation, portions of the eye, including the retina and the choroid, were further dissected to longitudinal strips posterior to the ora serrata and anterior to the macula. Tissues were rinsed in buffer and treated with 2% reduced osmium tetroxide for 1 hour. Tissues were rinsed then dehydrated in increasing graded series of ethanol and embedded in Eponate 12 (Ted Pella, Redding, CA) as in standard processing techniques for transmission electron microscopy. Cross-sections through the retina and choroid were obtained using a diamond knife and were contrasted with solutions of 3% uranyl acetate and lead citrate. Sections were viewed and photographed with a Philips CM-10 transmission electron microscope (Philips Electron Optics, FEI, Hillsboro, Oregon).
Platelets were harvested from the peripheral blood of C57BL/6J, pale ear, pearl, and pale ear/pearl double mutant mice in the presence of sodium citrate. Platelets were pelleted gently and fixed in 2% paraformaldehyde/1.5% glutaraldehyde. The pellets were osmicated, stained with tannic acid and embedded in Epon for ultrathin section electron microscopy.
Results
Breeding and pigmentation phenotypes of double mutant mice
As the first step in producing double mutant mice, homozygous pale ear and homozygous pearl mice were mated to obtain F1 (ep/+, pe/+) offspring. These F1 animals, of normal black coat and eye color, were intercrossed, and F2 animals were typed as homozygous pearl (pe/pe), pale ear (ep/ep), or double mutants (ep/ep, pe/pe) by both coat color and molecular genotyping of the ep or pe mutations. Double mutant pale ear/pearl mice appear robust for at least 5 months of age though breeding performance is somewhat depressed.
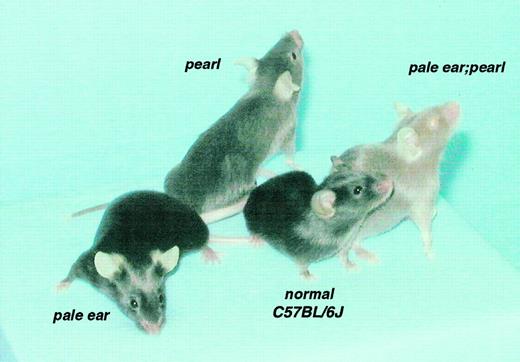
The majority of resultant F2 progeny had black coat and eye colors identical to C57BL/6J normal mice. Some exhibited the same eye (ie, dilute red in newborn and black in adults) and coat colors as either pale ear or pearl mice. Adult pale ear mice display hypopigmentation of the ears and tails with no effects on pigmentation of other body parts (Figure 1). The pearl mutant shows a brown-gray coat color on all body parts. In contrast, a third group of offspring displayed a new phenotype (Figure 1) with strikingly lighter coat color throughout than either parental single mutant and light red eye color in both newborn and adult animals. These animals were tentatively designated as double mutants (ep/ep, pe/pe), a classification subsequently confirmed by PCR genotyping of genomic DNA (not shown). By visual examination, the ep/+, pe/pe mouse is not different from the +/+, pe/pe mouse in coat color; neither is the ep/ep, pe/+ mouse different from the ep/ep, +/+ mouse (not shown).
Coat and eye pigmentations.
Coat and eye pigmentation are severely affected in double mutant pale ear/pearl mice (ep/ep, pe/pe) as compared with normal (C57Bl/6J +/+) and with single mutant pale ear (ep/ep) and pearl (pe/pe) mice.
Coat and eye pigmentations.
Coat and eye pigmentation are severely affected in double mutant pale ear/pearl mice (ep/ep, pe/pe) as compared with normal (C57Bl/6J +/+) and with single mutant pale ear (ep/ep) and pearl (pe/pe) mice.
Double mutants or those of genotypes ep/+, pe/pe orep/ep, pe/+ were selected for further breeding. As expected from mendelian genetics, self-matings of the double mutants produced the identical distinctive coat and eye color phenotype of the parents in all subsequent generations. Also, when double mutants were backcrossed to either pale ear or pearl mice, only pale ear or pearl phenotypes, respectively, were observed.
Ultrastructure of eye melanosomes
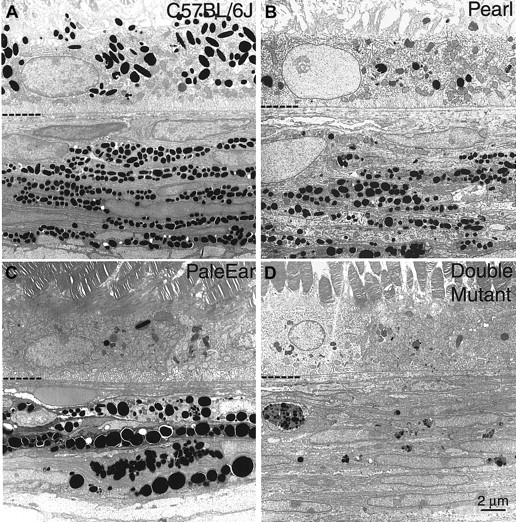
Eye tissues of control C57Bl/6J, pale ear, pearl, and double mutant mice were subjected to ultrastructural analysis by electron microscopy. Both quantitative and qualitative morphologic abnormalities in melanosomes of the retinal pigment epithelium (RPE) and choroid were apparent (Figure 2).
The ultrastructure of RPE and choroidal melanosomes of double mutant mice are severely altered.
Adult eye tissues from (A) control or wild-type C57Bl/6J (+/+), (B) pearl (pe/pe), (C) pale ear (ep/ep), and (D) mice doubly mutant for pale ear and pearl genes (ep/ep, pe/pe) were analyzed by electron microscopy. The interface between the RPE and choroid (CH) regions is indicated by the hyphenated partial line. The RPE is above the line and the CH is below.
The ultrastructure of RPE and choroidal melanosomes of double mutant mice are severely altered.
Adult eye tissues from (A) control or wild-type C57Bl/6J (+/+), (B) pearl (pe/pe), (C) pale ear (ep/ep), and (D) mice doubly mutant for pale ear and pearl genes (ep/ep, pe/pe) were analyzed by electron microscopy. The interface between the RPE and choroid (CH) regions is indicated by the hyphenated partial line. The RPE is above the line and the CH is below.
In the pearl mouse (Figure 2B), very small numbers of melanosomes were observed in the RPE. Melanosomes were present in the choroid of pearl mice, but they were reduced in number and exhibited greater size heterogeneity as compared with control tissues.
Melanized melanosomes were extremely rare in the RPE cells of the pale ear mouse (Figure 2C). Fewer but abnormally large melanosomes (macromelanosomes) were observed in the pale ear choroid relative to wild-type. Less pigmented intermediate structures were also observed.
In the double mutant mouse (Figure 2D), essentially no melanized melanosomes were observed in RPE cells, a situation similar to that of the pale ear RPE but different from that of pearl. Most striking, and in dramatic difference to that observed in either single mutant, was a drastic decrease in number of choroidal melanosomes in the double mutant. Only a very few multivesicular pigment granules with irregular boundaries and containing aggregates of melanosomes of various developmental stages were observed.
Platelet characteristics
As expected, double mutant mice had prolonged bleeding times. Four double mutants had bleeding times more than 15 minutes, a value at least as great as those observed in single mutant pale ear23 or pearl24 mice. It is technically difficult to determine if mutant mice have bleeding times longer than 15 minutes. Therefore, other platelet granule characteristics were assayed to determine if the presence of both mutant genes caused more severe effects. Platelet serotonin levels are an accurate monitor of the contents of platelet dense granules, which supply several low-molecular-weight compounds critical for normal hemostasis. As previously documented,23 24 platelet serotonin levels are significantly depressed in both the pale ear and pearl mutants with pale ear having intermediate levels (Table1). More notably, a particularly severe depression occurs in double mutant platelets to a level only 1% that of C57BL/6J controls. All mutant platelet serotonins are significantly different (P < .001), not only from C57BL/6J, but from all other mutant values.
Platelet serotonin concentrations in single and double mutant mice
| . | Serotonin (μg/109 platelets) . |
|---|---|
| C57BL/6J | 3.31 ± 0.19 (5) |
| ep/ep | 0.51 ± 0.067 (6) |
| pe/pe | 0.12 ± 0.012 (6) |
| ep/ep, pe/pe | 0.03 ± 0.008 (5) |
| . | Serotonin (μg/109 platelets) . |
|---|---|
| C57BL/6J | 3.31 ± 0.19 (5) |
| ep/ep | 0.51 ± 0.067 (6) |
| pe/pe | 0.12 ± 0.012 (6) |
| ep/ep, pe/pe | 0.03 ± 0.008 (5) |
Values represent the mean ± SEM of the number of mice in parentheses. All values differ by P ≤ .001.
Most mouse HPS mutants have abnormalities in secretion of the contents of platelet lysosomes, and similar abnormalities have been observed in patients with HPS.4 Previous studies23 24 had documented relatively high thrombin-stimulated secretion of lysosomal enzymes from platelets of pale ear and low secretion from platelets of pearl. The present analyses (Table 2) of the release of lysosomal enzymes from platelets of ep/ep, pe/pe(double mutant) mice revealed a phenotype similar to that of pearl, that is, low rates of secretion, for each of 2 lysosomal enzymes, β-glucuronidase and β-galactosidase. Double mutant and pearl mice secreted 15% to 20% of total platelet lysosomal enzymes, after treatment with thrombin, compared to the approximately 45% to 50% secretion observed for both pale ear and normal mice. Abnormalities in basal lysosomal secretion were not apparent for single or double mutants.
Thrombin-stimulated secretion of lysosomal enzymes from platelets of normal C57BL/6J and mutant mice
| . | Secretion (% total) . | |
|---|---|---|
| − thrombin . | 1.6 U thrombin . | |
| β-Glucuronidase | ||
| C57BL/6J | 5.12 ± 0.94 (6) | 46.8 ± 2.6 (5) |
| ep/ep | 4.03 ± 1.1 (6) | 56.7 ± 3.1 (6) |
| pe/pe | 4.10 ± 1.1 (6) | 15.8 ± 1.3 (6)* |
| ep/ep, pe/pe | 3.47 ± 0.68 (5) | 19.2 ± 1.1 (6)* |
| β-Galactosidase | ||
| C57BL/6J | 4.99 ± 0.77 (10) | 48.1 ± 3.0 (9) |
| ep/ep | 5.47 ± 0.96 (10) | 52.8 ± 3.3 (10) |
| pe/pe | 4.17 ± 0.37 (10) | 19.2 ± 3.6 (8)* |
| ep/ep, pe/pe | 4.21 ± 0.85 (9) | 19.6 ± 1.9 (10)* |
| . | Secretion (% total) . | |
|---|---|---|
| − thrombin . | 1.6 U thrombin . | |
| β-Glucuronidase | ||
| C57BL/6J | 5.12 ± 0.94 (6) | 46.8 ± 2.6 (5) |
| ep/ep | 4.03 ± 1.1 (6) | 56.7 ± 3.1 (6) |
| pe/pe | 4.10 ± 1.1 (6) | 15.8 ± 1.3 (6)* |
| ep/ep, pe/pe | 3.47 ± 0.68 (5) | 19.2 ± 1.1 (6)* |
| β-Galactosidase | ||
| C57BL/6J | 4.99 ± 0.77 (10) | 48.1 ± 3.0 (9) |
| ep/ep | 5.47 ± 0.96 (10) | 52.8 ± 3.3 (10) |
| pe/pe | 4.17 ± 0.37 (10) | 19.2 ± 3.6 (8)* |
| ep/ep, pe/pe | 4.21 ± 0.85 (9) | 19.6 ± 1.9 (10)* |
Platelets (1 × 108 cells/mL) were treated with thrombin for 3 min at 37°C. The reaction was terminated with Thromstop. Values represent the mean ± SEM of the individual determinations on the number of mice indicated in parentheses.
P ≤ .001.
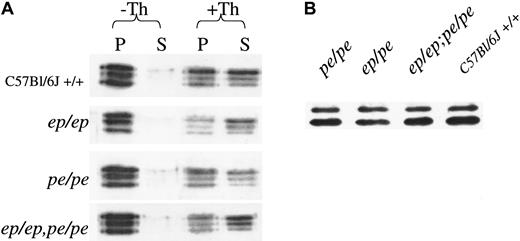
Contents of the major platelet subcellular organelle, the α granule, were monitored by immunoblotting (Figure3). Platelet contents of the α-granule protein fibrinogen were normal in both single and double mutants (Figure 3A). Likewise, secretion rates of this protein (Figure 3A) were equally robust in normal, single, and double mutant platelets after a 3-minute exposure to thrombin. Although levels of a second α-granule component, PF4, were too low to accurately measure secretion by blotting, it was apparent that steady-state levels (Figure 3B) of this protein were equivalent in normal, single, and double mutant platelets.
Steady-state concentrations and secretion rates of platelet α-granule components are normal in double mutant mice.
(A) Fibrinogen concentrations and secretion from untreated control (−Th) and thrombin (+Th)-treated platelets of mutant and normal mice. After treatment of platelets for 3 minutes with buffer or 1.6 U/mL thrombin, platelets were centrifuged and equivalent proportions of pellets (P) and supernatants (S) were electrophoresed on denaturing gels that were immunoblotted with antifibrinogen antibody. (B) Steady-state levels of PF4 in normal and mutant platelets. Platelet protein (30 μg) was electrophoresed on denaturing gels and immunoblotted with antibody to PF4.
Steady-state concentrations and secretion rates of platelet α-granule components are normal in double mutant mice.
(A) Fibrinogen concentrations and secretion from untreated control (−Th) and thrombin (+Th)-treated platelets of mutant and normal mice. After treatment of platelets for 3 minutes with buffer or 1.6 U/mL thrombin, platelets were centrifuged and equivalent proportions of pellets (P) and supernatants (S) were electrophoresed on denaturing gels that were immunoblotted with antifibrinogen antibody. (B) Steady-state levels of PF4 in normal and mutant platelets. Platelet protein (30 μg) was electrophoresed on denaturing gels and immunoblotted with antibody to PF4.
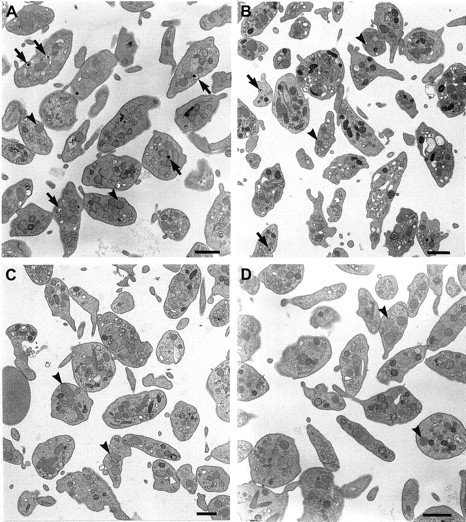
Ultrastructural analyses of platelets by electron microscopy (Figure4) were consistent with the above findings. Very few, if any, dense granules with their typical “bull's eye” appearance25 were found in the double mutant mouse platelets (Figure 4D), although these structures were readily apparent in normal mice (Figure 4A) and occasionally observed in pale ear mutants (Figure 4B). Further, consistent with the above immunoblotting analyses of α-granule components, no additional morphologic alterations were apparent in other subcellular structures, including α granules, in platelets of the double mutant.
Platelets of double mutant mice have normal ultrastructure, other than a lack of dense granules.
Platelets of normal C57BL/6J (A), pale ear (ep/ep) (B), pearl (pe/pe) (C), and double mutant (ep/ep, pe/pe) (D) mice were analyzed by electron microscopy. Dense granules (arrows) are common in normal platelets, less so in pale ear and are not apparent in pearl or double mutant cells. The α granules (arrowheads) are equally common in both qualitative and quantitative senses in all platelet genetic types. Bars represent 1 μm.
Platelets of double mutant mice have normal ultrastructure, other than a lack of dense granules.
Platelets of normal C57BL/6J (A), pale ear (ep/ep) (B), pearl (pe/pe) (C), and double mutant (ep/ep, pe/pe) (D) mice were analyzed by electron microscopy. Dense granules (arrows) are common in normal platelets, less so in pale ear and are not apparent in pearl or double mutant cells. The α granules (arrowheads) are equally common in both qualitative and quantitative senses in all platelet genetic types. Bars represent 1 μm.
Tissue steady-state lysosomal enzyme levels and secretion of kidney lysosomal enzymes
Measurements of tissue steady-state levels of lysosomal enzymes revealed significant and potentially important differences between the single and double mutants (Table3). Murine kidney undergoes significant hypertrophy, increases in lysosomal enzyme levels in proximal tubule cells and hypersecretion of lysosomal enzymes from these cells into urine in response to testosterone treatment.21 As previously documented,26 both pale ear and pearl testosterone-treated mice have significant (P < .001) increases in kidney lysosomal enzyme activity, as compared with normal C57BL/6J mice (Table 3). β-Glucuronidase is increased 2-fold in both mutants as is β-galactosidase in pale ear, whereas β-galactosidase is increased 30% in pearl compared to control C57BL/6J. The increases of kidney lysosomal enzymes in double mutant mice were even more pronounced. β-Glucuronidase levels were further elevated 1.6-fold (P < .001) over those of either pale ear or pearl, and β-galactosidase levels were 1.4-fold greater (P < .02) than pale ear. Altogether, β-glucuronidase and β-galactosidase concentrations of double mutant mice are increased a striking 3.7- and 2.5-fold, respectively, over that of normal C57BL/6J controls.
Lysosomal enzyme activities of various organs and platelets of normal C57BL/6J, single mutant (ep/ep andpe/pe), and double mutant (ep/ep,pe/pe) mice
| . | β-Glucuronidase (U/g) . | β-Galactosidase (U/g) . |
|---|---|---|
| Kidney | ||
| C57BL/6J (8) | 61.4 ± 4.5 | 14.8 ± .64 |
| ep/ep(6) | 143.0 ± 6.53-150 | 27.2 ± 1.13-150 |
| pe/pe(7) | 145.0 ± 5.03-150 | 19.2 ± 0.73-150 |
| ep/ep, pe/pe(8) | 229.0 ± 15.03-150 | 37.6 ± 2.83-150 |
| Lung | ||
| C57BL/6J | 4.73 ± 0.12 | 3.29 ± 0.14 |
| ep/ep | 5.20 ± 0.26 | 3.64 ± 0.18 |
| pe/pe | 6.08 ± 0.79 | 3.65 ± 0.28 |
| ep/ep,pe/pe | 12.2 ± 1.23-150 | 6.27 ± 0.593-150 |
| Liver | ||
| C57BL/6J | 25.4 ± 1.8 | 9.02 ± 0.79 |
| ep/ep | 27.7 ± 1.9 | 10.0 ± 0.80 |
| pe/pe | 23.7 ± 2.3 | 11.7 ± 0.58 |
| ep/ep, pe/pe | 29.0 ± 0.8 | 10.0 ± 1.1 |
| Brain | ||
| C57BL/6J | 0.84 ± 0.02 | 3.13 ± 0.11 |
| ep/ep | 0.96 ± 0.06 | 2.88 ± 0.06 |
| pe/pe | 0.96 ± 0.05 | 3.11 ± 0.07 |
| ep/ep,pe/pe | 1.06 ± 0.033-150 | 2.88 ± 0.14 |
| Spleen | ||
| C57BL/6J | 16.0 ± 0.77 | 17.5 ± 1.2 |
| ep/ep | 18.4 ± 2.3 | 18.2 ± 2.5 |
| pe/pe | 22.0 ± 1.53-150 | 19.3 ± 0.84 |
| ep/ep,pe/pe | 21.5 ± 2.83-150 | 20.0 ± 1.4 |
| Platelets | ||
| C57BL/6J | .0516 ± .0012 | .0693 ± .0044 |
| ep/ep | .0587 ± .0025 | .0885 ± .0029 |
| pe/pe | .0491 ± .0043 | .0723 ± .0088 |
| ep/ep,pe/pe | .0501 ± .0024 | .0796 ± .0075 |
| . | β-Glucuronidase (U/g) . | β-Galactosidase (U/g) . |
|---|---|---|
| Kidney | ||
| C57BL/6J (8) | 61.4 ± 4.5 | 14.8 ± .64 |
| ep/ep(6) | 143.0 ± 6.53-150 | 27.2 ± 1.13-150 |
| pe/pe(7) | 145.0 ± 5.03-150 | 19.2 ± 0.73-150 |
| ep/ep, pe/pe(8) | 229.0 ± 15.03-150 | 37.6 ± 2.83-150 |
| Lung | ||
| C57BL/6J | 4.73 ± 0.12 | 3.29 ± 0.14 |
| ep/ep | 5.20 ± 0.26 | 3.64 ± 0.18 |
| pe/pe | 6.08 ± 0.79 | 3.65 ± 0.28 |
| ep/ep,pe/pe | 12.2 ± 1.23-150 | 6.27 ± 0.593-150 |
| Liver | ||
| C57BL/6J | 25.4 ± 1.8 | 9.02 ± 0.79 |
| ep/ep | 27.7 ± 1.9 | 10.0 ± 0.80 |
| pe/pe | 23.7 ± 2.3 | 11.7 ± 0.58 |
| ep/ep, pe/pe | 29.0 ± 0.8 | 10.0 ± 1.1 |
| Brain | ||
| C57BL/6J | 0.84 ± 0.02 | 3.13 ± 0.11 |
| ep/ep | 0.96 ± 0.06 | 2.88 ± 0.06 |
| pe/pe | 0.96 ± 0.05 | 3.11 ± 0.07 |
| ep/ep,pe/pe | 1.06 ± 0.033-150 | 2.88 ± 0.14 |
| Spleen | ||
| C57BL/6J | 16.0 ± 0.77 | 17.5 ± 1.2 |
| ep/ep | 18.4 ± 2.3 | 18.2 ± 2.5 |
| pe/pe | 22.0 ± 1.53-150 | 19.3 ± 0.84 |
| ep/ep,pe/pe | 21.5 ± 2.83-150 | 20.0 ± 1.4 |
| Platelets | ||
| C57BL/6J | .0516 ± .0012 | .0693 ± .0044 |
| ep/ep | .0587 ± .0025 | .0885 ± .0029 |
| pe/pe | .0491 ± .0043 | .0723 ± .0088 |
| ep/ep,pe/pe | .0501 ± .0024 | .0796 ± .0075 |
Values are the mean ± SEM of the number of mice indicated in parentheses (all organs). Platelet values are U/109platelets and are from assays on platelets from 4 separate mice in each case.
Significant increase; see text for P values.
Large increases (2.6-fold for β-glucuronidase and 1.9-fold for β-galactosidase, P < .001, compared to C57BL/6J) were also observed for both lysosomal enzymes in lungs of double mutant mice (Table 3). In contrast, no significant elevation, over normal C57BL/6J values, of either lysosomal enzyme was apparent in lungs of either single mutant, pearl, or pale ear mice. Increases in lung lysosomal enzymes were not due to testosterone treatment because similar increases were observed in lung lysosomal enzymes of untreated double mutant females (not shown).
There were no significant alterations of liver, brain, or spleen lysosomal enzymes in either single or double mutants with the exceptions of small increases in brain β-glucuronidase (26%;P < .01) in the double mutant and spleen β-glucuronidase (38%; P < .02) in pearl and the double mutant.
The major mechanism for regulation of lysosomal enzyme levels in mouse kidney is secretion into urine.21 It is obvious from an analysis of urine levels (Table 4) that this process is highly abnormal in double mutants and is responsible for their increased kidney lysosomal enzyme levels. Normal C57BL/6J mice secreted approximately 15% of total kidney lysosomal enzyme levels daily. Secretion rates in contrast were about 8%, 40%, and 2.5% (P < .001 for all) of normal rates in ep/ep, pe/pe, and double mutants, respectively. Secretion rates of double mutants were 35% (P < .02) of that observed inep/ep mutants for both enzymes. Testosterone-mediated induction of kidney lysosomal enzymes is accompanied by large increases in kidney hypertrophy, and this process is accentuated in mutant mice with lysosomal enzyme secretion deficits.21 Kidney hypertrophy was in fact most pronounced in pearl and in double mutant mice (not shown), consistent with a major secretion defect. Mutant kidney hypertrophy is likely due in large part to an inability to eliminate by secretion material engorged within lysosomes.
Secretion of kidney lysosomal enzymes into urine of normal C57BL/6J, pale ear (ep/ep), pearl (pe/pe), and double mutant (ep/ep, pe/pe) mice
| . | % secreted/d . | |
|---|---|---|
| β-Glucuronidase . | β-Galactosidase . | |
| C57BL/6J | 18.4 ± 1.0 | 13.5 ± 1.0 |
| ep/ep | 1.28 ± 0.19 | 1.22 ± 0.18 |
| pe/pe | 8.73 ± 0.74 | 5.19 ± 0.36 |
| ep/ep,pe/pe | 0.432 ± .088 | 0.464 ± .078 |
| . | % secreted/d . | |
|---|---|---|
| β-Glucuronidase . | β-Galactosidase . | |
| C57BL/6J | 18.4 ± 1.0 | 13.5 ± 1.0 |
| ep/ep | 1.28 ± 0.19 | 1.22 ± 0.18 |
| pe/pe | 8.73 ± 0.74 | 5.19 ± 0.36 |
| ep/ep,pe/pe | 0.432 ± .088 | 0.464 ± .078 |
Urine was collected from 6 testosterone-treated female mice on 6 consecutive days. Values represent the mean ± SEM of total kidney lysosomal enzyme units.
Discussion
In the mouse, there are at least 15 models for HPS.4,5 The fact that all these mouse genes for HPS affect the same subcellular organelles, melanosomes, lysosomes, and platelet dense granules, indicates that the biosynthesis/function of these 3 organelles are under multiple genetic regulatory controls. Several of these 15 genes are involved in vesicle trafficking. These include (1) pallid, a novel 25-kd protein that interacts with syntaxin 13,27 (2) gunmetal, the α subunit of Rab geranylgeranyltransferase,28 (3) ashen, Rab27a,29 (4) mocha, the δ subunit of the AP-3 complex,30 and (5) pearl, the β subunit of the same AP-3 complex.13,15 Another gene, pale ear, encodes a novel 79-kd cytoplasmic protein of unknown function that is associated with tubulovesicular structures, vesicles, and nascent melanosomes in melanocytes.10 It is important to determine if these genes act by common or independent vesicle trafficking pathways. This is especially so for the pale ear and pearl genes which are the orthologs of 2 human HPS genes1-3 including the most common form (HPS1).
An experimental advantage of the mouse is that it is possible to test for interaction of recessive genes by producing and analyzing offspring that are genetically homozygous for both mutant alleles.16If the mutations are in a common pathway or within the subunits of a common protein complex, offspring will resemble one or the other parent. If the mutations are in independent pathways, the double mutant is expected to exhibit additive or interactive effects, and a new, usually more severe, phenotype occurs. Clearly, the present data for the pale ear and pearl genes support the latter possibility for nearly all organelle characteristics tested. Both pearl and pale ear are maintained as congenic mutations on a common C57BL/6J inbred background, a fact that greatly simplifies interpretation of new phenotypes because there is no contribution of differing background genes in the double mutant. Also, the molecular nature of each mutation predicts that the function of both proteins is essentially completely abrogated in the double mutant.11,12 15 Thus, there is minimal or no contribution of normal gene products in double mutant mice to confound interpretations.
Intercross matings of the pearl and pale ear mutants have produced a double mutant mouse with new severe phenotypes of platelet dense granules, lysosomes, and melanosomes re-emphasizing the interrelatedness of these organelles. The platelet effects of HPS mutations are very selective as they are confined to dense granules and lysosomes,4 and this pattern was maintained in double HPS mutants. The production of more severe granule phenotypes is especially apparent in the case of platelet dense granules where platelet serotonin levels were depressed to only 1% that of normal platelets. This is the lowest level documented in any mouse HPS mutant and predicts a very severe platelet storage pool deficiency and bleeding phenotype. Thrombin-stimulated platelet lysosomal enzyme secretion was severely impaired in the double mutant though in this case impairment was equivalent to that of the single mutant pearl. It remains possible therefore that the pale ear and pearl genes act within a common pathway for this process. Mutant effects on platelets are specific to dense granules and lysosomes. No effects on contents or rates of secretion of the α-granule components, fibrinogen or PF4, were apparent, and ultrastructural examination revealed no obvious abnormalities in organelles other than platelet dense granules.
Several lines of evidence support cooperation of the pale ear and pearl genes in the biogenesis of melanosomes. The gross pigmentation phenotype of the double mutant is unlike that of either single mutant. Double mutant mice are conspicuously more hypopigmented in both coat and eye color than either single mutant. The bases of the eye hypopigmentation became apparent upon ultrastructural analyses of the retinal pigment epithelium and choroidal melanocytes of the eye. Most notable was the near absence of pigmentation of the choroid. It is likely that the cause of the pink eye color of the adult double mutant originates in the choroid, because the near absence of RPE melanosomes in pale ear and pearl mice nevertheless produces a dark adult eye color. The observed near absence of RPE melanosomes in the pale ear12,31 and pearl31 32 mutants is consistent with previous observations by others.
In agreement with the pigmentation results, the lysosomal phenotype of the double mutant is considerably more severe than that of either single mutant. This is apparent in kidney proximal tubule cells where the constitutive secretion of the soluble lysosomal enzymes β-glucuronidase and β-galactosidase was reduced to vanishingly low levels and a corresponding hyperincrease in kidney hypertrophy and lysosomal enzyme activities occurs. Although the physiologic function of secretion of mouse kidney lysosomal enzymes remains elusive, it is clear that this is a massive process21 with an accompanying appearance of large numbers of striking multilamellar lysosomes within proximal tubule cells together with proteinuria. Proximal tubule cells have the ordered subcellular structure of typical secretory cells. The secretion effect is further magnified by testosterone treatment which increases the rates of synthesis of certain lysosomal enzymes such as β-glucuronidase more than 100-fold.21
A very interesting lysosomal phenotype of the double mutant, in terms of known clinical features of human HPS, is the increase in lysosomal enzyme concentrations in lung. A significant fraction of patients with HPS1 die in midlife due to lung abnormalities including lung fibrosis.33,34 Similarly reductions in life span and abnormalities of lung structure occur in selected mouse HPS mutants, including pearl35 and pale ear.5Accumulations of lysosomal ceroid or aging pigment has been postulated as the cause of the lung abnormalities.36 It is possible that increased number or size of lysosomes leads to increased fragility of lysosomes followed by more severe lung abnormalities including death of lung cells. Although further experiments are required to establish the basis of the high lysosomal enzyme concentrations in lung, a reasonable hypothesis is that they may derive, as observed for kidney of the double mutant, from abnormally low secretion rates. This scenario is consistent with the fact that secretion of lysosomal contents is an important process in lungs. The contents, including pulmonary surfactant, of lamellar bodies or lysosomes, are regularly secreted from type II cells in delivery of surfactant to the air-liquid interface to lower surface tension in the lung.37 It is clear that lysosomal enzyme secretion is important in a wide variety of physiologic processes in higher organisms.4,21 38-41
The conclusion that the pale ear and pearl gene products interact indirectly and independently to produce more severe melanosome, lysosome, and platelet dense granule phenotypes is consistent with other recent studies at the cell and molecular levels. For example, the intracellular location of the AP-3 complex is not changed in fibroblasts of patients with HPS17 or in normal fibroblasts overexpressing a His6-HPS1 fusion protein.9Similarly, the abnormal trafficking of the lysosomal membrane proteins CD63 and LAMP1 commonly observed in HPS2 cells is not observed in HPS1 fibroblasts,9 arguing against a role for the HPS1 protein in the AP-3 dependent pathway for lysosomal membrane protein trafficking. Finally, immunoprecipitation-recapture experiments failed to show an association of the HPS1 protein with AP-3.9
Mice with the new phenotype are viable, breed true, and are by molecular tests doubly homozygous for both the pearl and pale ear recessive genes. Doubly mutant HPS mice provide the practical advantage that they accentuate the mutant phenotype. This may allow, for example, analysis of abnormalities at a considerably earlier age than possible in single mutants, which typically require aging to more than 1 year for appearance of HPS abnormalities such as abnormal lung manifestations.35 The reduced breeding performance of the double mutants suggests that the combined mutant HPS genes have additional general deleterious physiologic effects. This suggestion is consistent with shortened life spans observed with other mouse strains doubly homozygous for other combinations of HPS genes.35
Analyses of double mutants of mouse models for the HPS (pale ear) and Chediak-Higashi (beige) genes similarly concluded that these genes acted on independent pigmentation pathways.42 However, the results of these double mutant experiments clearly differ from those involving double mutants of 2 other mouse HPS mutants, light ear and pale ear.43 In the latter case, the phenotypes of the double mutants were identical to the single mutant parents, suggesting that the pale ear and light ear gene products may directly interact in a common path or common protein complex.
Together, these results indicate that the pale ear and pearl genes are functionally interactive at the level of the whole organism in related, yet independent pathways to affect the synthesis/function of specialized mammalian organelles such as melanosomes, lysosomes, and platelet dense granules. The interactive effect of the double mutant on the morphology and physiology of several subcellular organelles is consistent with recent molecular evidence that other mouse genes for HPS, in addition to pale ear and pearl, encode genes that regulate vesicle trafficking. It appears likely that HPS proteins form a physiologic module44 of directly and indirectly interacting proteins necessary for the synthesis of these specialized mammalian organelles.
We thank Aaron Mammoser and Donna Reddington for excellent technical assistance.
Supported by National Institutes of Health grants HL51480, HL31698, and EY12104 (to R.T.S.), by a Medical Research Council program grant awarded to Colin Hopkins, and by the Roswell Park Cancer Institute Cancer Center Support Grant CA 16056.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard T. Swank, Molecular and Cellular Biology Dept, Roswell Park Cancer Institute, Carlton and Elm Sts, Buffalo, NY 14263; e-mail: richard.swank@roswellpark.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal