Antithrombin (AT) prevents Escherichia coli–induced hypotension in animal models of sepsis, and it further reduces the mortality of patients with septic shock. In the present study, we examined whether AT may prevent the endotoxin (ET)-induced hypotension by promoting the endothelial release of prostacyclin (PGI2) in rats. Intravenous administration of AT (250 U/kg) prevented both hypotension and the increases in plasma levels of NO2−/NO3− in rats given ET. Lung expression of messenger RNA (mRNA) for tumor necrosis factor-α (TNF-α) was transiently increased after ET administration, followed by the increases in lung tissue levels of TNF-α. Both the lung activity of the inducible form of nitric oxide synthase (iNOS) and the lung expression of iNOS mRNA in animals administered ET were gradually increased after the TNF-α mRNA expression had peaked. Administration of AT significantly inhibited these increases. Neither DEGR-F.Xa, a selective inhibitor of thrombin generation, nor Trp49-modified AT, which is not capable of promoting the endothelial release of PGI2, showed any effects on these changes induced by ET. Administration of antirat TNF-α antibody produced effects similar to those induced by AT. Indomethacin pretreatment abrogated the effects induced by AT. Iloprost, a stable derivative of PGI2, produced effects similar to those of AT. These findings suggested that AT prevents the ET-induced hypotension by inhibiting the induction of iNOS through inhibiting TNF-α production. These effects of AT could be mediated by the promotion of endothelial release of PGI2 and might at least partly explain the therapeutic effects for septic shock.
Introduction
Antithrombin (AT) is an important serine protease inhibitor of the coagulation system.1 Inhibition by AT of thrombin and the other serine proteases generated from the coagulation cascade is markedly accelerated by its interaction with the endothelial surface glycosaminoglycans (GAGs).1 Patients with congenital AT deficiency and those with the variant AT that lacks affinity for GAGs develop thrombosis, showing the importance of the interaction of AT with the endothelial cell surface GAGs for regulation of the coagulation cascade.2 3
AT has been shown to promote endothelial release of prostacyclin (PGI2) by interacting with the endothelial surface GAGs in vitro4,5 and in vivo.6 PGI2potently inhibits platelet aggregation7 and induces vasodilation,8 thereby maintaining proper organ microcirculation. In addition, PGI2 inhibits the endotoxin (ET)-induced monocytic production of tumor necrosis factor-α (TNF-α).9 TNF-α plays a role in the propagation of inflammation by activating neutrophils10 and by inducing nitric oxide synthase (NOS).11
Excessive production of NO by the inducible form of NOS (iNOS) plays critical roles in the pathophysiology of septic shock.12Septic shock, associated with infection with Gram-negative and Gram-positive bacteria and with fungus, is characterized by hypotension, organ dysfunction, and disseminated intravascular coagulation, leading to multiple organ failure.13 The mechanism of septic shock is now considered to be the marked reduction of vascular reactivity to vasoconstrictors.14 The hyporeactivity has been shown to be attributable to the action of excessively produced NO by iNOS expressed within the vasculature.15 NO activates soluble guanylyl cyclase, thereby increasing the cytoplasmic concentration of cyclic guanosine monophosphate followed by the reduction of the intracellular calcium concentration.16 The cyclic guanosine monophosphate–independent mechanism for the vasodilation has also been postulated. For example, peroxynitrite, an oxidant produced by the reaction of NO and superoxide, has been suggested to activate membrane potassium channels, leading to vasodilation.17-19Furthermore, myocardial depression induced by NO might also contribute to hypotension induced by ET.20
AT significantly reduced the lethal effects of Escherichia coli infusion in baboons, and the therapeutic effects could not be explained by its anticoagulant activities.21,22 In the study using the baboon model of sepsis, AT significantly inhibited the decrease in the mean systemic arterial pressure.21 We previously reported that AT reduced both ET-induced pulmonary vascular injury23 and ischemia/reperfusion-induced liver injury by inhibiting leukocyte activation in rats.24 Neither DEGR-F.Xa, an inactive derivative of F.Xa that selectively inhibits thrombin generation, nor Trp49-modified AT, which is incapable of promoting the endothelial release of PGI2 due to the lack of the affinity for heparin, reduced these organ injuries.23,24 These observations strongly suggested that the therapeutic effects induced by AT could not be mediated by its anticoagulant effects but could be mediated by the increase in the endothelial production of PGI2.23 24Taken together, these observations suggest the possibility that AT prevents ET-induced hypotension by inhibiting induction of iNOS and that such effects can be mediated by PGI2, which inhibits the monocytic production of TNF-α.
In the present study, we examined this possibility using a rat model of septic shock. Because the lung is one of the main organs expressing large amounts of iNOS in response to ET,15 we investigated whether AT inhibits the induction of iNOS by inhibiting TNF-α production in lungs of rats administered ET.
Materials and methods
Materials
AT was kindly provided by WelFide (Osaka, Japan). AT was purified from heat-treated pooled human plasma by absorption on fixed heparin according to a modification of the technique of Miller-Anderson et al.25 The AT concentrate used in the experiment revealed a single band on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Further characterization of the AT concentrate demonstrated that the heparin concentration was less than 0.01 U/mL and that it was free of pyrogen. Iloprost was kindly supplied by Eizai Pharmaceutical, Tokyo, Japan. Dimethyl-(2-hydroxy-5-nitrobenzyl) sulfonium bromide (DHNBSB) and indomethacin were obtained from Sigma (St Louis, MO). Anti-rat TNF-α antibody (Ab) was purchased from Genzyme-Techne (Minneapolis, MN), and ET (lipopolysaccharide,E coli, serotype 055:B5) was from Difco (Detroit, MI). All reagents used were of analytical grade.
Preparation of active site-blocked factor Xa
Human factor X was kindly provided by Chemo-sero-therapeutic Research Institute (Kumamoto, Japan). Factor X was purified from human plasma26 and activated with Russell viper venom27 as described previously. Activated factor X was inactivated by incubation with a 20-fold molar excess of [5-(dimethylamino)1-naphthalenesulfonyl]-glutamylglycylarginyl chloromethyl ketone (DEGR) for 30 minutes at 25°C. Thereafter, the mixture was subjected to extensive dialysis against a solution containing 20 mM Tris-HCl (pH 7.4) and 100 mM NaCl. The DEGR-treated factor Xa (DEGR-F.Xa) thus prepared showed no clotting activity and a prolonged activated partial thromboplastin time in a concentration-dependent (0-300 μg/mL) manner (data not shown).
Preparation of Trp49-modified AT
The Trp49 residue of AT was chemically modified by a version of the method of Karp et al.28 In brief, DHNBSB was mixed with a continuously stirred solution containing 40 μM AT, 0.1 M Tris-HCl (pH 8.0), and 0.15 M NaCl. The final concentration of DHNBSB was calculated as 8 mM. After 15 minutes at 22°C, the insoluble hydroxynitrobenzyl alcohol, which formed as a hydrolysis product, was removed by centrifugation. The solution was subjected to chromatography on a column (2.6 × 60 cm) of Sephacryl S-200HR (Pharmacia, Uppsala, Sweden) that had been equilibrated with 0.1 M Tris-HCl (pH 8.0) and 0.15 M NaCl and then subjected to chromatography on a column (3 × 6 cm) of heparin-Sepharose CL6B (Pharmacia), as previously described.3 The AT that was present in the void volume was collected and concentrated by filtration with an Amicon YM-10 membrane (Amicon, Danvers, MA). The concentration of AT in the sample was determined immunologically as previously described.29 The extent of derivatization of AT was determined spectrophotometrically in 2 M NaOH at 410 nm (molar extinction coefficient 1.85 × 104M−1).28 Although the heparin cofactor activity of Trp49-modified AT was markedly decreased, the progressive AT activity measured in the absence of heparin was virtually identical to that of native AT (data not shown). The study of thrombin inhibition was performed as previously described.29 Because Trp49-modified AT prevented the coagulation abnormalities induced by ET in rats to the same extent as native AT,23 the anticoagulant activity of Trp49-modified AT could be comparable to that of native AT in vivo.
ET shock in rats and measurement of MAP
The study protocol was approved by the Kumamoto University School of Medicine Animal Care and Use Committee, and the care and handling of the animals were in accordance with the guidelines of the National Institutes of Health. Specific pathogen-free male Wistar rats weighing 220 to 280 g were obtained from Kyudo (Kumamoto, Japan). Animals were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally). Mean arterial pressure (MAP) equals the diastolic pressure plus one third of the pulse pressure, which is the difference between the systolic and diastolic pressure. The right femoral artery was cannulated and connected to a pressure transducer for the measurement of MAP. The right jugular vein was cannulated for administration of reagents. Upon completion of the surgical procedure, MAP was allowed to stabilize for 15 minutes. After recording the baseline MAP, animals were treated with vehicle alone (saline) or reagents such as AT (250 U/kg). Thirty minutes after administration of vehicle or reagents, animals intravenously received ET (5 mg/kg) as a slow injection over 5 minutes. MAP was continuously monitored for 180 minutes after ET administration.
Measurement of plasma levels of NO2−/NO3−
NO2− and NO3−are the primary oxidized products of NO reacting with water, and therefore total concentration of NO2−/NO3− in plasma was used as an indicator of NO production in vivo.30 Blood was collected from the abdominal aorta into tubes containing 3.8% sodium citrate. The blood samples were placed on ice and centrifuged at 1000g for 20 minutes to prepare plasma. Total concentration of NO2−/NO3− was measured using a Griess reaction kit (Roche Diagnostics, Mannheim, Germany). Briefly, plasma protein was removed by adding 5% ZnSO4 and NaOH (0.3 M) solution. After centrifugation (2300g for 10 minutes), the supernatant was incubated with nitrate reductase, NADPH, and flavin adenine dinucleotide for 30 minutes at room temperature to reduce NO3− to NO2−. After incubation, the samples were placed in 96-well plates and incubated with 1% sulfanilic acid and 0.2% naphthylethylenediamine dihydrochloride/20% H3PO4 for 5 minutes at room temperature. The absorbance of the mixture at 550 nm was determined using a microplate reader. NO2− concentration was calculated by comparison with the absorbance of a standard solution of KNO2.
Measurement of lung level of NOS activity
A total of 180 minutes after ET administration, rats were anesthetized by pentobarbital sodium and the lung vasculature was perfused via the right cardiac ventricle with 10 mL cold 0.9% NaCl. The lungs were removed and frozen in liquid nitrogen. These lung samples were homogenized on ice in HEPES buffer (pH 7.5, 30 mM). The homogenate was sonicated and centrifuged at 12 500g for 15 minutes at 4°C. Conversion of [3H]-l-arginine to [3H]-l-citrulline was measured in the supernatant as described by Szabó et al.15 Briefly, tissue homogenate was incubated in the presence of [3H]- l-arginine (0.5 mCi/μM [18.5 MBq/μM]), NADPH (1 mM), calmodulin (30 nM), tetrahydrobiopterin (50 μM), flavin mononucleotide (20 μM), flavin adenine dinucleotide (20 μM), l-valine (60 mM), and CaCl2 (2 mM) for 20 minutes at 37°C in HEPES buffer (pH 7.5, 30 mM). Reactions were stopped by dilution with HEPES buffer (pH 5.5, 100 mM) containing ethyleneglycotetraacetic acid (2 mM) and ethylenediaminetetraacetic acid (2 mM). Reaction mixtures were applied to Dowex 50 W (sodium form) columns (Bio-Rad Laboratories, Hercules, CA), and the radioactivity of eluted [3H]-l-citrulline was measured using a scintillation counter (TRI-CARB 2300TR, Packard, Meriden, CT). Reaction mixtures were prepared without calcium and with ethyleneglycotetraacetic acid (5 mM) to determine the level of calcium-independent iNOS activity. Protein concentration of lung homogenate was measured spectrophotometrically by the Lowry method with bovine serum albumin as a standard.
Measurement of lung levels of TNF-α
Lung samples were obtained from rats and homogenized on ice in HEPES buffer (pH 7.5, 30 mM). After sonication and centrifugation of lung homogenate (12 500g for 15 minutes), the levels of TNF-α in the supernatant were determined using an enzyme-linked immunosorbent assay kit for rat TNF-α (Genzyme, Cambridge, MA).
Isolation of RNA and Northern blotting analysis
Total RNA from rat lungs was prepared by the acid-guanidinium-phenol-chloroform extraction procedure.31After electrophoresis in formaldehyde-containing agarose gels, RNAs were transferred onto nylon membranes. Hybridization was performed using digoxigenin-labeled rat iNOS antisense RNA32 or rat TNF-α antisense RNA as the probe. The antisense RNA was synthesized using complementary DNA (cDNA) cloned by reverse transcription–polymerase chain reaction and subcloned into pcDNAII as the template and a digoxigenin-RNA labeling kit (Roche Diagnostics). Chemiluminescence signals derived from hybridized probe were detected on X-ray film using a digoxigenin luminescence detection kit (Roche Diagnostics) and quantified by densitometry.
Data analysis
Data are presented as means ± SD. The results were compared using either analysis of variance followed by Scheffé post hoc test or an unpaired t test. A level of P < .05 was accepted as statistically significant.
Results
Effect of AT, DEGR-F.Xa, or Trp49-modified AT on MAP and plasma levels of NO2−/NO3− in rats administered ET
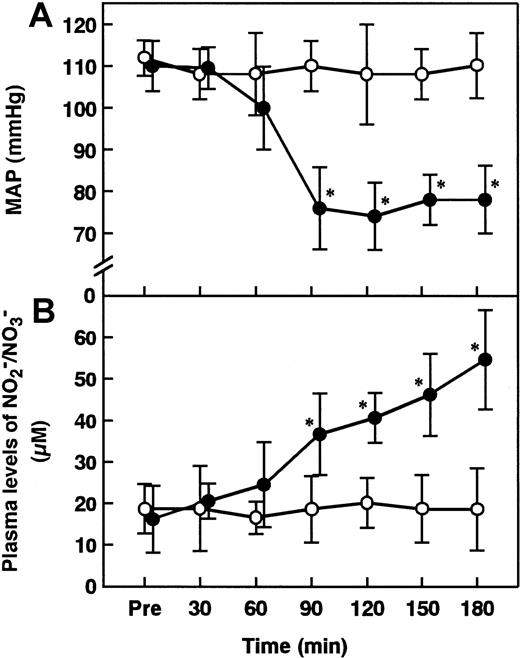
MAP decreased from the prelevel (110 ± 6 mmHg) to 75 ± 10 mmHg 90 minutes after ET administration (Figure1A). This hypotension was sustained until 180 minutes after ET administration. Plasma levels of NO2−/NO3− were significantly increased 90 minutes after ET administration compared with those of control animals, reaching the maximum at 180 minutes (Figure 1B).
Changes in MAP and plasma levels of NO2−/NO3− in rats administered saline or ET.
Changes in MAP (A) and plasma levels of NO2−/NO3−, stable metabolites of NO, (B) were determined at the indicated time points after the administration of saline (control, open circles) or ET (5 mg/kg, closed circles). Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus control.
Changes in MAP and plasma levels of NO2−/NO3− in rats administered saline or ET.
Changes in MAP (A) and plasma levels of NO2−/NO3−, stable metabolites of NO, (B) were determined at the indicated time points after the administration of saline (control, open circles) or ET (5 mg/kg, closed circles). Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus control.
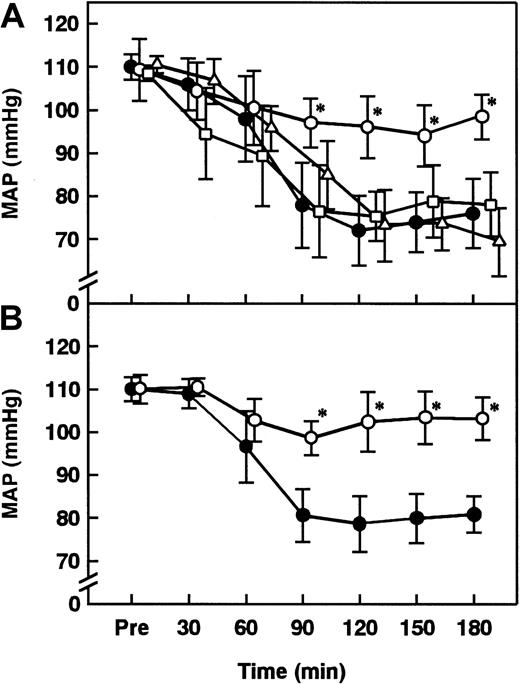
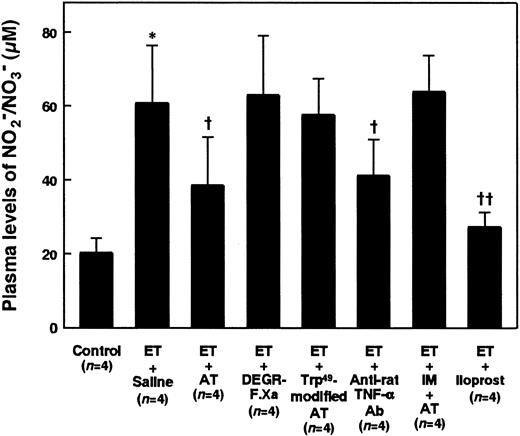
AT showed a significant inhibitory effect on the ET-induced decrease in MAP, while neither DEGR-F.Xa nor Trp49-modified AT showed any effect (Figure 2A). Although intravenous administration of AT 60 minutes after the ET challenge did not inhibit the ET-induced hypotension (data not shown), AT administered 30 minutes after ET administration significantly prevented the hypotension (Figure 2B). Although AT (250 U/kg) significantly inhibited increases in plasma levels of NO2−/NO3− 180 minutes after ET administration, neither DEGR-F.Xa (3 mg/kg) nor Trp49-modified AT (250 U/kg) showed any effects (Figure 3).
Effect of AT, DEGR-F.Xa, or Trp49-modified AT on changes in MAP in rats administered ET.
Changes in MAP after the administration of ET (5 mg/kg) were determined. (A) AT (250 U/kg, open circles), DEGR-F.Xa (3 mg/kg, open squares), Trp49-modified AT (250 U/kg, open triangles), or saline (closed circles) was administered intravenously 30 minutes before ET administration. (B) AT (250 U/kg, open circles) or saline (closed circles) was intravenously administered 30 minutes after ET administration. Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus ET plus saline.
Effect of AT, DEGR-F.Xa, or Trp49-modified AT on changes in MAP in rats administered ET.
Changes in MAP after the administration of ET (5 mg/kg) were determined. (A) AT (250 U/kg, open circles), DEGR-F.Xa (3 mg/kg, open squares), Trp49-modified AT (250 U/kg, open triangles), or saline (closed circles) was administered intravenously 30 minutes before ET administration. (B) AT (250 U/kg, open circles) or saline (closed circles) was intravenously administered 30 minutes after ET administration. Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus ET plus saline.
Effect of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, AT pretreated with indomethacin (IM), or iloprost on increases in plasma levels of NO2−/NO3− 180 minutes after ET administration in rats.
Plasma levels of NO2−/NO3− were determined 180 minutes after ET administration. Concentration of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, IM, or iloprost was as in Figures 2A and 12. Control animals were administered saline alone. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .05 versus ET plus saline. ††P < .01 versus ET plus saline.
Effect of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, AT pretreated with indomethacin (IM), or iloprost on increases in plasma levels of NO2−/NO3− 180 minutes after ET administration in rats.
Plasma levels of NO2−/NO3− were determined 180 minutes after ET administration. Concentration of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, IM, or iloprost was as in Figures 2A and 12. Control animals were administered saline alone. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .05 versus ET plus saline. ††P < .01 versus ET plus saline.
Effect of AT, DEGR-F.Xa, or Trp49-modified AT on increase in iNOS activity in the lungs of rats administered ET
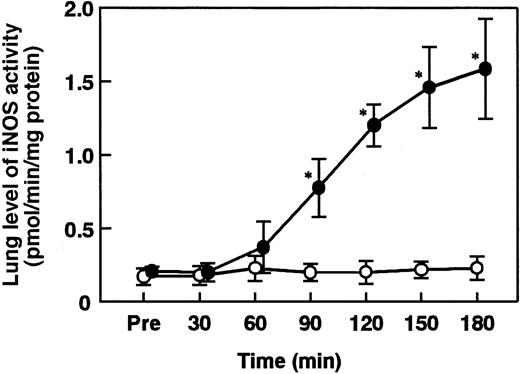
Lung iNOS activity was significantly increased 90 minutes after ET administration and reached its maximum at 180 minutes (Figure4). AT (250 U/kg) significantly inhibited increase in lung iNOS activity 180 minutes after ET administration, while neither DEGR-F.Xa (3 mg/kg) nor Trp49-modified AT (250 U/kg) showed any effects (Figure 5).
Change in the lung level of iNOS activity in rats administered saline or ET.
Change in the lung level of iNOS activity was determined at the indicated time points after the administration of saline (control, open circles) or ET (5 mg/kg, closed circles). Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus control.
Change in the lung level of iNOS activity in rats administered saline or ET.
Change in the lung level of iNOS activity was determined at the indicated time points after the administration of saline (control, open circles) or ET (5 mg/kg, closed circles). Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus control.
Effect of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, AT pretreated with indomethacin (IM), or iloprost on increases in the lung level of iNOS activity 180 minutes after ET administration in rats.
Lung activity of iNOS was determined 180 minutes after ET administration. Concentration of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, IM, or iloprost was as in Figures 2A and 12. Control animals were administered saline alone. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .01 versus ET plus saline.
Effect of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, AT pretreated with indomethacin (IM), or iloprost on increases in the lung level of iNOS activity 180 minutes after ET administration in rats.
Lung activity of iNOS was determined 180 minutes after ET administration. Concentration of AT, DEGR-F.Xa, Trp49-modified AT, anti-rat TNF-α Ab, IM, or iloprost was as in Figures 2A and 12. Control animals were administered saline alone. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .01 versus ET plus saline.
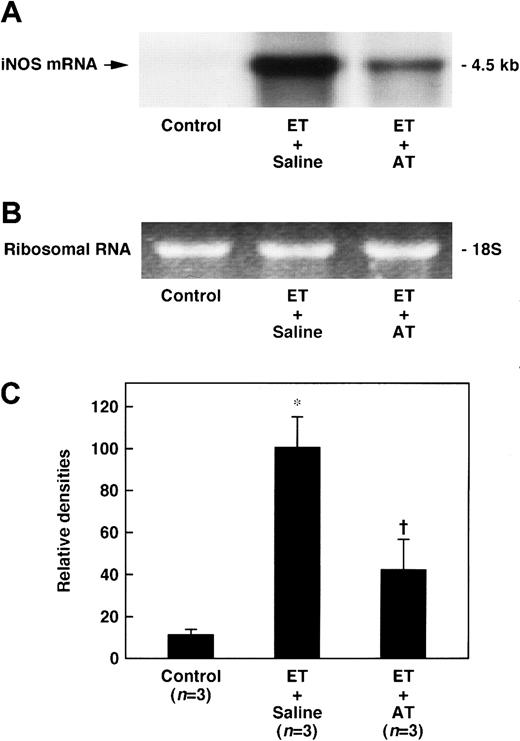
Effect of AT on the expression of iNOS mRNA in the lungs of rats administered ET
Expression of iNOS mRNA in the lungs was increased 60 minutes after ET administration and reached its maximum at 180 minutes (Figure6). The expression of iNOS mRNA in the lungs 180 minutes after ET administration was significantly inhibited by administration of AT (250 U/kg) (Figure 7).
Changes in lung level of iNOS mRNA in rats administered ET.
Expression of iNOS mRNA was examined in the lungs at the indicated time points after ET (5 mg/kg) administration. Chemiluminograms of typical expression of iNOS mRNA (4.5 kilobases [kb]) from 4 determinations (A) and ethidium bromide staining of 18S ribosomal RNA (1 μg of total RNA per each lane) (B) at each time point are shown. (C) The chemiluminograms of iNOS mRNA expression were quantified, and the results are presented as means ± SD of 4 animals. Pre indicates the time just before ET administration. Mean level of maximal value is arbitrarily set at 100. *P < .01 versus pre.
Changes in lung level of iNOS mRNA in rats administered ET.
Expression of iNOS mRNA was examined in the lungs at the indicated time points after ET (5 mg/kg) administration. Chemiluminograms of typical expression of iNOS mRNA (4.5 kilobases [kb]) from 4 determinations (A) and ethidium bromide staining of 18S ribosomal RNA (1 μg of total RNA per each lane) (B) at each time point are shown. (C) The chemiluminograms of iNOS mRNA expression were quantified, and the results are presented as means ± SD of 4 animals. Pre indicates the time just before ET administration. Mean level of maximal value is arbitrarily set at 100. *P < .01 versus pre.
Effect of AT on increase in the lung level of iNOS mRNA 180 minutes after ET administration in rats.
AT (250 U/kg) was administered intravenously 30 minutes prior to ET (5 mg/kg) administration. Control animals were administered saline alone. The iNOS mRNA expression in the lungs was determined 180 minutes after ET administration. (A) Chemiluminograms of typical expression of iNOS mRNA (4.5 kb) in each experimental group; (B) ethidium bromide staining of 18S ribosomal RNA (1 μg of total RNA per each lane). (C) The chemiluminograms of expression of iNOS mRNA were quantified by comparison with the mean value seen in the ET plus saline group, arbitrarily set at 100. Data are expressed as means ± SD of 3 animals. *P < .01 versus control. †P < .05 versus ET plus saline.
Effect of AT on increase in the lung level of iNOS mRNA 180 minutes after ET administration in rats.
AT (250 U/kg) was administered intravenously 30 minutes prior to ET (5 mg/kg) administration. Control animals were administered saline alone. The iNOS mRNA expression in the lungs was determined 180 minutes after ET administration. (A) Chemiluminograms of typical expression of iNOS mRNA (4.5 kb) in each experimental group; (B) ethidium bromide staining of 18S ribosomal RNA (1 μg of total RNA per each lane). (C) The chemiluminograms of expression of iNOS mRNA were quantified by comparison with the mean value seen in the ET plus saline group, arbitrarily set at 100. Data are expressed as means ± SD of 3 animals. *P < .01 versus control. †P < .05 versus ET plus saline.
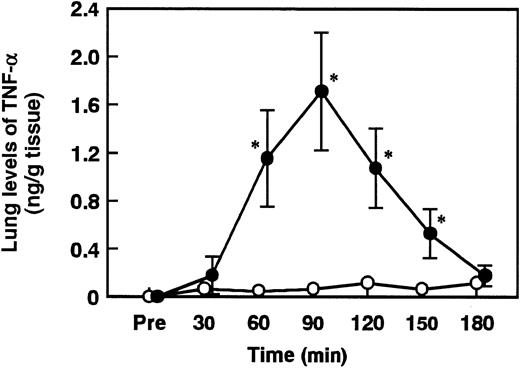
Effect of AT, DEGR-F.Xa, or Trp49-modified AT on the tissue levels of TNF-α in the lungs of rats administered ET
The lung tissue levels of TNF-α began to increase 60 minutes after ET administration, peaking at 90 minutes (Figure8). Intravenous administration of 50 or 100 U/kg AT did not inhibit the increases in the tissue levels of TNF-α 90 minutes after ET administration (data not shown). However, AT at the dosage of 250 U/kg significantly inhibited these increases (Figure 9). Neither DEGR-F.Xa (3 mg/kg) nor Trp49-modified AT (250 U/kg) showed any effect on increases in the lung tissue levels of TNF-α (Figure 9).
Changes in lung levels of TNF-α in rats administered saline or ET.
Animals were administered saline (control, open circles) or ET (5 mg/kg, closed circles). Lung tissue was obtained from rats and homogenized in HEPES buffer. After centrifugation of lung homogenate, the supernatant was assayed using a rat TNF-α enzyme-linked immunosorbent assay kit. Lung levels of TNF-α were determined at the indicated time points after the administration of saline or ET. Pre indicates the time just before ET administration. Data are expressed as means ± SD of 4 animals. *P < .01 versus control.
Changes in lung levels of TNF-α in rats administered saline or ET.
Animals were administered saline (control, open circles) or ET (5 mg/kg, closed circles). Lung tissue was obtained from rats and homogenized in HEPES buffer. After centrifugation of lung homogenate, the supernatant was assayed using a rat TNF-α enzyme-linked immunosorbent assay kit. Lung levels of TNF-α were determined at the indicated time points after the administration of saline or ET. Pre indicates the time just before ET administration. Data are expressed as means ± SD of 4 animals. *P < .01 versus control.
Effect of AT, DEGR-F.Xa, Trp49-modified AT, AT pretreated with indomethacin (IM), or iloprost on increases in lung levels of TNF-α 90 minutes after ET administration in rats.
Lung levels of TNF-α were determined 90 minutes after ET administration. Concentration of AT, DEGR-F.Xa, Trp49-modified AT, IM, or iloprost was as in Figures 2A and12. Control animals were administered saline alone. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .01 versus ET plus saline. ††P < .05 versus ET plus saline.
Effect of AT, DEGR-F.Xa, Trp49-modified AT, AT pretreated with indomethacin (IM), or iloprost on increases in lung levels of TNF-α 90 minutes after ET administration in rats.
Lung levels of TNF-α were determined 90 minutes after ET administration. Concentration of AT, DEGR-F.Xa, Trp49-modified AT, IM, or iloprost was as in Figures 2A and12. Control animals were administered saline alone. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .01 versus ET plus saline. ††P < .05 versus ET plus saline.
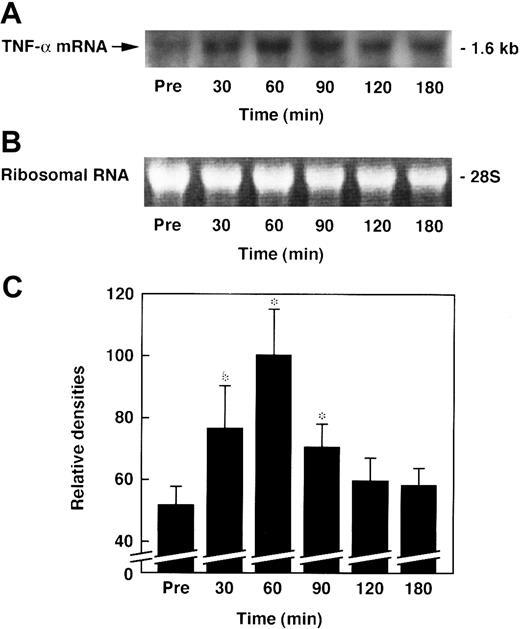
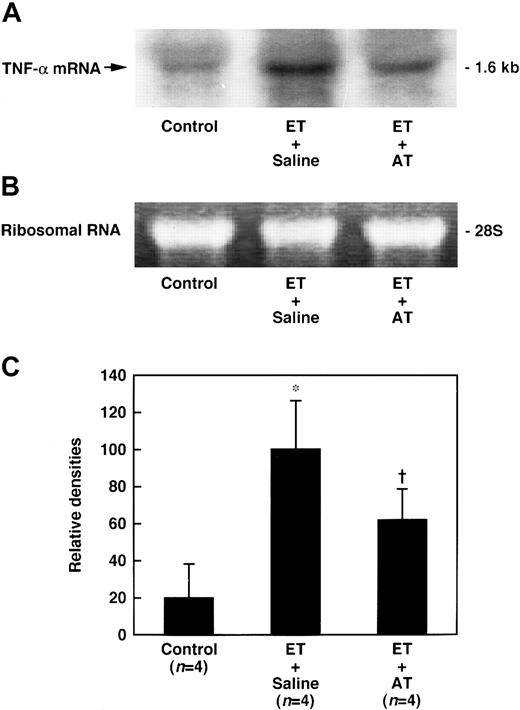
Effect of AT on the expression of TNF-α mRNA in the lungs of rats administered ET
The expression of lung tissue TNF-α mRNA began to increase 30 minutes after ET administration, peaking at 60 minutes, and gradually decreased to prelevels (Figure 10). The expression of lung tissue TNF-α mRNA 60 minutes after ET administration was significantly inhibited by AT (250 U/kg) (Figure 11).
Changes in lung level of TNF-α mRNA in rats administered ET.
Expression of TNF-α mRNA was examined in the lungs at the indicated time points after ET (5 mg/kg) administration. Chemiluminograms of typical expression of TNF-α mRNA (1.6 kb) from 4 determinations (A) and ethidium bromide staining of 28S ribosomal RNAs (3 μg of total RNA per each lane) (B) at each time point are shown. (C) The chemiluminograms of TNF-α mRNA expression were quantified, and the results are presented as means ±SD of 4 animals. Pre indicates the time just before ET administration. Mean level of maximal value is arbitrarily set at 100. *P < .01 versus pre.
Changes in lung level of TNF-α mRNA in rats administered ET.
Expression of TNF-α mRNA was examined in the lungs at the indicated time points after ET (5 mg/kg) administration. Chemiluminograms of typical expression of TNF-α mRNA (1.6 kb) from 4 determinations (A) and ethidium bromide staining of 28S ribosomal RNAs (3 μg of total RNA per each lane) (B) at each time point are shown. (C) The chemiluminograms of TNF-α mRNA expression were quantified, and the results are presented as means ±SD of 4 animals. Pre indicates the time just before ET administration. Mean level of maximal value is arbitrarily set at 100. *P < .01 versus pre.
Effect of AT on increase in the lung level of TNF-α mRNA 60 minutes after ET administration in rats.
AT (250 U/kg) was administered intravenously 30 minutes prior to ET (5 mg/kg) administration. Control animals were administered saline instead of ET. Expression of TNF-α mRNA in the lungs was determined 60 minutes after ET administration. (A) Chemiluminograms of typical expression of TNF-α mRNA (1.6 kb) 60 minutes after ET administration; (B) ethidium bromide staining of 28S ribosomal RNAs (3 μg of total RNA per each lane). (C) The chemiluminograms were quantified by comparison with the mean value seen in the ET plus saline group, arbitrarily set at 100. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .05 versus ET plus saline.
Effect of AT on increase in the lung level of TNF-α mRNA 60 minutes after ET administration in rats.
AT (250 U/kg) was administered intravenously 30 minutes prior to ET (5 mg/kg) administration. Control animals were administered saline instead of ET. Expression of TNF-α mRNA in the lungs was determined 60 minutes after ET administration. (A) Chemiluminograms of typical expression of TNF-α mRNA (1.6 kb) 60 minutes after ET administration; (B) ethidium bromide staining of 28S ribosomal RNAs (3 μg of total RNA per each lane). (C) The chemiluminograms were quantified by comparison with the mean value seen in the ET plus saline group, arbitrarily set at 100. Data are expressed as means ± SD of 4 animals. *P < .01 versus control. †P < .05 versus ET plus saline.
Effect of anti-rat TNF-α Ab on changes in MAP, plasma levels of NO2−/NO3−, and lung tissue level of iNOS activity in rats administered ET
Both the hypotension and increases in plasma levels of NO2−/NO3− seen 180 minutes after ET administration were inhibited in animals treated with antirat TNF-α Ab (Figures 12A and 3). The increase in lung tissue level of iNOS activity was significantly inhibited in animals treated with anti-rat TNF-α Ab 180 minutes after ET administration (Figure 5).
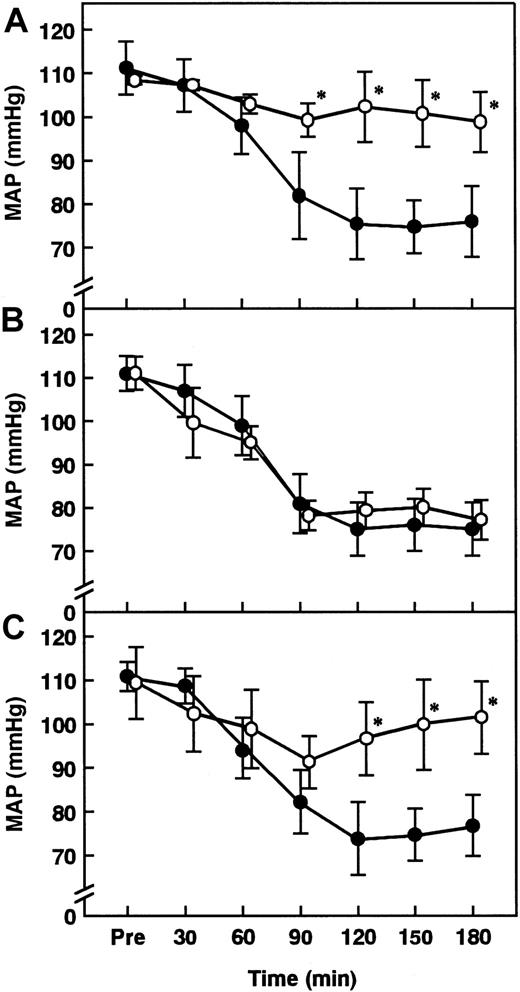
Effect of anti-rat TNF-α Ab, AT pretreated with indomethacin, or iloprost on changes in MAP in rats administered ET.
Changes in MAP after the administration of ET (5 mg/kg) were determined. (A) Anti-rat TNF-α Ab (0.25 mg/kg, open circles) was administered intraperitoneally 30 minutes prior to ET administration. Control rats were injected with saline plus ET (closed circles). (B) Indomethacin (5 mg/kg) was administered subcutaneously 30 minutes before the administration of AT (250 U/kg), which was administered 30 minutes prior to the administration of ET (open circles). Control rats were injected with saline plus ET (closed circles). (C) Iloprost (100 ng/kg/min, open circles) or saline (closed circles) was continuously injected from 30 minutes before the administration of ET until the end of the experiment. Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus ET plus saline.
Effect of anti-rat TNF-α Ab, AT pretreated with indomethacin, or iloprost on changes in MAP in rats administered ET.
Changes in MAP after the administration of ET (5 mg/kg) were determined. (A) Anti-rat TNF-α Ab (0.25 mg/kg, open circles) was administered intraperitoneally 30 minutes prior to ET administration. Control rats were injected with saline plus ET (closed circles). (B) Indomethacin (5 mg/kg) was administered subcutaneously 30 minutes before the administration of AT (250 U/kg), which was administered 30 minutes prior to the administration of ET (open circles). Control rats were injected with saline plus ET (closed circles). (C) Iloprost (100 ng/kg/min, open circles) or saline (closed circles) was continuously injected from 30 minutes before the administration of ET until the end of the experiment. Pre indicates the time just before ET administration. Data are expressed as means ± SD of 5 animals. *P < .01 versus ET plus saline.
Effect of indomethacin pretreatment on AT-induced effects
Neither the inhibitory effect of AT on the ET-induced decrease in MAP nor that on the ET-induced increases in the plasma levels of NO2−/NO3− were observed in animals pretreated with indomethacin (5 mg/kg) (Figures 12B and 3). When administered to animals pretreated with indomethacin (5 mg/kg), AT (250 U/kg) did not inhibit increase in the lung tissue level of iNOS activity 180 minutes after ET administration (Figure 5). Increases in the lung tissue levels of TNF-α 90 minutes after ET administration were not inhibited by AT in animals pretreated with indomethacin (Figure 9).
Effect of iloprost, a stable derivative of PGI2, on changes in MAP, plasma levels of NO2−/NO3−, lung tissue level of iNOS activity, and lung tissue levels of TNF-α in rats administered ET
Although intravenous infusion of iloprost decreased MAP about 20 mmHg 90 minutes after ET administration, it significantly inhibited the ET-induced decrease in MAP from 120 to 180 minutes after ET administration (Figure 12C). Iloprost inhibited the ET-induced increases in the plasma levels of NO2−/NO3− and lung tissue level of iNOS activity 180 minutes after ET administration (Figures 3 and 5). Iloprost significantly inhibited the ET-induced increases in the lung tissue levels of TNF-α 90 minutes after ET administration (Figure 9).
Discussion
In the present study, AT significantly inhibited both the increases in plasma levels of NO2−/NO3− and the hypotension induced by ET. Because excessive production of NO by iNOS has been shown to play a major role in ET-induced hypotension,12,33 AT might inhibit ET-induced hypotension by inhibiting excessive production of NO. Aminoguanidine, a selective inhibitor of iNOS,34 significantly inhibited the increases in plasma levels of NO2−/NO3− as well as the hypotension in this animal model (data not shown), suggesting that iNOS might play a role in ET-induced hypotension in the present study. The lung is one of the main organs expressing large amounts of iNOS in response to ET.15 Consistent with this observation, lung iNOS activity began to increase 90 minutes after ET administration, peaking at 180 minutes after ET administration when plasma levels of NO2−/NO3− reached maximum levels. Expression of iNOS mRNA in the lung began to increase 60 minutes after ET administration, peaking at 180 minutes after ET administration. Because AT inhibited the ET-induced increases in both lung iNOS activity and the expression of iNOS mRNA in the lung, AT might inhibit the excessive production of NO by inhibiting induction of iNOS in the lungs of animals administered ET.
Because endothelial NOS (eNOS) was shown to be induced in rat brain astrocytes by ET administration,35 eNOS could be induced in the lungs of rats given ET, contributing to the development of hypotension by producing NO. We measured eNOS activity in lung samples of rats 180 minutes after ET administration according to the method described by Szabó et al.15 However, there was no significant increase in the lung eNOS activity 180 minutes after ET administration (data not shown). Thus, NO implicated in the development of ET-induced hypotension in this rat model could be derived from iNOS.
AT also inhibited the ET-induced increases in both tissue levels of TNF-α and the expression of TNF-α mRNA in the lungs of animals given ET. Because ET increases the production of TNF-α in circulating monocytes and resident macrophages,36 the increases in the lung tissue levels of TNF-α in animals administered ET might have been due to the increase in the production of TNF-α by these cells. TNF-α has been shown to play a major role in the development of ET-induced shock by inducing iNOS.11 Consistent with this notion, recombinant human TNF-α has been shown to cause hypotension and death in a rat sepsis model.37 Fahey et al38 reported that anti–TNF-α Ab inhibited sustained hypotension induced by ET in rats. Thiemermann et al11reported that a monoclonal Ab against TNF-α ameliorated the fall in blood pressure induced by ET in rats. Mice in which the TNF-α receptor gene has been disrupted are resistant to ET-induced shock.39 40 Because treatment with anti-rat TNF-α Ab significantly inhibited increases in both plasma levels of NO2−/NO3− and lung tissue level of iNOS activity and further ameliorated the subsequent hypotension, TNF-α plays a causal role in ET-induced hypotension by inducing iNOS in this animal model of septic shock. These observations strongly suggest that TNF-α is involved in the pathologic processes leading to ET-induced hypotension. Thus, the inhibition of TNF-α production by AT might contribute to prevent the hypotension through inhibition of iNOS induction in the present study.
Because neither AT nor iloprost completely inhibited the hypotension and production of TNF-α and NO in this rat model, some other processes that could not be regulated by AT might be involved in the ET-induced hypotension. Platelet-activating factor has been shown to play important roles in the ET-induced hypotension by enhancing TNF-α production41 and by increasing vascular permeability.42 Because inhibition of platelet-activating factor production by AT has not been shown, platelet-activating factor could also be implicated in the ET-induced hypotension in this animal model.
DEGR-F.Xa, a selective inhibitor of thrombin generation, did not show any effects induced by AT in this animal model of septic shock. DEGR-F.Xa (3 mg/kg) inhibited ET-induced coagulation abnormalities to similar extents as AT (250 U/kg).23 Thus, the effects of AT seen in the present animal model might not be mediated by the anticoagulant effects of AT.
AT has been shown to promote the endothelial release of PGI2 by interacting with heparinlike GAGs on the endothelial cell surface in vitro4,5 and in vivo.6 The effects induced by AT in this rat model of septic shock appeared to be mediated by PGI2, which may have been released from the endothelial cells. This conclusion was supported by the following observations. (1) Trp49-modified AT (250 U/kg) is a chemically modified AT that shows AT activity similar to that of unmodified AT but has no ability to promote the endothelial release of PGI2 due to a lack of affinity for heparin.6 This compound did not show any effects such as those induced by AT. (2) Indomethacin pretreatment abrogated the effects induced by AT. (3) Iloprost, a stable derivative of PGI2, showed effects similar to those of AT. PGI2 inhibits TNF-α biosynthesis by inhibiting its transcription through regulation of NF-κB activation.43-45 This is consistent with the observation that AT inhibits TNF-α production by inhibiting its transcriptional level as shown in the present study.
Consistent with the observations in the present study, Taylor et al21 also demonstrated that AT prevents hypotension in baboons challenged with E coli independent of its anticoagulant effect. Because administration of AT after, in addition to its administration before, significantly inhibited the ET-induced hypotension in rats, AT might be effective for the treatment of patients with septic shock. Consistent with this hypothesis are the findings of a previous study showing that AT significantly reduced the mortality of patients with septic shock.46
Because TNF-α activates neutrophils, it increases the release of various inflammatory mediators such as neutrophil elastase and oxygen-free radicals, which are capable of damaging endothelial cells.10 Thus, AT might reduce the endothelial cell injury by inhibiting neutrophil activation by inhibiting TNF-α production. Consistent with this hypothesis are our previous findings showing that AT reduced the activated neutrophil-induced pulmonary vascular injury.23 In addition, AT has been shown to reduce the ischemia/reperfusion-induced liver injury by inhibiting leukocyte activation by increasing the hepatic level of PGI2.24 These observations in animal experiments might at least partly explain why AT reduces respiratory failure and liver dysfunction seen in patients with sepsis.47
Although AT has been shown to reduce organ injury by increasing the endothelial production of PGI2 in vivo, the precise mechanism(s) by which AT promotes the endothelial release of PGI2 have not been well understood. In this process, interaction of AT with the endothelial heparinlike GAGs is critical for the promotion of the endothelial release of PGI2.5 6 Further studies are necessary to clarify the mechanism(s).
We thank Drs Masataka Mori and Akitoshi Nagasaki for providing the iNOS cDNA probe and Drs Yasuo Yamaguchi and Kazutoshi Okabe for providing the TNF-α cDNA probe. We also thank Ms Yumi Sakamoto for the technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenji Okajima, Department of Laboratory Medicine, Kumamoto University School of Medicine, Honjo 1-1-1, Kumamoto, 860-0811, Japan; e-mail: whynot@kaiju.medic.kumamoto-u.ac.jp.


![Fig. 6. Changes in lung level of iNOS mRNA in rats administered ET. / Expression of iNOS mRNA was examined in the lungs at the indicated time points after ET (5 mg/kg) administration. Chemiluminograms of typical expression of iNOS mRNA (4.5 kilobases [kb]) from 4 determinations (A) and ethidium bromide staining of 18S ribosomal RNA (1 μg of total RNA per each lane) (B) at each time point are shown. (C) The chemiluminograms of iNOS mRNA expression were quantified, and the results are presented as means ± SD of 4 animals. Pre indicates the time just before ET administration. Mean level of maximal value is arbitrarily set at 100. *P < .01 versus pre.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1638/5/m_h80522219006.jpeg?Expires=1763651667&Signature=VuyBthThFavd7adFipeh7eoUIRB-StDwE7aKLIqD0Z~5dJhK-xWmhjogaDmZAlr87m5D4yQMqQjDzfEYZ4-BH62DD8a~Y68qz6BEAPgSqVbp6XK0AhnPO6~DY98RmgFtnU-EFF5BRENgKQO5ZkM8bSZBvjx2eZz8KY1m6XZUq3R7GsC4lVOZKfNmopu5UK3bB1UiP6m8KjRXQP1NoywqwA89PUppR-AM3ZVChXMAaa7JCdIitEJ-WxJywGfZmUQTNqo20nMdFWGT15yh5h1g2WOhPiEEweh5V-H0yWle3eXp7PW8k3voLsdqbfQp0BXxCtj~apAWVGGsyYO-UYULbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal