Human T-cell leukemia virus type I (HTLV-I) is the etiologic agent of adult T-cell leukemia (ATL) and of tropical spastic paraparesis/HTLV-I–associated myelopathy. Infiltration of various tissues by circulating leukemic cells is a characteristic of ATL. Matrix metalloproteinases (MMPs), which mediate the degradation of the basement membrane and extracellular matrix, play an important role in metastasis and tumor cell dissemination. The aim of this study was to explore whether expression of MMP-2 and MMP-9 was deregulated by HTLV-I infection. The data showed that HTLV-I–infected T-cell lines expressed high levels of MMP-9 compared with uninfected T-cell lines. In contrast, the levels of the related MMP-2 were not significantly altered by HTLV-I infection. In addition, the elevated expression of MMP-9 in HTLV-I–infected cells was attributable to the action of the viral transactivator protein Tax. The results show that Tax can activate the MMP-9 promoter and induce MMP-9 expression in T cells, indicating that the constitutive expression of MMP-9 in virus-infected cell lines is at least in part mediated by Tax. Activation of the MMP-9 promoter by Tax occurs mainly through the action of NF-κB and SP-1. The biologic significance of these observations was validated by the following 2 findings: MMP-9 expression was increased in primary ATL cells, and plasma MMP-9 levels were elevated in ATL patients. In addition, plasma levels of MMP-9 correlated with organ involvement in ATL patients. Together these data suggest that overexpression of MMP-9 in HTLV-I– infected cells may be in part responsible for the invasiveness of ATL cells.
Introduction
Adult T-cell leukemia (ATL) is a malignancy of peripheral CD4+ T cells initiated by infection with the human T-cell leukemia virus type I (HTLV-I).1-3Infiltration by leukemic cells of various organs, such as lymph nodes, liver, spleen, lungs, skin, and intestinal tract, is a frequent manifestation of ATL. This type of cell infiltration often poses serious clinical problems for ATL patients, affecting the disease profile and prognosis. Tissue infiltration by ATL cells is most likely a sequential multistep process, which may be attributable to the cells' biologic properties, such as the deregulated expression of certain chemokines, chemokine receptors, and adhesion molecules. Deregulated adhesion molecule expression may enhance ATL cell interactions with endothelial cells. After transendothelial migration, ATL cells encounter the extracellular matrix (ECM), where they must pass through the basement membrane (BM) and migrate into the interstitial matrix. Many proteolytic enzymes degrade components of the ECM and BM.4,5 Among these, the matrix metalloproteinases (MMPs) may play important roles in tumor invasion and metastasis. Proteolytic disruption of ECM and BM by MMPs may represent a key process mediating ATL cell invasion. The role of MMPs in the dissemination of other hematologic malignancies, such as human non-Hodgkin lymphomas and acute myelogenous leukemia, has been described recently.6-10
Depending on their substrate specificity, MMPs are broadly divided into collagenases, stomelysins, and gelatinases. The latter group, consisting of gelatinase A (MMP-2) and gelatinase B (MMP-9), degrades denatured collagens (gelatin), native type IV and V collagens, and elastin. Because type IV collagen is one of the integral components of BM, the uncontrolled expression of 2 type IV collagenases, MMP-2 and MMP-9, is believed to play a critical role in the invasion of BM by tumor cells.11 The expression patterns of MMP-2 and MMP-9 do not completely overlap, indicating that they are regulated by distinct mechanisms.12 The MMP-2 and MMP-9 promoters differ markedly, with the MMP-9 promoter containing several putative activator protein-1 (AP-1) and NF-κB binding sites that are not present in the MMP-2 promoter.13-15
HTLV-I is also associated with a chronic progressive disease of the central nervous system termed tropical spastic paraparesis/HTLV-I–associated myelopathy.16,17 Previous immunohistochemical studies revealed that MMP-2 and MMP-9 are frequently immunostained in perivascular mononuclear cells in inflammatory lesions in the central nervous system of tropical spastic paraparesis/HTLV-I–associated myelopathy.18 However, systematic investigations of MMP expression patterns in ATL cells have not been reported. We hypothesized that deregulated MMP expression may influence the ability of ATL cells to invade healthy organs. We demonstrate that T-cell lines infected with HTLV-I have elevated expression of MMP-9. In contrast, expression of the related MMP-2 was not significantly increased in HTLV-I–infected T-cell lines. We further show that elevated expression of MMP-9 is attributable to the HTLV-I transactivator, Tax. Importantly, MMP-9 expression was also markedly increased in primary leukemic cells derived from ATL patients. Furthermore, we found that levels of MMP-9 were elevated in plasma from patients with ATL compared with healthy controls. Finally, plasma levels of MMP-9 in ATL patients with organ involvement were even higher than those in ATL patients without organ involvement. These data support the notion that aberrant expression of MMP-9 may increase the invasiveness of HTLV-I–infected T cells.
Materials and methods
Cell lines
Human leukemic T-cell lines (Jurkat, MOLT-4, and CCRF-CEM) and HTLV-I–infected T-cell lines (MT-1,19HUT-102,1 HPB-ATL-2,20HPB-CTL-I,20 and HLN-ATL-O20) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomycin. JPX-9 and JPX/M (kindly provided by Dr M. Nakamura, Tokyo Medical and Dental University, Tokyo, Japan) are subclones of Jurkat cells expressing either Tax wild type or a nonfunctional Tax mutant, respectively, under the control of the metallothionein promoter.21 22
Patient samples
Leukemic cells from 8 patients diagnosed with either acute-type (cases 1, 4, 6, and 8) or chronic-type (cases 2, 3, 5, and 7) ATL were analyzed. The diagnosis of ATL was based on clinical features, hematologic findings, and the presence of anti–HTLV-I antibodies in patient sera. Monoclonal HTLV-I provirus integration into the DNA of leukemic cells was confirmed by Southern blot hybridization in all cases (data not shown). Peripheral blood mononuclear cells (PBMCs) from healthy volunteers or patients with ATL were purified by Ficoll-Hypaque gradient centrifugation (Pharmacia LKB, Uppsala, Sweden) and washed with phosphate-buffered saline (PBS). Each patient sample contained more than 90% leukemic cells at the time of analysis. All samples were obtained after informed consent was received.
Plasmids and transfections
Mammalian expression vectors for HTLV-I Tax and the Tax M22 mutant, which fails to activate NF-κB, were described previously.23,24 IκBαΔN25 and IκBβΔN26 (kindly provided by Dr D. W. Ballard, Vanderbilt University School of Medicine, Nashville, TN) are deletion mutants of IκBα and IκBβ lacking the NH2-terminal 36 amino acids and 23 amino acids, respectively. The kinase-deficient K44M IκB kinase α (IKKα), K44A IKKβ, and KK429/430AA NF-κB–inducing kinase (NIK) mutants have been described previously.27 A series of chloramphenicol acetyltransferase (CAT) reporter gene constructs containing 5′ flanking sequences derived from the MMP-9 gene were used to map Tax-responsive regions.15 Transient transfections were performed in Jurkat cells by electroporation using 5 × 106 cells and 10 μg of appropriate reporter and effector plasmids. Preliminary studies with measurement of CAT activities from cell lysates at 12, 24, and 48 hours after transfection indicated that the greatest CAT activity was at 48 hours following transfection. Therefore, after 48 hours, transfected cells were collected by centrifugation, washed with PBS, and lysed in reporter lysis buffer (Promega, Madison, WI). To correct for variation in cell number, equal amounts of cellular protein were used as determined by the Bio-Rad protein assay. Lysates were assayed for reporter gene activity with the CAT enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim GmbH, Mannheim, Germany).
Reverse transcriptase–polymerase chain reaction
Total cellular RNA was extracted with Trizol (Life Technologies, Gaithersburg, MD) according to the protocol provided by the manufacturer. First-strand cDNA was synthesized from 1 μg total cellular RNA in a 20-μL reaction volume using an RNA-PCR kit (Takara Shuzo, Kyoto, Japan) with random primers. Thereafter, cDNA was amplified for 30 or 40 cycles for MMPs and 28 cycles for β-actin. The oligonucleotide primers used were as follows: for MMP-2, sense, 5′-GCGACAAGAAGTATGGCTTC-3′ and antisense, 5′-TGCCAAGGTCAATGTCAGGA-3′28; for MMP-9, sense, 5′-CGCAGACATCGTCATCCAGT-3′ and antisense, 5′-GGATTGGCCTTGGAAGATGA-3′28; and for β-actin, sense, 5′-GTGGGGCGCCCCAGGCACCA-3′ and antisense, 5′-CTCCTTAATGTCACGCACGATTTC-3′. Product sizes were 390 bp for MMP-2, 406 bp for MMP-9, and 548 bp for β-actin. Cycling conditions were as follows: denaturing at 95°C (60 seconds for MMPs and 30 seconds for β-actin), annealing at 62°C (60 seconds for MMPs and 30 seconds for β-actin), and extension at 72°C (60 seconds for MMPs and 90 seconds for β-actin). The PCR products were fractionated on 2% agarose gels and visualized by ethidium bromide staining.
Quantitative reverse transcriptase–polymerase chain reaction
Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using an XpressPack mRNA Expression Analysis kit (Chemicon International, Temecula, CA) according to the manufacturer's instructions. This assay is a hybridization capture assay designed to detect amplified nucleic acid specific to the target of interest. Briefly, first-strand cDNA was synthesized using an RNA-PCR kit as described earlier. Five microliters of the cDNA mixture was subjected to PCR amplification incorporating a biotinylated primer set for MMP-2, MMP-9, or β-actin. Ten microliters of the amplification product was added directly to the capture plate coated with a specific oligonucleotide probe and denatured under alkali conditions. The resultant hybrid complex was then reacted with streptavidin conjugated to horseradish peroxidase. Unbound conjugate was removed by washing. Substrate (3,3′,5,5′-tetramethylbenzidine) was added and incubated at 37°C for 10 minutes. The reaction was stopped by the addition of an acid. The amount of amplification product was measured with a microplate reader at a reference wavelength of 650 nm and test wavelength of 450 nm. The relative expression levels of MMP genes in each sample represented the amount of amplification product of MMPs relative to that of β-actin.
Northern blot analysis
Total RNA (20 μg) was subjected to electrophoresis through a formaldehyde-agarose gel and transferred to a nylon filter. Filters were prehybridized (in 0.5 M sodium phosphate, 0.1% bovine serum albumin, 7% sodium dodecyl sulfate [SDS], 100 μg/mL salmon testis DNA, and 100 μg/mL yeast RNA) for 2 hours at 65°C. Hybridization was then carried out in prehybridization buffer overnight containing the following α32P-radiolabeled probes: cDNA of HTLV-I Tax and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Radiolabeled probes were generated using a Megaprime DNA Labeling System (Amersham, Arlington Heights, IL).
Cell-conditioned media and gelatin zymography
The human T-cell lines were harvested at the exponential growth phase, washed in serum-free RPMI 1640, and cultured at a concentration of 1 × 106 cells/mL for 72 hours. The cell-conditioned media (supernatants) were collected, concentrated 10-fold using Centricon concentrators (Amicon, Beverly, MA), and analyzed by zymography using a modification of the method described previously.7 29 Conditioned serum-free medium from phorbol 12-myristate 13-acetate (PMA)–activated PBMCs was used as a positive control for zymographic analysis. Gelatinolytic activities were assessed under nonreducing conditions using a modified SDS-polyacrylamide gel electrophoresis procedure. Twelve microliters of supernatant was mixed with 4 μL loading buffer (0.19 M Tris-HCl, 30% glycerol, 6% SDS, and 0.01% bromophenol blue, pH 6.8) and then applied onto a 10% polyacrylamide gel copolymerized with 1 mg/mL gelatin (Sigma, St Louis, MO). Electrophoresis was performed under constant current at 10 mA per gel at 4°C. The gels were washed twice for 30 minutes each with 2.5% Triton X-100 (Sigma) to remove SDS and to allow the electrophoresed enzymes to renature before being incubated in zymography buffer (150 mM NaCl, 5 mM CaCl2, 0.05% NaN3, and 50 mM Tris-HCl, pH 7.5) for 18 hours at 37°C. The gels were then stained with 0.2% Coomassie brilliant blue G-250 (Nakarai Chemicals, Kyoto, Japan) in 5:1:5 ethanol/acetic acid/water for 2 hours and destained with 2:3:35 ethanol/acetic acid/water for 3 hours at room temperature. Gelatinolytic signals were quantified by densitometry and shown as relative activities.
Electrophoretic mobility shift assay
NF-κB and SP-1 binding activities to κB or SP-1 elements were examined by electrophoretic mobility shift assay (EMSA), as described previously.30 In brief, 5 μg of nuclear extracts were preincubated in a binding buffer containing 1 μg poly-deoxy-inosinic-deoxy-cytidylic acid (Pharmacia, Piscataway, NJ), followed by addition of α32P-labeled oligonucleotide probes containing κB or SP-1 elements (approximately 50 000 cpm). These mixtures were incubated for 15 minutes at room temperature. The DNA protein complexes were separated on a 4% polyacrylamide gel and visualized by autoradiography. To examine the specificity of the κB and SP-1 element probes, we preincubated unlabeled competitor oligonucleotides with nuclear extracts for 15 minutes before incubation with probes. The probes or competitors used were prepared by annealing the sense and antisense synthetic oligonucleotides as follows: κB element of the MMP-9 gene, 5′-gatcGGGTTGCCCCAGTGGAATTCCCCAGCCTT-3′; κB mutant, 5′-gatcGGGTTGCCCCAGTttAATTCCCCAGCCTT-3′; SP-1 element of the MMP-9 gene, 5′-gatcGCCCATTCCTTCCGCCCCCAGATGAAGCAG-3′; SP-1 mutant, 5′-gatcGCCCATTCCTTCCaaCCCCAGATGAAGCAG-3′; and a typical κB element from the interleukin-2 receptor (IL-2R) α gene, 5′-gatcCGGCAGGGGAATCTCCCTCTC-3′. Underlined sequences represent the NF-κB or SP-1 binding sites, and mutations are indicated in lowercase. To identify NF-κB/Rel proteins in the DNA protein complex revealed by EMSA, we used antibodies specific for various NF-κB/Rel family proteins, including RelA/p65, NF-κB1 p50, c-Rel, and NF-κB2 p52 (Santa Cruz Biotechnology, Santa Cruz, CA), to elicit a supershift and/or to inhibit DNA protein complex formation. These antibodies were incubated with the nuclear extracts for 45 minutes at room temperature before incubation with radiolabeled probes.
Immunohistochemical localization of MMP-9
A lymph node biopsy was obtained from an ATL patient who manifested lymph node involvement. Serial sections of this lymph node tissue were produced, deparaffinized in xylene, and dehydrated through a graded ethanol series. For better detection, sections were pretreated with 0.03% trypsin for 1 hour at 37°C. This procedure increases the number of antigenic sites available for binding by the antibody. In the next step, the tissues were placed in 3% hydrogen peroxide and absolute methanol for 5 minutes to reduce endogenous peroxidase activity, followed by washing in PBS. The tissue sections were covered with mouse anti–human MMP-9 monoclonal antibody (mAb) (56-2A4) (Fuji Chemical, Takaoka, Japan) (diluted 1:250) or control mouse IgG for 3 hours at 37°C. To ensure that the expression seen was due to recognition of MMP-9, blocking was done with MMP-9 (Boehringer Mannheim GmbH). The tissue sections were stained with MMP-9 mAb after incubation with MMP-9. After PBS washing, sections were covered with EnVision plus (Dako, Santa Barbara, CA) for 40 minutes at 37°C and washed in PBS. Antigenic sites bound by antibody were revealed by reacting these sections with a mixture of 0.05% 3,3′-diaminobenzidine tetrahydrochloride in 50 mM Tris-HCl buffer and 0.01% hydrogen peroxide for 7 minutes. Sections were then counterstained with methyl green for 10 minutes, hydrated in ethanol, cleaned in xylene, and mounted.
Detection of MMP-2 and MMP-9 by ELISA
Plasma from heparinized peripheral blood was obtained from healthy volunteers or each ATL patient at diagnosis but before chemotherapy and stored at −80°C until use. The concentrations of MMP-2 and MMP-9 in the plasma samples were measured using ELISA kits (Biotrack; Amersham) according to the manufacturer's instructions. ELISA systems for MMP-2 and MMP-9 recognize both the free form of pro-MMPs and the complex form with tissue inhibitor of MMPs.
Statistical analysis
Statistical analyses were performed by means of the unpaired 2-tailed Student t test. When the P value was less than .05, the difference was considered significant.
Results
MMP-9 mRNA expression and enzymatic activity in HTLV-I–infected T-cell lines
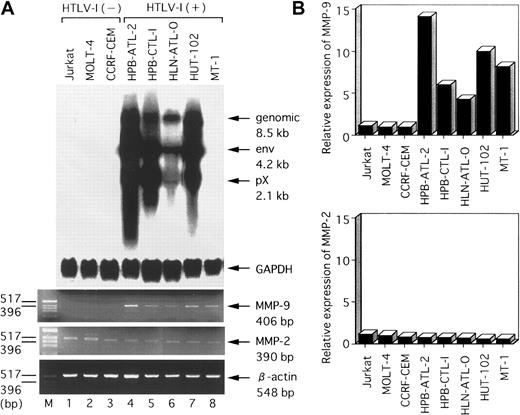
To determine whether MMP-2 and MMP-9 expression is deregulated after HTLV-I infection of T cells, we examined mRNA levels of these 2 MMPs in several HTLV-I–infected or uninfected cell lines. The results of RT-PCR analysis of MMP-2 and MMP-9 mRNA levels in several T-cell lines are shown in Figure 1A. MMP-9 transcripts (406 bp) were detected in all 5 HTLV-I–infected T-cell lines (HPB-ATL-2, HPB-CTL-I, HLN-ATL-O, HUT-102, and MT-1), but were not detected in the uninfected T-cell lines (Jurkat, MOLT-4, and CCRF-CEM). In contrast, MMP-2 transcripts (390 bp) were detected in all T-cell lines. We verified these RT-PCR results quantitatively by a hybridization capture assay designed to detect amplified nucleic acid specific to the target (Figure 1B). There was good qualitative agreement between the RT-PCR and hybridization capture assay results. These results demonstrate that the MMP-9 gene is selectively expressed in HTLV-I–infected T-cell lines. The HTLV-I–encoded transactivator protein, Tax, activates expression of a number of cellular genes. Thus, to examine whether MMP-9 expression correlated with Tax expression, we next measured Tax mRNA levels in these T-cell lines by Northern blot analysis. We found that Tax-related mRNAs (pX 2.1 kb) were expressed strongly in the HPB-ATL-2, HPB-CTL-I, and HUT-102 cell lines; weakly expressed in HLN-ATL-O; and not expressed at all in MT-1. Hybridization with the GAPDH probe confirmed comparable RNA loading in these lanes. We reanalyzed T-cell lines for Tax mRNA using highly sensitive RT-PCR. RT-PCR revealed that Tax mRNA was expressed in MT-1, as described previously.31
Northern blot analysis of HTLV-I mRNA expression and RT-PCR analysis of MMP-2 and MMP-9 mRNA levels in various HTLV-I–infected and uninfected human T-cell lines.
(A) Total RNA was prepared from the indicated T-cell lines. Predominant HTLV-I mRNA species of 2.1, 4.2, and 8.5 kb were detected in HPB-ATL-2, HPB-CTL-I, HLN-ATL-O, and HUT-102 cell lines (lanes 4-7). GAPDH and β-actin expression served as controls. (B) Quantitative hybridization capture assay to detect amplified nucleic acid of MMPs. Expression levels of MMP mRNA were calculated from the ratios of the amount of amplification product of MMPs to that of β-actin. Results show relative levels of the MMPs/β-actin in each line compared with that in HTLV-I–negative Jurkat cells.
Northern blot analysis of HTLV-I mRNA expression and RT-PCR analysis of MMP-2 and MMP-9 mRNA levels in various HTLV-I–infected and uninfected human T-cell lines.
(A) Total RNA was prepared from the indicated T-cell lines. Predominant HTLV-I mRNA species of 2.1, 4.2, and 8.5 kb were detected in HPB-ATL-2, HPB-CTL-I, HLN-ATL-O, and HUT-102 cell lines (lanes 4-7). GAPDH and β-actin expression served as controls. (B) Quantitative hybridization capture assay to detect amplified nucleic acid of MMPs. Expression levels of MMP mRNA were calculated from the ratios of the amount of amplification product of MMPs to that of β-actin. Results show relative levels of the MMPs/β-actin in each line compared with that in HTLV-I–negative Jurkat cells.
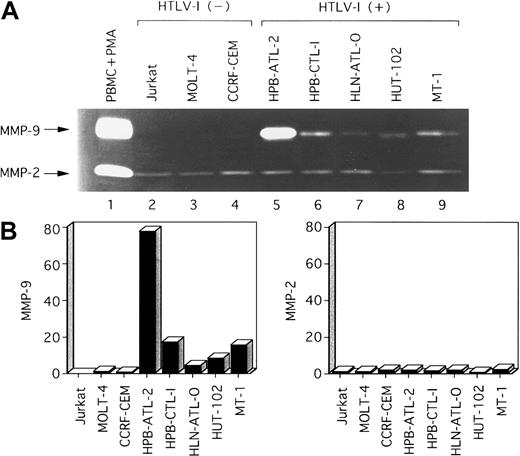
We next investigated whether MMP-2 and MMP-9 were secreted into the culture media of these HTLV-I–infected T-cell lines. Analysis by zymography indicated that MMP-9 was constitutively secreted into serum-free media by HTLV-I–infected T-cell lines, but was not or was faintly secreted by uninfected T-cell lines (Figure2). MMP-2 was secreted in all T-cell lines examined. The secretion of MMP-9 and MMP-2 correlated with their transcriptional activities (Figure 1). In summary, both MMP-9 enzymatic activity and mRNA expression correlated with HTLV-I infection.
Zymographic analysis of conditioned media from various T-cell lines.
(A) A total of 12 μL conditioned medium was subjected to electrophoresis on 10% SDS-polyacrylamide gels containing 1 mg/mL gelatin. SDS was removed following electrophoresis, and gels were incubated to restore gelatinase activity. Lane 1, conditioned media from PBMCs activated by PMA (positive control for MMP-2 and MMP-9). Major gelatinolytic activities corresponding to pro–MMP-9 (92 kd) and pro–MMP-2 (68 kd) are indicated. (B) Densitometric scan of MMP bands in (A) shows relative levels of the amounts of MMPs in each line compared with those in HTLV-I–negative control MOLT-4 cells.
Zymographic analysis of conditioned media from various T-cell lines.
(A) A total of 12 μL conditioned medium was subjected to electrophoresis on 10% SDS-polyacrylamide gels containing 1 mg/mL gelatin. SDS was removed following electrophoresis, and gels were incubated to restore gelatinase activity. Lane 1, conditioned media from PBMCs activated by PMA (positive control for MMP-2 and MMP-9). Major gelatinolytic activities corresponding to pro–MMP-9 (92 kd) and pro–MMP-2 (68 kd) are indicated. (B) Densitometric scan of MMP bands in (A) shows relative levels of the amounts of MMPs in each line compared with those in HTLV-I–negative control MOLT-4 cells.
Tax induces MMP-9 gene expression in T cells
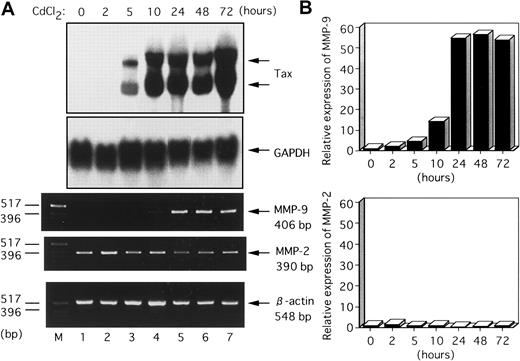
Because HTLV-I Tax expression correlated with elevated MMP-9 expression and enzymatic activity, we next examined whether Tax itself caused up-regulation of MMP-9 gene expression. To investigate the effects of Tax expression on MMP-9 gene expression, we used JPX-9, which stably carries Tax expression plasmid, pMAXneo, in which expression of Tax is inducible by the addition of CdCl2.21,22 The level of expression of Tax mRNA in these cells was determined by Northern blot analysis, and expression of the MMP-9 and MMP-2 genes was assayed by RT-PCR. As shown in Figure 3A, the addition of CdCl2 (20 μM) to the culture medium of JPX-9 cells induced the expression of Tax within 5 hours, which persisted until 72 hours after treatment. pMAXneo contains an intron with splice donor and acceptor sites in the Tax coding region.22 The 2 hybridized bands correspond to spliced and unspliced Tax mRNA, respectively. A concomitant increase in the expression of MMP-9 within 10 hours of treatment with CdCl2 was observed in JPX-9 cells. In contrast, MMP-2 mRNA expression was not significantly affected by induced Tax expression. We also analyzed the expression of these 2 MMP genes by quantitative hybridization capture assay. As shown in Figure 3B, the induction of MMP-9 was dramatic in JPX-9 cells. The induction of MMP-9 could not be attributed to CdCl2treatment because MMP-9 expression was not induced in JPX/M cells, expressing a nonfunctional Tax mutant protein, after treatment with CdCl2 (data not shown). These results indicate that Tax itself is capable of causing elevated expression of the MMP-9 gene in Jurkat T cells.
Induction kinetics of the MMP-9 gene in JPX-9 cells treated with CdCl2.
(A) Total RNA samples were prepared from CdCl2-treated JPX-9 cells at the indicated time points (0-72 hours). The expression of Tax and MMPs in the extracted RNA was analyzed by Northern blot and RT-PCR analysis, respectively. GAPDH served as a loading control for Northern analysis, whereas β-actin served as an internal control in the RT-PCR procedure. (B) Quantitative hybridization capture assay to detect amplified nucleic acid of MMPs. Results are shown quantitatively as fold increase over the amount at time zero.
Induction kinetics of the MMP-9 gene in JPX-9 cells treated with CdCl2.
(A) Total RNA samples were prepared from CdCl2-treated JPX-9 cells at the indicated time points (0-72 hours). The expression of Tax and MMPs in the extracted RNA was analyzed by Northern blot and RT-PCR analysis, respectively. GAPDH served as a loading control for Northern analysis, whereas β-actin served as an internal control in the RT-PCR procedure. (B) Quantitative hybridization capture assay to detect amplified nucleic acid of MMPs. Results are shown quantitatively as fold increase over the amount at time zero.
Tax transactivation of the MMP-9 promoter in T cells
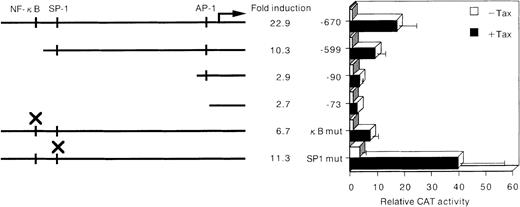
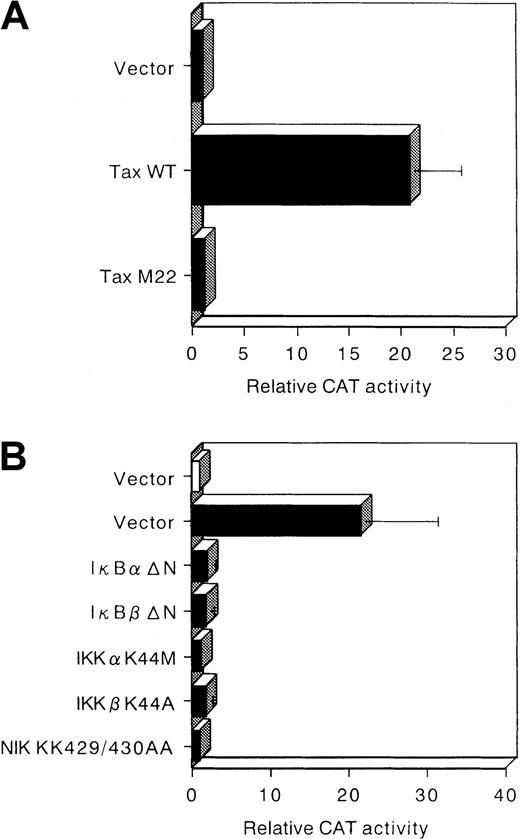
MMP activity is mainly regulated at the level of gene transcription, but also by posttranslational mechanisms.32,33 On the basis of these observations, we investigated whether the possible mechanism of Tax-mediated up-regulation of MMP-9 gene expression could be direct enhancement of its promoter activity. Jurkat cells were transiently transfected with a Tax expression vector together with a reporter gene construct containing the −670 to +53 segment of the MMP-9 upstream regulatory sequences. Coexpression of Tax caused a 23-fold elevation in the activity of this MMP-9–driven reporter construct (Figure4). Three transcription factors—NF-κB, SP-1, and AP-1—have been identified as transactivators of MMP-9 when cells were treated with 12-O-tetradecanoyl-phorbol-13-acetate and/or tumor necrosis factor-α.15 To test the relative contributions of these binding sites to Tax-mediated transactivation of MMP-9, plasmids with deletions and point mutations in these sites of the MMP-9 promoter were cotransfected with the Tax expression vector (Figure 4). Deletion of the NF-κB binding site (−599 CAT) considerably reduced Tax transactivation of the MMP-9 promoter. The −90 CAT construct, which contains only the AP-1 binding site and TATA box, completely abolished Tax-mediated transactivation of this reporter construct. Mutational analysis of these binding sites revealed that the NF-κB binding site (κB mut CAT) represented a major Tax-responsive element within the MMP-9 transcriptional regulatory sequences. In fact, the κB mut CAT construct was less responsive to Tax than the NF-κB deleted construct (−599 CAT). Mutation of the SP-1 site also decreased Tax transactivation (SP-1 mut CAT). Next, through the use of a mutant Tax protein that is incapable of activating NF-κB/Rel factors (M22),23 24 we investigated whether Tax-mediated activation of NF-κB was required for induction of the MMP-9 promoter in T cells. Whereas wild-type Tax increased MMP-9–driven reporter gene activity, no significant activation of this reporter was observed with the M22 mutant (Figure 5A). Therefore, both the NF-κB and SP-1 binding sites contribute to activation of the MMP-9 promoter by Tax.
Deletion and mutational analysis of the cis-elements required for HTLV-I Tax-induced MMP-9 promoter activity.
Arrow indicates transcription start site, and × indicates the sites of mutation in the MMP-9 5′ flanking sequence inserted upstream of the CAT gene. Open and solid bars represent CAT activity of pHβAPr-1-neo (−Tax) and β-actin–Tax (+Tax)–transfected Jurkat cells, respectively. The activities are given relative to the activity of cells transfected with the empty vector (pHβAPr-1-neo) and −73 CAT, which was defined as 1. Data are also expressed as fold inductions in CAT activity in Tax-transfected cells over that in empty vector-transfected cells. The mean values and SD bars represent the results of 3 experiments.
Deletion and mutational analysis of the cis-elements required for HTLV-I Tax-induced MMP-9 promoter activity.
Arrow indicates transcription start site, and × indicates the sites of mutation in the MMP-9 5′ flanking sequence inserted upstream of the CAT gene. Open and solid bars represent CAT activity of pHβAPr-1-neo (−Tax) and β-actin–Tax (+Tax)–transfected Jurkat cells, respectively. The activities are given relative to the activity of cells transfected with the empty vector (pHβAPr-1-neo) and −73 CAT, which was defined as 1. Data are also expressed as fold inductions in CAT activity in Tax-transfected cells over that in empty vector-transfected cells. The mean values and SD bars represent the results of 3 experiments.
Tax transactivates the MMP-9 promoter mainly through the NF-κB pathway.
(A) Jurkat cells were transfected with HTLV-I Tax (Tax WT), M22 Tax, or pHβAPr-1-neo vector and a CAT reporter construct containing the full-length MMP-9 promoter (−670 CAT). The results are expressed as fold induction relative to the basal level measured in cells transfected with the empty vector (pHβAPr-1-neo). (B) The indicated effector plasmids were cotransfected with −670 CAT. Open bar represents CAT activity of empty vector (pCMV4) without Tax. Solid bars represent CAT activity of IκBα and IκBβ mutants and kinase-deficient IKKα, IKKβ, and NIK mutants in the presence of Tax. The activities are given relative to the activity of empty vector (pCMV4) without Tax, which was defined as 1. The mean values and SDs represented were obtained from 3 experiments.
Tax transactivates the MMP-9 promoter mainly through the NF-κB pathway.
(A) Jurkat cells were transfected with HTLV-I Tax (Tax WT), M22 Tax, or pHβAPr-1-neo vector and a CAT reporter construct containing the full-length MMP-9 promoter (−670 CAT). The results are expressed as fold induction relative to the basal level measured in cells transfected with the empty vector (pHβAPr-1-neo). (B) The indicated effector plasmids were cotransfected with −670 CAT. Open bar represents CAT activity of empty vector (pCMV4) without Tax. Solid bars represent CAT activity of IκBα and IκBβ mutants and kinase-deficient IKKα, IKKβ, and NIK mutants in the presence of Tax. The activities are given relative to the activity of empty vector (pCMV4) without Tax, which was defined as 1. The mean values and SDs represented were obtained from 3 experiments.
Dominant interfering components of the NF-κB pathway inhibit Tax-mediated transactivation of MMP-9 gene expression
We next examined whether Tax-mediated transactivation of MMP-9 gene expression involves signal transduction components in NF-κB activation. Dominant interfering mutants of IκBα and IκBβ and kinase-deficient mutants of IKKα, IKKβ, and NIK were tested for the ability to inhibit Tax-mediated transactivation of MMP-9–driven reporter gene activity. Expression of these various inhibitory mutants abolished the induction of the MMP-9 promoter by Tax (Figure 5B). These data demonstrate that signaling components involved in the activation of NF-κB are necessary for Tax transactivation of the MMP-9 promoter.
Binding of NF-κB/Rel and SP-1 to the Tax-responsive elements within the MMP-9 upstream regulatory sequences
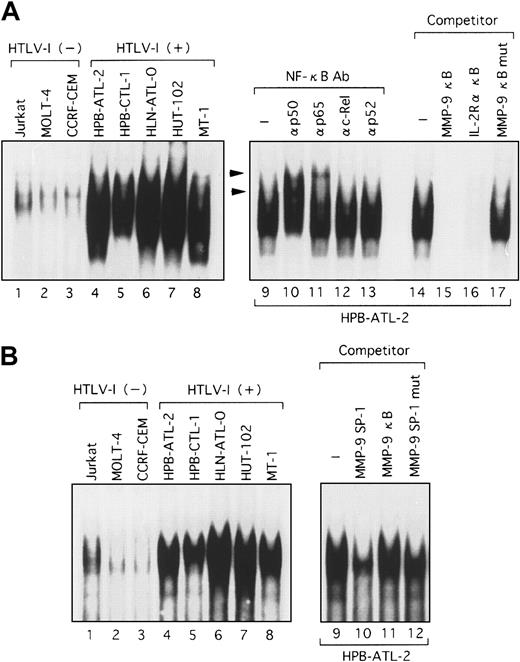
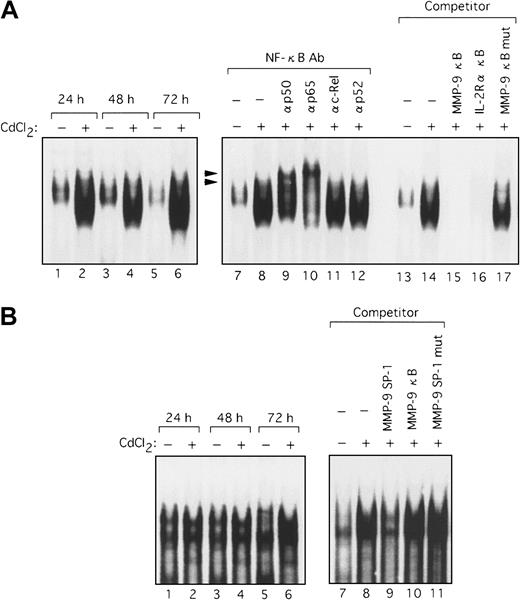
Because the deletional and mutational analyses of the MMP-9 promoter indicated that Tax activated transcription through both the NF-κB and SP-1 sites, it was important to identify the nuclear factors that bind to these sites. Using the NF-κB sequence derived from the MMP-9 promoter as a probe in an EMSA, we observed a clear shifted band when this probe was incubated with nuclear extracts from HTLV-I–infected T-cell lines, but not with nuclear extracts from uninfected cells (Figure 6A, lanes 1-8). This shifted complex was specific to the NF-κB sequence because complex formation was reduced by the addition of excess cold probe and the typical NF-κB sequence of the IL-2Rα enhancer but not by a mutant sequence of NF-κB (Figure 6A, lanes 14-17). To identify which NF-κB/Rel family members were binding to the NF-κB element from the MMP-9 promoter, we performed EMSA using antibodies specific for members of the NF-κB/Rel family. Supershifts were seen with anti-p65 and anti-p50 antibodies in complexes formed with nuclear extracts from HPB-ATL-2 cells and the MMP-9 NF-κB probe, illustrating that these complexes contain the p50 and p65 subunits of NF-κB (Figure 6A, lanes 9-13). An increase in binding of nuclear factors to the SP-1 sequence of the MMP-9 promoter was also detected when extracts from HTLV-I–infected T-cell lines were used. Such complexes were not visualized when nuclear extracts from uninfected T-cell lines were assayed (Figure 6B, lanes 1-8). This complex formation was inhibited by cold SP-1 probe, but not by a mutated SP-1 site or the MMP-9 NF-κB oligonucleotide (Figure 6B, lanes 9-12). We next examined nuclear extracts from the Tax-inducible T-cell line JPX-9. Induction of Tax expression resulted in the formation of a complex with the MMP-9 NF-κB probe within 24 hours (Figure7A, lanes 1-6). This binding activity was reduced by the addition of either cold probe or the typical NF-κB sequence derived from the IL-2Rα enhancer, but not by an oligonucleotide containing a mutated NF-κB sequence (Figure 7A, lanes 13-17). The Tax-induced complexes identified by the MMP-9 NF-κB probe were also characterized. These complexes were supershifted by the addition of anti-p50 or anti-p65 antibodies (Figure 7A, lanes 7-12). These data demonstrate that the MMP-9 NF-κB binding activity that is induced by Tax is composed of p50 and RelA/p65. In agreement with the MMP-9 promoter deletional and mutational analyses, implicating that Tax transactivation of the MMP-9 promoter involves SP-1, an increase in binding to the MMP-9 SP-1 sequence was also detected in nuclear extracts from JPX-9 cells within 72 hours of Tax induction (Figure 7B, lanes 1-6). This complex formation was inhibited by cold probe, but not by an oligonucleotide containing a mutated SP-1 site or the MMP-9 NF-κB oligonucleotide (Figure 7B, lanes 7-11). Therefore, Tax induces MMP-9 gene expression, at least in part through the induced binding of p50 and RelA/p65 to the MMP-9 NF-κB site and an SP-1 binding activity in the MMP-9 promoter region.
Binding of nuclear proteins from HTLV-I–infected T cells to the NF-κB and SP-1 probes derived from the MMP-9 promoter.
Nuclear extracts were prepared from HTLV-I–infected (lanes 4-8) and uninfected T cells (lanes 1-3) and incubated with either NF-κB (A) or SP-1 32P-labeled probes (B). Competition assays were performed with 100-fold excess amounts of each specific competitor oligonucleotide in nuclear extracts from HPB-ATL-2 cells (A, lanes 14-17; B, lanes 9-12). Supershift assay of NF-κB DNA-binding complexes in HPB-ATL-2 cells was also performed. Where indicated, appropriate antibodies were added to the reaction mixture before addition of 32P-labeled probes (A, lanes 9-13). Arrowheads show the DNA-binding complexes supershifted by antibodies.
Binding of nuclear proteins from HTLV-I–infected T cells to the NF-κB and SP-1 probes derived from the MMP-9 promoter.
Nuclear extracts were prepared from HTLV-I–infected (lanes 4-8) and uninfected T cells (lanes 1-3) and incubated with either NF-κB (A) or SP-1 32P-labeled probes (B). Competition assays were performed with 100-fold excess amounts of each specific competitor oligonucleotide in nuclear extracts from HPB-ATL-2 cells (A, lanes 14-17; B, lanes 9-12). Supershift assay of NF-κB DNA-binding complexes in HPB-ATL-2 cells was also performed. Where indicated, appropriate antibodies were added to the reaction mixture before addition of 32P-labeled probes (A, lanes 9-13). Arrowheads show the DNA-binding complexes supershifted by antibodies.
Tax-induced NF-κB and SP-1 binding activity.
Nuclear extracts from JPX-9 cells, treated with or without CdCl2 (20 μM) for the indicated time periods, were mixed with either NF-κB (A) or SP-1 32P-labeled probes (B) (lanes 1-6). Competition assays were performed with nuclear extracts from JPX-9 cells treated with CdCl2 for 72 hours. Where indicated, 100-fold excess amounts of each specific competitor oligonucleotide were added to the reaction mixture with labeled probes NF-κB (A, lanes 13-17) or SP-1 (B, lanes 7-11). Supershift assay of NF-κB DNA-binding complexes in the same nuclear extracts was performed as in Figure 6 (A, lanes 7-12). Arrowheads show the DNA-binding complexes supershifted by antibodies.
Tax-induced NF-κB and SP-1 binding activity.
Nuclear extracts from JPX-9 cells, treated with or without CdCl2 (20 μM) for the indicated time periods, were mixed with either NF-κB (A) or SP-1 32P-labeled probes (B) (lanes 1-6). Competition assays were performed with nuclear extracts from JPX-9 cells treated with CdCl2 for 72 hours. Where indicated, 100-fold excess amounts of each specific competitor oligonucleotide were added to the reaction mixture with labeled probes NF-κB (A, lanes 13-17) or SP-1 (B, lanes 7-11). Supershift assay of NF-κB DNA-binding complexes in the same nuclear extracts was performed as in Figure 6 (A, lanes 7-12). Arrowheads show the DNA-binding complexes supershifted by antibodies.
High levels of MMP-9 expression in leukemic cells from ATL patients
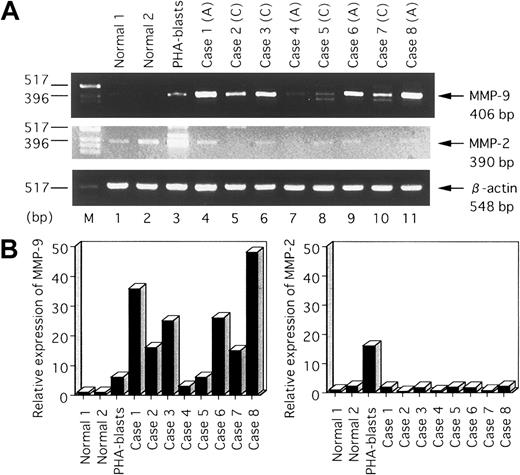
To assess the relevance of our findings, we analyzed the levels of expression of MMP-2 and MMP-9 in uncultured primary blood cells from ATL patients. As shown in Figure 8A, using RT-PCR, we demonstrated that both MMP-2 and MMP-9 transcripts were present in phytohemagglutinin (PHA)–stimulated normal PBMCs (PHA blasts). Analysis of ATL cell RNA revealed very low to undetectable levels of MMP-2 mRNA, which were comparable to that observed in resting PBMCs. In contrast, MMP-9 mRNA levels in cells derived from ATL patients were either similar to or greater than that observed in normal PHA blasts. In agreement with these RT-PCR results, quantitative hybridization capture assay revealed high expression of MMP-9 mRNA in ATL cells (Figure 8B). This finding is consistent with our previous observation that MMP-9 mRNA levels were elevated in HTLV-I–infected T-cell lines.
Detection of MMP-9 mRNA in leukemic cells obtained from patients with ATL by RT-PCR analysis.
(A) Lanes 1 and 2, RNA from normal resting PBMCs; lane 3, RNA from PHA blasts; lanes 4-11, RNA from PBMCs from patients with ATL. (A) indicates acute type; (C), chronic type. Bottom, ethidium bromide–stained gel showing β-actin PCR products. Arrows indicate positions of the specifically amplified DNA. (B) Quantitative hybridization capture assay to detect amplified nucleic acid of MMPs. Results show relative levels of the MMPs/β-actin compared with MMPs/ β-actin in normal 1.
Detection of MMP-9 mRNA in leukemic cells obtained from patients with ATL by RT-PCR analysis.
(A) Lanes 1 and 2, RNA from normal resting PBMCs; lane 3, RNA from PHA blasts; lanes 4-11, RNA from PBMCs from patients with ATL. (A) indicates acute type; (C), chronic type. Bottom, ethidium bromide–stained gel showing β-actin PCR products. Arrows indicate positions of the specifically amplified DNA. (B) Quantitative hybridization capture assay to detect amplified nucleic acid of MMPs. Results show relative levels of the MMPs/β-actin compared with MMPs/ β-actin in normal 1.
Immunohistochemical studies
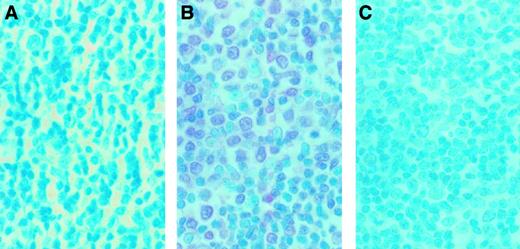
To examine whether MMP-9 was expressed in infiltrating ATL cells from a patient with organ involvement, immunohistochemical detection of MMP-9 was performed on a lymph node section. A sample from a lymph node biopsy from an ATL patient who manifested lymph node involvement was analyzed by enzyme-linked immunostaining for MMP-9 expression. A representative study is shown in Figure9. Massive mononuclear cell infiltration was observed. Staining of the serial section with 56-2A4 mAb revealed that most of the infiltrating cells expressed MMP-9 (Figure 9B). To ensure the specificity of 56-2A4 mAb, we performed the staining with control mouse IgG and blocking with MMP-9. No staining could be detected with the control mouse IgG (Figure 9A) or with MMP-9 blocking (Figure 9C).
Immunohistochemical findings of the lymph node biopsy from an ATL case.
The serial sections of lymph node biopsy specimens were stained with control mouse IgG (A) and 56-2A4 mAb (B). (C) To ensure the specificity of 56-2A4 mAb, blocking was done with MMP-9. The tissue sections were stained with 56-2A4 mAb after incubation with MMP-9 (C). Sections were counterstained with methyl green; original magnification × 400. Mononuclear cells infiltrating are clearly positive for MMP-9 (B).
Immunohistochemical findings of the lymph node biopsy from an ATL case.
The serial sections of lymph node biopsy specimens were stained with control mouse IgG (A) and 56-2A4 mAb (B). (C) To ensure the specificity of 56-2A4 mAb, blocking was done with MMP-9. The tissue sections were stained with 56-2A4 mAb after incubation with MMP-9 (C). Sections were counterstained with methyl green; original magnification × 400. Mononuclear cells infiltrating are clearly positive for MMP-9 (B).
Plasma MMP-9 levels correlate with organ involvement in ATL
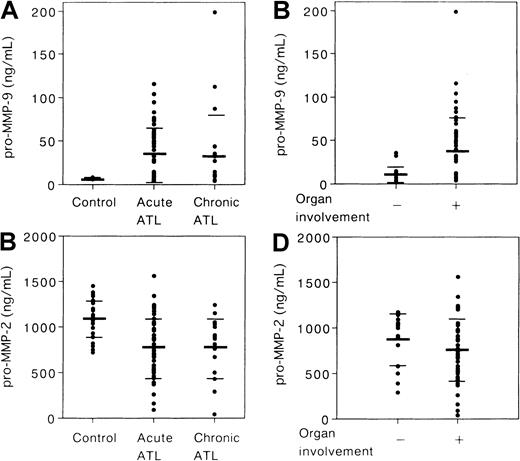
Finally, to address the biologic and clinical significance of elevated MMP-9 expression in ATL leukemic cell infiltration of tissues, we measured the concentrations of pro–MMP-9 in the plasma of ATL patients by ELISA (acute type, 51 cases; chronic type, 20 cases). The pro–MMP-9 levels in healthy control subjects ranged from 6.1 to 8.9 ng/mL (n = 24; mean ± SD, 7.1 ± 1.1 ng/mL). As shown in Figure 10A, the pro–MMP-9 levels at diagnosis of acute-type ATL (range, 4.2-116.0 ng/mL; 33.9 ± 31.0) and chronic-type ATL (range, 4.2-198.1 ng/mL; 32.9 ± 48.5) were significantly higher than those of healthy controls (P < .05). The infiltration of lymphoid organs, skin, bone, and other organs by ATL cells was confirmed in 45 patients. Interestingly, the pro–MMP-9 level of ATL patients with organ involvement (n = 45; range, 4.2-198.1 ng/mL; 38.5 ± 40.2) was significantly higher than that of ATL patients without organ involvement (n = 14; range, 4.2-35.4 ng/mL; 11.4 ± 10.2) (P < .05) (Figure 10B). The levels of pro–MMP-2 in the plasma of patients with acute-type ATL (range, 89.8-1557.3 ng/mL; 774.5 ± 336.4) and chronic-type ATL (range, 36.0-1242.9 ng/mL; 784.3 ± 325.8) were significantly lower than those of healthy control subjects (range, 721.8-1375.9 ng/mL; 1099.4 ± 204.8) (P < .05) (Figure 10C). There was no significant correlation between plasma levels of pro–MMP-2 and organ involvement in ATL patients (Figure 10D). Taken together, our observations suggest that pro–MMP-9 plasma levels closely correlate with organ involvement in ATL.
Amounts of pro–MMP-2 and pro–MMP-9 in the plasma from patients with ATL.
Plasma pro–MMP-9 (A) and pro–MMP-2 levels (C) in 24 healthy volunteers, in 51 patients with acute ATL, and in 20 patients with chronic ATL. Plasma pro–MMP-9 (B) and pro–MMP-2 levels (D) in 14 ATL patients without organ involvement (−) and in 45 ATL patients with organ involvement (+). The bars indicate the mean value ± SD of each group.
Amounts of pro–MMP-2 and pro–MMP-9 in the plasma from patients with ATL.
Plasma pro–MMP-9 (A) and pro–MMP-2 levels (C) in 24 healthy volunteers, in 51 patients with acute ATL, and in 20 patients with chronic ATL. Plasma pro–MMP-9 (B) and pro–MMP-2 levels (D) in 14 ATL patients without organ involvement (−) and in 45 ATL patients with organ involvement (+). The bars indicate the mean value ± SD of each group.
Discussion
The involvement of the MMPs in development of the metastatic phenotype in solid tumors is now well established. Previous studies indicated that these gene products may also be involved in the dissemination and progression of hematopoietic neoplasms.6-10 Several studies on the localization and infiltration of ATL cells in healthy organs have been published. Overexpression of certain MMPs found in ATL cells may explain why these malignant cells are capable of organ infiltration. However, there has not been a thorough and systematic study of MMP expression in HTLV-I–infected T-cell lines and in ATL patients.
In this study, we investigated the expression levels of 2 MMPs, MMP-2 and MMP-9, in various HTLV-I–infected T-cell lines as well as in uncultured primary leukemic cells isolated from ATL patients. We demonstrate that levels of MMP-9 expression are constitutively higher in all HTLV-I–infected T-cell lines examined in this study. Using the zymogram technique, we detected a 92-kd gelatinase activity consistent with MMP-9 in all HTLV-I–infected T-cell lines investigated, but not in uninfected T-cell lines. In contrast, there were no significant differences in MMP-2 transcript levels or activity observed between HTLV-I–infected and uninfected T-cell lines. The finding that fresh leukemic cells from ATL patients also had higher levels of MMP-9 expression when compared with PBMCs from uninfected donors validates the observations made in HTLV-I–infected T-cell lines. Interestingly, we observed that the HTLV-I–encoded oncoprotein Tax up-regulates MMP-9 mainly through the NF-κB pathway. Tax-mediated NF-κB activation may be central to increasing the expression of MMP-9 in HTLV-I–infected T cells.
The expression of Tax in ATL cells can be detected only by RT-PCR, as observed in an ATL-derived cell line, MT-1. A trace amount of Tax might be sufficient for the induction of MMP-9 in ATL cells and MT-1 cells. Alternatively, in the absence of Tax, ATL cells and MT-1 cells might maintain high MMP-9 expression. The NF-κB pathway is constitutively activated despite the low level of Tax expression in both ATL cells and MT-1 cells.30 It is questionable whether Tax alone explains in vivo NF-κB activation in ATL cells. Tax is crucial for the leukemic transformation of infected T cells at the early stage. However, continuous expression of the viral antigens would not be possible because such cells would be rejected by the host immune response; therefore, expression has to be low, or infected cells would not be able to survive in vivo. Tax might not be essential in the maintenance of the leukemic phenotype. The NF-κB activation rather than the high level of Tax expression might be necessary for the constitutive expression of MMP-9. Tax-independent mechanisms might operate for constitutive activation of NF-κB and subsequent expression of MMP-9 in ATL cells.
Increased MMP-9 levels (both free pro–MMP-9 and pro–MMP-9 complexed with tissue inhibitor of MMP-1) in plasma samples from patients with ATL are consistent with our observation that MMP-9 expression levels are elevated by HTLV-I infection. In this study, we have revealed a correlation between organ involvement in ATL disease and MMP-9 levels in the plasma of patients with ATL. Our immunohistochemical study showed that infiltrating ATL cells in a lymph node from an ATL patient expressed MMP-9. Taken together, these data suggest that MMP-9, which seems to be released from ATL cells as evidenced by its presence in plasma of ATL patients, may be important for the promotion of infiltration and accumulation of leukemic cells into several tissues in ATL.
Interestingly, Giraudon et al34 showed that transient contact between astrocytes and T lymphocytes activated by HTLV-I infection led to increased production of MMP-3 and MMP-9 in astrocytes. This change in MMP expression was mediated via T-cell–produced inflammatory cytokines and integrins. ATL cells are known to express cytokines and integrins. The induction of MMP-9 expression may also occur in stromal cells of tissues infiltrated by ATL cells in vivo, and the mechanism involves signals from ATL cells, in particular integrin-mediated adhesion and cytokines, as shown in the above studies. MMP-9 detected in the plasma may originate from ATL cells as well as from stromal cells within the tissues where ATL cells infiltrate.
It has been demonstrated recently that Tax binds to IKKγ, a component of a 700-kd IKK complex, which normally phosphorylates IκBs.35-37 Phosphorylation of IκBs by IKKs leads to their ubiquitination and degradation, resulting in activation and nuclear localization of NF-κB.38 We showed that Tax-mediated enhancement of MMP-9 promoter activity is blocked by the overexpression of dominant interfering mutants of IκBα and IκBβ and kinase-deficient mutants of IKKα, IKKβ, and NIK. Therefore, agents that specifically inhibit NF-κB activation may be effective in preventing the organ infiltration of ATL.
In addition to their capacity for matrix degradation, MMPs have been found to utilize some atypical substrates. For example, active tumor necrosis factor-α is liberated from its inactive precursor form, and Fas ligand is shed from the leukocyte cell surface by the action of MMPs.39 40 Thus, the constitutive release of MMP-9 that we found in ATL cell cultures may also influence ATL cell growth and behavior through the processing of cellular regulatory proteins.
In conclusion, this work provides the first evidence for the constitutive production of MMP-9 in HTLV-I–infected T cells in vitro and in vivo. Our findings suggest that MMP-9 represents a marker of malignant transformation of T cells with potential prognostic significance for the progression of disease in ATL. The release of MMP-9 from ATL cells may contribute to ATL cell dissemination by local digestion of ECM barriers similar to the invasion and metastasis processes of solid tumor cells. Currently, MMP-2 and MMP-9 are promising targets in an adjuvant therapy of solid tumor spread.41 Synthetic MMP inhibitors such as Batimastat and Marimastat have proven effective in various models for tumor metastasis and are currently being used in clinical studies on human cancers.42 In conclusion, our results suggest that MMPs, especially MMP-9, may be a target molecule in the strategy of treatment for ATL.
We are deeply indebted to the many patients with ATL and the control subjects who donated blood for these studies. We thank Drs K. Matsumoto and D. W. Ballard for providing expression vectors for HTLV-I Tax and the Tax M22 mutant and for deletion mutants of IκBs, respectively. We also thank Dr M. Nakamura for providing JPX-9 and JPX/M and Fujisaki Cell Center, Hayashibara Biomedical Laboratories (Okayama, Japan) for providing Jurkat, HUT-102, and MT-1 cell lines. We are grateful to M. Yamamoto and M. Sasaki for excellent technical assistance.
Supported in part by a grant-in-aid for Scientific Research (C) from the Japan Society for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Naoki Mori, Department of Preventive Medicine and AIDS Research, Institute of Tropical Medicine, Nagasaki University, 1-12-4, Sakamoto, Nagasaki 852-8523, Japan; e-mail:n-mori@net.nagasaki-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal