Granulocytopenia is thought to be the sole mechanism underlying the increased susceptibility to bacterial infection in hosts with anticancer chemotherapy. Little is known about the functional state of tissue macrophage populations in such hosts. Using a model of chemotherapy-induced leukopenia, the number and function of alveolar macrophages (AMS) were examined during and after multiple injections of an anticancer agent, cyclophosphamide (CP). Although CP quickly reduced peripheral blood leukocytes, the number of these cells rebounded quickly 3 to 4 days after the withdrawal of CP. Accompanying blood leukopenia was a profound reduction in the number of ams. Contrary to the rapid onset of blood leukopenia, tissue macrophage deficiency was a more chronic process that worsened gradually as the CP regimen continued. Of importance, in contrast to blood leukopenia, which restored itself shortly after CP withdrawal, tissue macrophage deficiency was not immediately self-recoverable in spite of a restored number of circulating leukocytes. Although AMS had a decreased ability to proliferate during, but not after, the CP regimen, these cells retained a normal ability to release tumor necrosis factor-α and nitric oxide. To identify the potential therapeutics for recovering macrophages, a gene vector expressing granulocyte macrophage–colony-stimulating factor (GM-CSF) was delivered either systemically or locally. GM-CSF transgene was able to expand macrophage populations only when delivered to the lung after, but not during, the CP regimen. This study thus identifies tissue macrophage deficiency as a mechanism of weakened innate immunity by chemotherapy and suggests the usefulness of topical GM-CSF transgene expression for restoring innate immunity in the lung.
Introduction
Patients on chemotherapy with anticancer agents such as cyclophosphamide (CP) often experience a diminished number of peripheral blood leukocytes, especially neutrophils (granulocytopenia or neutropenia), and weakened innate immunity.1-4 These patients are thus especially susceptible to opportunistic infections, including gram-negative bacterial pneumonia.2 Such opportunistic infections are a common cause of death in cancer patients who are undergoing chemotherapy. Neutropenia, or the deficiency of circulating neutrophils (PMNs), has been thought to be the sole mechanism underlying weakened host innate immunity.
During acute bacterial infection, PMNs migrate rapidly from the circulation to the tissue site of infection, where they carry out bacterial phagocytosis and killing. PMNs are also a cellular source of inflammatory cytokines and other mediators.5,6 Indeed, experimental animals rendered leukopenic or neutropenic by cyclophosphamide4,7-9 or antineutrophil antibodies10 were shown to be susceptible to pulmonary bacterial infection. In addition to PMNs, however, the tissue resident macrophage is also an important player of innate immunity. Alveolar macrophages are capable of active microbial phagocytosis, killing, and biosynthesis of a wide range of immune molecules, including cytokines.11 Mice rendered deficient only in alveolar macrophages or their functions were also shown to be susceptible to pulmonary gram-negative bacterial infection.12-15Together, these lines of evidence indicate that tissue macrophages and circulating neutrophils are required for host defense against acute bacterial infection.
Although peripheral blood leukopenia or neutropenia is a well-recognized cause of weakened innate immunity by anticancer agents, whether such anticancer agents have any detrimental effect on tissue macrophage population has remained largely unknown. The lung exposes directly to the external environment and thus is a common site for opportunistic bacterial infection in hosts undergoing chemotherapy. Alveolar macrophages (ams) are a tissue macrophage population residing in the lung with a half-life of approximately 1 to 5 weeks.16-18 The maintenance of this cell population is believed to depend on the migration and subsequent differentiation of blood monocytes and the replication of ams in the lung.ams are not only the first line of host defense in the lung, they also play an important role in triggering PMN influx into the lung by secreting neutrophil chemotactic factors in the early stages of acute infection.6 19 Examination of the functional state of the am population will help us to fully understand the mechanisms of weakened innate immunity in hosts during and after chemotherapy and to develop better strategies to improve the innate immunity in these hosts.
In this study, we established a mouse model of peripheral blood leukopenia induced by subcutaneously repeated doses of CP, and we examined the number and function of lung macrophages during and after CP regimen. In particular, we compared the kinetic changes in the number of lung macrophages with those of peripheral blood leukocytes. We also investigated whether transgene expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) could help restore and activate the CP-impaired tissue macrophage population.
Materials and methods
Mice and reagents
C57BL/6 mice aged 8 to 14 weeks were purchased from Harlan Laboratories (Indianapolis, IN). These mice were housed at the McMaster Central Animal facility under a 12-hour light-dark cycle and level B-specific pathogen-free conditions. All experiments described in this study were approved by the Animal Ethics Research Board of McMaster University. Cyclophosphamide was purchased from Sigma (St Louis, MO) and reconstituted in sterile phosphate-buffered saline (PBS) and was stored at 4°C. Macrophage culture RPMI 1640 media contained 10% fetal bovine serum and 1% penicillin–streptomycin. A recombinant replication-deficient adenoviral vector (AdGM-CSF) was previously developed in our laboratory20 21 and was used to transfer GM-CSF transgene into the lung. An adenoviral vector (Addl70.3), which does not contain transgene, was used as control.
Induction of peripheral blood leukopenia
Leukopenia was induced according to a published protocol with modification.7 Mice were anesthetized and injected subcutaneously with CP. The initial injection was carried out at a dose of 150 mg/kg body weight. The second dose was 100 mg/kg and was given 3 days later (second dose) and subsequently every other day (Figure 1A-B, experimental regimen). Induction and maintenance of leukopenia were examined by analyzing blood specimens extracted through the retro-orbital plexus during the CP regimen and at the time of death.
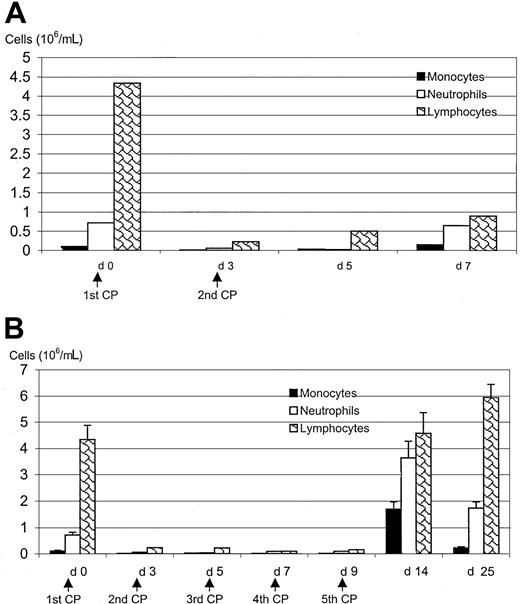
Peripheral blood leukopenia induced by CP.
Mice were injected subcutaneously with CP 2 (A) or 5 (B) times and were bled at various time points after the first CP at intervals indicated on the graphs. Differential numbers of monocytes, neutrophils, and lymphocytes were determined on blood smears as described in “Materials and methods.” Results are expressed as mean from 2 mice (A) or as mean ± SEM from 2 to 7 mice per time point (B).
Peripheral blood leukopenia induced by CP.
Mice were injected subcutaneously with CP 2 (A) or 5 (B) times and were bled at various time points after the first CP at intervals indicated on the graphs. Differential numbers of monocytes, neutrophils, and lymphocytes were determined on blood smears as described in “Materials and methods.” Results are expressed as mean from 2 mice (A) or as mean ± SEM from 2 to 7 mice per time point (B).
Preparation and analysis of peripheral blood samples
Total numbers of peripheral blood leukocytes were determined on a hemocytometer after red blood cell lysis with a lysis buffer, as previously described.22 Differential counts were determined on Diff-Quik–stained blood smears made from whole blood.
Analysis of alveolar macrophage population
PBS-treated C57BL/6 control mice or mice injected 5 times with CP were killed at days 10, 14, 18, 25, and 35, and alveolar macrophages were obtained by a bronchoalveolar lavage procedure as previously described.22 23 Briefly, the mouse lung was lavaged with aliquots to a total of 1.8 mL PBS through a polyethylene tube cannulated into the trachea. Collected lavage fluids were centrifuged to pellet cells. Cells from bronchoalveolar lavage were spun onto a slide using a cytospin for cytologic analysis. Slides were stained using Diff-Quik, and 300 to 400 cells per cytospin were counted to determine differential cell types, including macrophages, neutrophils, and lymphocytes.
Culture of alveolar macrophages
Alveolar macrophages were resuspended in RPMI culture media and cultured in 96-well plates at a density of 0.1 × 106cells/well with or without stimuli, including lipopolysaccharide (LPS), various concentrations of interferon (IFN)–γ, GM-CSF, or combinations of LPS and IFN-γ in a total volume of 0.3 mL for 72 hours at 37°C. Supernatants were stored at −20°C until cytokine and nitric oxide (NO) assays. In the experiments involving the use of GM-CSF gene transfer, vector cell culture was enriched by the removal of nonadherent cells 1 hour after the initial culture period.
Measurement of macrophage proliferation
Macrophages were cultured with or without GM-CSF stimulation in a 96-well plate for 3 days at 37°C and 5% CO2 in a total volume of 0.25 mL RPMI media. Macrophage proliferation was assessed by using a 3H-incorporation assay as previously described.24 Briefly, cells were pulsed with 0.037 MBq/well of 3H-thymidine and were further incubated for 20 hours. After 3 freeze–thaw cycles, the cells were harvested on a cell harvester. 3H-thymidine incorporation was then quantified on a beta-counter and expressed in counts per minute.
Measurement of macrophage phagocytosis of Pseudomonas aeruginosa
Alveolar macrophages were allowed to interact with gram-negative bacterium Pseudomonas aeruginosa for the determination of phagocytosis. Alveolar macrophages (0.3 × 105) were cultured for 4 hours in RPMI media containing 5% fetal bovine serum to allow the formation of a monolayer on Labteck Chamber Slides (Nunc). Non-adherent cells were removed and RPMI media containing 0.15 × 106P aeruginosa (ratio of 5:1,P aeruginosa:am) was added.25 The reaction was stopped after 1 hour of incubation at 37°C and 5% CO2. Slides were stained using Giemsa and examined under the microscope to determine the number of macrophages that had phagocytosed bacteria.
Isolation of total lung mononuclear cells
In separate experiments, mice injected 5 times with CP and PBS-treated control mice were killed at day 10, and total lung tissue–derived mononuclear cells were isolated as previously described.23 Briefly, lungs were perfused through the left ventricle with buffer to remove peripheral blood cells. The lungs were digested using collagenase, and the remaining tissue was forced through a metal screen. Cells were collected and filtered through nylon mesh. Total tissue cells were centrifuged on a Percoll gradient to purify total mononuclear cells.
Cytokine measurement
The level of tumor necrosis factor (TNF)–α was measured using a mouse-specific enzyme-linked immunosorbent assay (ELISA) kit purchased from R&D Systems (Minneapolis, MN). The sensitivity of the kit was less than 5 pg/mL.
Measurement of nitric oxide production
The release of NO by macrophages was determined by measuring the concentration in culture supernatants of the end product of NO, nitrite, as previously described.26 Diluted supernatants were mixed in a 1:1 ratio with Griess reagent buffer (Sigma). Absorbency was measured at 540 nm using an ELISA reader. Final concentrations were calculated from a standard curve that was derived from prepared solutions of NaNO2 of known concentrations.
GM-CSF gene transfer
AdGM-CSF or Addl70.3 was administered intranasally to the lung of mice as previously described.21,27 Briefly, a dose of 0.2 × 109 plaque-forming units of gene transfer vector was diluted in a total volume of 30 μL PBS and was administered to the mouse using a fine-tip pipette in 2 aliquots (15 μL each). We have previously shown that after intrapulmonary gene transfer, transgene is expressed primarily by bronchial epithelial cells and to a lesser extent by alveolar macrophages.27,28 In some experiments, the same dose of AdGM-CSF or Addl70.3 was administered intramuscularly to the hind leg of the mice as previously described.29 We have previously shown that intramuscular gene transfer leads to transgene mRNA expression localized to the muscle and active release of transgene protein to the circulation.29 Mice were killed, and cellular responses were analyzed 5 days after gene transfer.
Statistical analysis
Wherever applicable, a Student t test was carried out to analyze the differences between samples. The difference was considered statistically significant when P ≤ .05.
Results
Induction of peripheral blood leukopenia by repeated doses of cyclophosphamide
To establish a mouse model of chemotherapy-induced leukopenia of clinical relevance, we administered CP subcutaneously to mice and examined its effect on peripheral blood leukocytes after 2 separate CP regimens. We found that the initial dose of CP quickly reduced the number not only of neutrophils but of monocytes and lymphocytes in the peripheral blood (Figure 1A), and such leukopenic status was maintained up to day 5 after a second dose of CP given on day 3. By day 7, however, the number of neutrophils and monocytes recovered to normal, though the number of lymphocytes was still reduced (Figure1A).
To establish a model involving repeated doses of CP, similar to human situations, and to examine whether multiple doses of CP would sustain leukopenia, CP injections were repeated 5 times. Again, this regimen resulted in a decrease in all 3 subsets of leukocytes. Monocyte, neutrophil, and lymphocyte populations deteriorated from a normal range of 0.1 × 106/mL, 0.7 × 106/mL, and 4.3 × 106/mL blood to population sizes of 1 × 103/mL, 1 × 104/mL, and 1 × 105/mL blood, respectively. Five days after completion of the 5-CP regimen, there was a marked recovery in monocytes (1.6 × 106) and neutrophils (3.6 × 106), to a level that exceeded the normal level (Figure 1B). In comparison, the number of lymphocytes (4.6 × 106) was restored to the normal level. By day 16 after the last dose of CP, the number of monocytes and neutrophils returned to near normal levels. These results suggest that CP ablates not only peripheral blood neutrophils, it ablates monocytes and lymphocytes, and that the number of these leukocytes rebounds approximately 3 days after the last CP dose.
Reduction in the number of lung macrophages induced by CP regimen
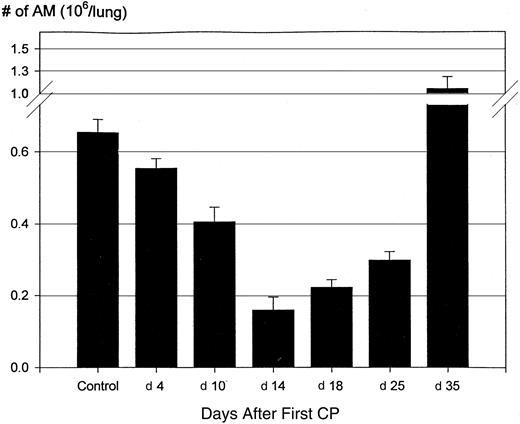
To investigate the potential effect of CP regimen on tissue residential macrophage population in the lung, mice were injected 2 or 5 times with CP according to the regimens delineated above (Figure 1A-B), and the size of alveolar macrophage population was determined at days 4, 10, 14, 18, 25, and 35 after the first dose of CP. At day 4 after the first CP dose (day 1 after the second CP dose), there was already a small but significant decrease in the number of alveolar macrophages (P = .03) (Figure2). At day 10 after the first CP (day 1 after the fifth or last CP dose), the AM population was reduced to 0.4 × 106/lung from 0.65 × 106(P = .0001) in a naive mouse lung (Figure 2). At day 14 (day 5 after the last CP), the number of AMs was further reduced to 0.16 × 106 (P < .001 compared to day 10). At days 18 (day 9 after the last CP dose) and 25 (day 16 after the last CP dose), the overall size of the AM population remained markedly reduced (50% to 70%; P < .0001 compared to normal control), though it was larger than at day 14. The intra-alveolar macrophage population, reduced by CP regimen, was well mirrored by a reduced size of total lung tissue mononuclear cell population, determined by an enzymatic dispersion method (0.11 × 106/lung vs 0.37 × 106/lung). However, by day 35 after the first CP dose (day 26 after the last CP dose), the size of the AM population was restored to a level of 1.2 × 106, which was even greater than the control (P ≤ .05). These findings suggest first that a systemic CP regimen had a chronic and profound effect on the size of tissue residential macrophage populations. Second, they suggest that recovery of the number of peripheral blood leukocytes, particularly monocytes, as a result of withdrawal of CP regimen, does not immediately help the recovery of reduced tissue macrophage population.
Reduction of alveolar macrophage population by CP.
Mice were injected subcutaneously with CP as described in Figure 1B. At various times after the first CP, mice were killed and alveolar macrophages were obtained from bronchoalveolar lavage fluids. The number of alveolar macrophages was determined on cytospins. Results are expressed as mean ± SEM from 20 (control), 16 (day 4), 14 (day 10), 5 (day 14), 8 (day 18), 7 (day 25), and 6 (day 35) mice. When compared with the control, the differences at various times are all statistically significant.
Reduction of alveolar macrophage population by CP.
Mice were injected subcutaneously with CP as described in Figure 1B. At various times after the first CP, mice were killed and alveolar macrophages were obtained from bronchoalveolar lavage fluids. The number of alveolar macrophages was determined on cytospins. Results are expressed as mean ± SEM from 20 (control), 16 (day 4), 14 (day 10), 5 (day 14), 8 (day 18), 7 (day 25), and 6 (day 35) mice. When compared with the control, the differences at various times are all statistically significant.
Reduced proliferative responses of lung macrophages by CP regimen
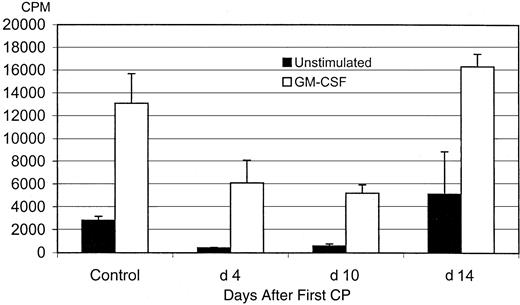
To examine whether the reduced lung macrophage population was attributed to decreased proliferative responses, alveolar macrophages were isolated at days 4, 10, and 14 after the first CP (day 1 after the second CP and days 1 and 5 after the last CP). They were examined for their proliferative responses to stimulation by GM-CSF in vitro by the use of a 3H-thymidine incorporation assay. Compared with cells from normal control mice, macrophages taken at day 4 (day 1 after the second CP) or at day 10 (day 1 after the last CP) demonstrated decreased spontaneous proliferation and reduced ability to proliferate on stimulation with GM-CSF (Figure 3) (P < .001 and P < .05, compared with control, respectively). However, by day 14 (day 5 after the last CP), spontaneous and GM-CSF–stimulated proliferation by these cells appeared comparable to those by control macrophages. These findings suggest that the impaired proliferative ability by the CP regimen of macrophages accounts for a reduced macrophage population only during the CP regimen but unlikely after the CP regimen. Furthermore, the improved proliferative response in macrophages later, after the withdrawal of CP regimen, provides the opportunity for using macrophage growth factors to restore the size of macrophage population in the lung.
Impaired macrophage proliferative responses by CP.
Alveolar macrophages were obtained from PBS-treated control mice or from mice treated with CP (days 4, 10, and 14 after the first CP). Macrophages were cultured without or with stimulation by GM-CSF (4 ng/mL) for 3 days and were pulsed with 3H-thymidine. Cell proliferation rate is expressed as counts per minute by beta-counting. Results are expressed as mean ± SEM from 2 independent experiments (replicate wells per condition in each experiment). The difference between the control and mice at days 4 and 10 is statistically significant.
Impaired macrophage proliferative responses by CP.
Alveolar macrophages were obtained from PBS-treated control mice or from mice treated with CP (days 4, 10, and 14 after the first CP). Macrophages were cultured without or with stimulation by GM-CSF (4 ng/mL) for 3 days and were pulsed with 3H-thymidine. Cell proliferation rate is expressed as counts per minute by beta-counting. Results are expressed as mean ± SEM from 2 independent experiments (replicate wells per condition in each experiment). The difference between the control and mice at days 4 and 10 is statistically significant.
Unaltered cytokine and nitric oxide responses in lung macrophages by CP regimen
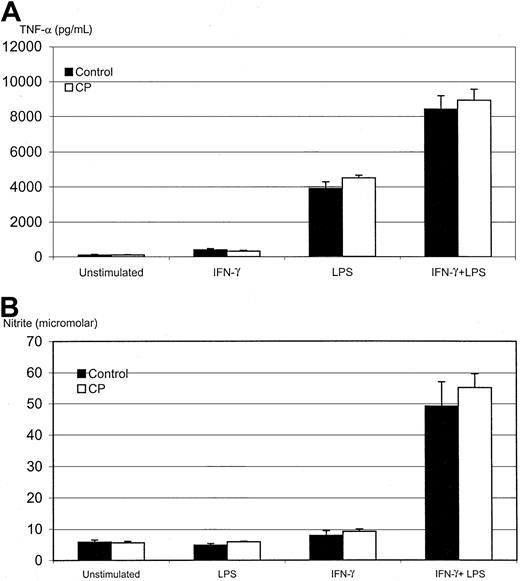
We next examined the ability of lung macrophages to release soluble factors in response to different stimuli in vitro. Because TNF-α and nitric oxide are important antimicrobial molecules released from activated macrophages,11,19,30 we chose to evaluate the release of these 2 mediators from AMs isolated from CP-treated mice at day 10 after the first CP (day 1 after the last CP). AMs were cultured for 72 hours under various stimulatory conditions and were assayed for TNF-α and NO production. We found that AMs from control and CP-treated mice released similar amounts of TNF-α on stimulation by IFN-γ, LPS, or IFN-γ–LPS combined (Figure4A). The production of NO was also similarly induced by these stimuli in cells from control and CP-treated mice (Figure 4B). In agreement with our previous findings,26 LPS stimulation alone did not increase NO release, whereas IFN-γ alone only moderately stimulated NO release. In contrast, IFN-γ and LPS in combination had a potent synergistic effect on NO release (Figure 4B). In line with such unaltered functional activities in macrophages, we also observed similar rates of bacterial phagocytosis by macrophages from control and CP-treated mice (data not shown). These observations suggest that the CP regimen has an effect primarily on the number, but not on the functional activities, of tissue macrophages.
Unimpaired TNF-α and NO responses in alveolar macrophages in CP-treated mice.
Macrophages were obtained from PBS-treated control mice or mice that had 5 consecutive treatments of CP (day 10 after the first CP) and were cultured for 3 days without or with stimulation by IFN-γ (800 pg/mL), LPS (1.2 μg/mL), or IFN-γ plus LPS (same concentrations). Supernatants were measured by ELISA for TNF-α (A) and by a biochemical method for NO (B). Results are expressed as mean ± SEM from 3 independent experiments (triplicate wells per condition in each experiment).
Unimpaired TNF-α and NO responses in alveolar macrophages in CP-treated mice.
Macrophages were obtained from PBS-treated control mice or mice that had 5 consecutive treatments of CP (day 10 after the first CP) and were cultured for 3 days without or with stimulation by IFN-γ (800 pg/mL), LPS (1.2 μg/mL), or IFN-γ plus LPS (same concentrations). Supernatants were measured by ELISA for TNF-α (A) and by a biochemical method for NO (B). Results are expressed as mean ± SEM from 3 independent experiments (triplicate wells per condition in each experiment).
Restoration of reduced macrophage population by GM-CSF gene transfer to the lung
GM-CSF is a well-known growth factor for monocytes and macrophages,31,32 and we have previously demonstrated that GM-CSF transgene expression in the lung stimulates macrophage proliferation.20 21 Thus, we investigated whether GM-CSF gene transfer could improve down-sized AM populations in CP-treated hosts.
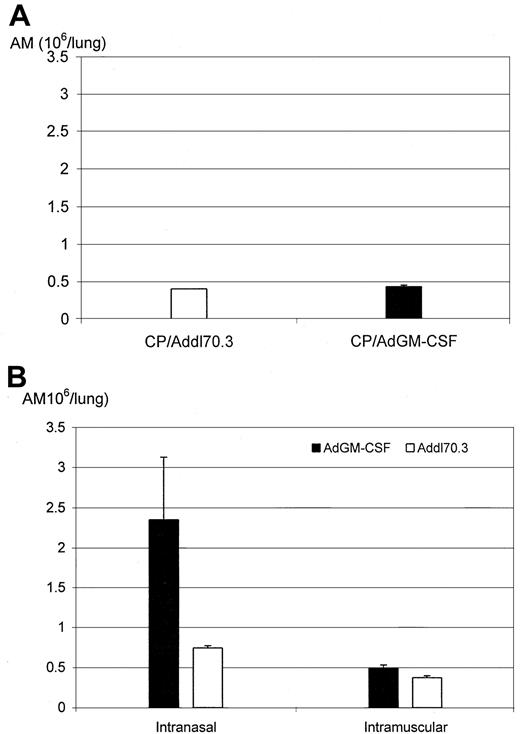
To examine the effect of GM-CSF transgene during CP regimen, a dose of adenoviral gene transfer vector expressing GM-CSF (AdGM-CSF) was delivered intranasally to mice at day 5 after first CP, and these mice were killed at day 10 after receiving 2 additional CP doses. We found that in this case, GM-CSF transgene delivery had little effect on the number of AMs (Figure 5A), suggesting that when delivered during the CP regimen, GM-CSF is ineffective perhaps because of the impaired ability by CP regimen of macrophages to proliferate in response to GM-CSF stimulation (Figure 3).
Expanded alveolar macrophage population by GM-CSF gene transfer.
(A) Mice received intranasally a dose of control gene transfer vector (Addl70.3) or GM-CSF gene transfer vector (AdGM-CSF) at day 5 after the first CP and received 2 additional doses of CP and were then killed at day 10 after the first CP (day 1 after the last CP). (B) Mice received Addl70.3 or AdGM-CSF gene transfer vector either intranasally or intramuscularly at day 10 after the first CP (1 day after the last CP) and were killed at day 15 after the first CP. The number of alveolar macrophages was determined on cytospins. Results are expressed as mean ± SEM from 4 mice (CP/AdGM-CSF) for panel A. The results (intranasal delivery) presented in panel B are expressed as mean ± SEM from 3 mice per group and are representative of 3 independent experiments.
Expanded alveolar macrophage population by GM-CSF gene transfer.
(A) Mice received intranasally a dose of control gene transfer vector (Addl70.3) or GM-CSF gene transfer vector (AdGM-CSF) at day 5 after the first CP and received 2 additional doses of CP and were then killed at day 10 after the first CP (day 1 after the last CP). (B) Mice received Addl70.3 or AdGM-CSF gene transfer vector either intranasally or intramuscularly at day 10 after the first CP (1 day after the last CP) and were killed at day 15 after the first CP. The number of alveolar macrophages was determined on cytospins. Results are expressed as mean ± SEM from 4 mice (CP/AdGM-CSF) for panel A. The results (intranasal delivery) presented in panel B are expressed as mean ± SEM from 3 mice per group and are representative of 3 independent experiments.
We next investigated whether GM-CSF transgene, when delivered right after the withdrawal of CP (day 10 after first CP), had any effect on the recovery of macrophage population. Indeed, GM-CSF transgene during the lung delivery significantly increased the number of AMs (Figure5B). Intranasal GM-CSF delivery did not affect the size of the peripheral blood leukocyte population (data not shown). These findings suggest that GM-CSF gene transfer to the lung, after withdrawal of CP regimen, is beneficial to the recovery of persistently impaired lung macrophage population.
We next examined whether heightened circulating levels of GM-CSF after intramuscular transgene delivery had any effect on the recovery of am population. When the same dose of AdGM-CSF was given and the responses were examined at the same time point, no significant increase in macrophage population was observed, which is in sharp contrast to the effect produced when GM-CSF transgene was delivered locally to the lung (Figure 5B). However, the systemic delivery of GM-CSF did cause a significant increase in the number of circulating neutrophils compared with control (16.82 × 106 ± 1.1 × 106 vs 3.628 × 106 ± 0.64 × 106, P = .0001). These findings suggest that the systemic administration of GM-CSF had little effect on the residential AM population.
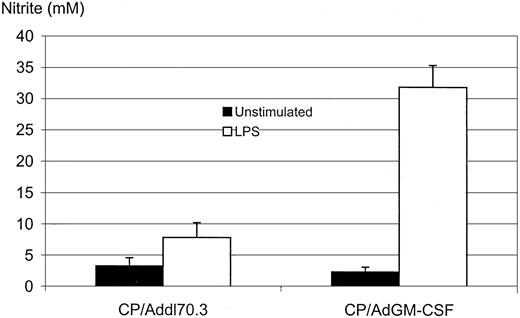
We examined whether GM-CSF transgene expression in the lung rendered after CP withdrawal could also activate alveolar macrophages. Alveolar macrophages, obtained as described above, were cultured with LPS, and the amount of NO was measured in the supernatants. We found that macrophages isolated from the lung of mice after intranasal AdGM-CSF released 3 times as much NO as those from the control group (Figure6; P = .0013). Because inactivated AMs cannot release increased NO in response to LPS stimulation (Figure 4B), such markedly increased NO release by LPS from these cells serves as a good indicator of cell activation in vivo. This suggests that GM-CSF transgene delivered in such a mode could not only restore the number of impaired macrophages by CP regimen, it could also activate these cells.
Activation of alveolar macrophages by GM-CSF transgene expression.
Mice were treated as in as the intranasal group in Figure 5B. Macrophages were purified by adherence and cultured without or with LPS (1.2 μg/mL) for 3 days, and supernatants were measured for NO by a biochemical method. Results are expressed as a mean ± SEM from triplicate wells and are representative of 2 independent experiments. The difference between CP/Addl70.3 and CP/Ad/GM-CSF is statistically significant (P = .0013).
Activation of alveolar macrophages by GM-CSF transgene expression.
Mice were treated as in as the intranasal group in Figure 5B. Macrophages were purified by adherence and cultured without or with LPS (1.2 μg/mL) for 3 days, and supernatants were measured for NO by a biochemical method. Results are expressed as a mean ± SEM from triplicate wells and are representative of 2 independent experiments. The difference between CP/Addl70.3 and CP/Ad/GM-CSF is statistically significant (P = .0013).
Discussion
In this study, we have developed a mouse model of leukopenia by repeated systemic administrations of an anticancer agent, cyclophosphamide, to dissect the mechanisms of weakened host innate immunity, which patients often experience during and after anticancer chemotherapy. We have found that although CP has a quick ablating effect on peripheral blood leukocytes, the number of these cells undergoes a speedy recovery that takes place between 3 and 4 days after the withdrawal of the CP regimen. Of importance, we have found that a profound reduction in the size of tissue macrophage population accompanies blood leukopenia. In contrast to the rapid onset of peripheral blood leukopenia induced by CP regimen, tissue macrophage deficiency is a chronic process that starts to manifest during the CP regimen and worsens as the regimen continues. Furthermore, in sharp contrast to blood leukopenia, which self-restores shortly after the withdrawal of CP regimen, tissue macrophage deficiency lasts considerably longer. Such reduced macrophage population was accounted for in part by its decreased proliferative potential. To identify a potential therapeutic means to recover macrophage populations, we tested the effect of a gene transfer vector expressing a macrophage growth factor, GM-CSF, and found that when delivered to the lung after CP withdrawal, it could help expand macrophage populations. When administered intramuscularly, however, GM-CSF could not expand the alveolar macrophage population regardless of increased peripheral blood leukocytes.
During and after anticancer chemotherapy, patients are at a particularly high risk for opportunistic infection, including gram-negative bacterial pneumonia.1-4 Indeed, experimental studies carried out by us (data not shown) and others7-9demonstrate that mice subjected to a CP regimen easily succumb to pulmonary gram-negative bacterial infection elicited by otherwise nonlethal inocula of bacteria. It has long been believed that granulocytopenia–neutropenia is the only cause of such increased susceptibility.7-9 Lung tissue residential macrophages, however, are also an integral part of innate immunity,19,30 and the lack of these cells, regardless of a normal level of peripheral blood neutrophils, predisposes hosts to acute lung bacterial infection.12-14 The critical requirement of a normal number of AMs in the lung for innate immunity was further demonstrated in a recent study by Broug-Holub et al15 showing that the host deficient in AMs succumbed toKlebsiella pneumonia despite a higher than normal level of neutrophilic infiltration in the lung. Given the importance of lung macrophages in host innate immunity, the potential effect of chemotherapeutics on these cells has not been investigated. Our current study has provided evidence to reveal the functional state of lung macrophages during and after the chemotherapeutic regimen. Our results indicate that compared to blood leukopenia induced by a CP regimen, lung macrophage deficiency is a persistent process that remains unrecoverable for a lengthy period after the cessation of the CP regimen. Such macrophage deficiency by chemotherapeutics thus may represent an important mechanism accounting for weakened innate immunity in patients undergoing chemotherapy, particularly after the withdrawal of such therapy (though it takes longer for humans to recover the number of peripheral blood leukocytes after CP treatment than it does for mice33). We have found that, in addition to the deficiency in AMs, the number of peritoneal macrophages in CP mice was also markedly reduced. However, this cell population was fully recovered by day 25 after first CP (data not shown), in contrast to the lung macrophage population that did not fully recover until day 35. Together, these findings suggest that tissue macrophage repopulation may take time after the CP regimen and that different tissues repopulate macrophages at different rates. Of interest, functional activities, including TNF-α and NO production, and bacterial phagocytosis, other than GM-CSF–stimulated proliferation, of these macrophages remained unaltered in CP mice. These results suggest that the primary effect of anticancer agents such as CP is on the number, not on the function, of tissue macrophages, in contrast to the enhancing effect of CP on cytokine release by peripheral blood leukocytes.34
In the last several years, a number of hematopoietic growth factors, including G-CSF and GM-CSF, have been administered systemically to patients during chemotherapy or after chemotherapy with only varying beneficial effects achieved.35-39 The use of these cytokines has been intended to prevent infection, allow intensified chemotherapy, and treat infection in conjunction with the use of antibiotics in these patients. We believe that our results will help design an improved clinical regimen involving the use of such cytokine-based therapeutics in 2 respects: (1) a cytokine such as GM-CSF that is active on neutrophils and macrophages will be advantageous over the one that is active only on neutrophils; and (2) the topical administration of such cytokine will most effectively help restore the tissue macrophage population ablated by chemotherapy, which is particularly worthy of consideration in patients after chemotherapy. Indeed, the latter point is supported by 2 of our observations, the first that GM-CSF transgene expressed systemically had no effect on the number of alveolar macrophages and the second that though it had minimal effect during the CP regimen, GM-CSF transgene, when expressed in the lung after the CP regimen, increased the number and function of alveolar macrophages. This in vivo effect of GM-CSF was consistent with our in vitro finding that isolated macrophages from the lung of mice 5 days after the withdrawal of CP regimen had a recovered ability to proliferate in response to GM-CSF stimulation. Along a similar line, Worgall et al40 have recently demonstrated that murine AMs, after being transduced ex vivo to express the GM-CSF transgene with an adenoviral vector and engrafted back to the mouse lung, underwent increased proliferation in the lung. In further support of the concept of local delivery of GM-CSF, recombinant GM-CSF has been delivered recently by aerosol to patients with lung cancer.37
The replication-deficient adenoviral system that we used to deliver the GM-CSF transgene has a remarkable ability to shuttle the transgene to airway epithelial cells, which express the transgene for 10 to 14 days in the lung.21,27,28 Such sustained but relatively transient expression may well suit the therapeutic setting that aims to repopulate the lung with tissue macrophages in patients for a limited period of time after chemotherapy. GM-CSF is ineffective in stimulating macrophage proliferation during CP regimen perhaps because CP is an alkylating anticancer agent that affects DNA synthesis; thus, it has a relatively specific effect on cells with cycling potential.41 It has been thought that the maintenance of local AM population is dependent on 2 events, local proliferation of mature macrophages and differentiation of newly arrived blood monocytes into macrophages.16-19 However, we observed that the self-restoration of peripheral blood leukocytes, including monocytes, on withdrawal of the CP regimen apparently failed to stimulate the immediate recovery of the local lung macrophage population. This finding suggests that local proliferation of mature macrophages represents an important mechanism for maintaining the macrophage population in the lung.
We thank Duncan Chong, Xueya Feng, Scott Alexander, Mohamed Panju, and Jun Wang for their technical assistance, help, and useful discussions.
Supported by funds from the Ontario Thoracic Society and McMaster University, Hamilton Health Sciences Corporation, and St. Joseph's Hospital. Z.X. is a recipient of a Canadian Institutes of Health Research Scholarship and an Ontario Premier's Research Excellence Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Zhou Xing, Dept of Pathology and Molecular Medicine, Health Sciences Center, Rm 4H19, McMaster University, 1200 Main St W, Hamilton, ON L8N 3Z5, Canada; e-mail: xingz@mcmaster.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal