Serum amyloid A (SAA) is an acute phase reactant, and its level in the blood is elevated to 1000-fold in response of the body to trauma, infection, inflammation, and neoplasia. SAA was reported to inhibit platelet aggregation and to induce adhesion of leukocytes. This study looked at adhesion of human platelets to SAA. Immobilized SAA supported the adhesion of human washed platelets; level of adhesion to SAA was comparable to fibronectin and lower than to fibrinogen. Adhesion to SAA was further enhanced by Mn2+ and the physiological agonist, thrombin. Platelet adhesion to SAA was completely abolished by anti-SAA antibody. SAA-induced adhesion was inhibited by antibodies against the integrin receptor αIIbβ3, by the peptide GRGDSP and by SAA-derived peptide containing YIGSR-like and RGD-like adhesion motifs (amino acids 29 to 42). Adhesion was not inhibited by control immunoglobulin G, by antibody against the integrin receptor αVβ3, by the peptide GRGESP, and by SAA-derived peptide that includes incomplete RGD motif. SAA-derived peptide 29 to 42 also inhibited platelet adhesion to fibronectin. Transfected human melanoma cells expressing αIIbβ3 adhered to SAA, whereas transfected cells expressing αVβ3 did not. By using flow cytometry, the αIIbβ3 cells displayed significantly higher levels of binding of soluble SAA than the αVβ3 cells. These data indicate that human platelets specifically adhere to SAA in an RGD- and αIIbβ3-dependent manner. Thus, SAA may play a role in modulating platelet adhesion at vascular injury sites by sharing platelet receptors with other platelet-adhesive proteins.
Introduction
Serum amyloid A (SAA) is composed of a family of proteins, and its level in the blood is elevated to 1000-fold as part of the response of the body to various injuries, including trauma, infection, inflammation, and neoplasia.1,2 The SAA proteins are expressed primarily in the liver, but extrahepatic expression was described, including in cells of human atherosclerotic lesions, ie, smooth muscle cells, endothelial cells, and monocytes/macrophages,3,4 and in many histologically normal human tissues, predominantly by the epithelium.5SAA contains binding sites for the extracellular matrix (ECM) components laminin6 and heparin/heparan sulfate7 as well as YIGSR-like and RGD-like adhesion motifs (YIGSDKYFHARGNY, residues 29 to 42). The latter is present at the turn of an assumed β-sheet, in a region that is highly conserved through evolution.8 SAA was reported to inhibit platelet aggregation,9 to induce adhesion of mononuclear and polymorphonuclear leukocytes,10,11 to bind to ECM proteins,12,13 to induce matrix metalloproteinases, and to serve as their substrate.14,15 SAA-derived peptides were reported to inhibit T-lymphocyte attachment to ECM proteins.16 Nevertheless, the physiologic role of SAA as an ECM-associated and/or adhesion protein is not well established.
The major integrin receptor expressed on platelet membrane, αIIbβ3 (platelet receptor GPIIb/IIIa), plays a critical role in hemostasis and thrombosis by mediating platelet-platelet and platelet-matrix protein interactions.17 The RGD (arginine-glycine-aspartic acid) adhesion motif is present in fibronectin and other matrix adhesive proteins, including fibrinogen, vitronectin, and von Willebrand factor, and mediates the adhesive interactions of platelets and cells (ie, leukocytes and endothelial cells) by cell surface integrin receptors, including αIIbβ3.18 In this study we demonstrate the adhesion of human platelets to SAA and show that human washed platelets adhere to immobilized SAA in an RGD- and αIIbβ3-dependent manner. Furthermore, SAA-derived peptide inhibited platelet adhesion to the subendothelial ECM protein, fibronectin.
Materials and methods
Antibodies and peptides
Antibodies used in the adhesion assays were as follows: anti-αIIbβ3 (c7E3Fab/abciximab/ReoPro [Eli-Lilly, Centocor, Leiden, The Netherlands]) and 10E5 (a gift from Dr B. S. Coller, Mount Sinai School of Medicine, New York, NY), anti-αVβ3 LM609 (Chemicon International, Temecula, CA), anti-SAA antibodies (clones mc29 and mcl) prepared and characterized as previously described,19,20 and control normal mouse immunoglobulin G (IgG; Zymed Laboratories, San Francisco, CA). Antibodies used in flow cytometry were as follows: anti-αIIb/CD41 (clone 5B12) and control mouse IgG were from DAKO Corporation (Carpinteria, CA) and fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). The secondary antibody used in Western blot analysis was a horseradish peroxidase–conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories). RGD containing peptides GRGDSP and GRGESP were purchased from GibcoBRL (Gaithersburg, MD). SAA-derived peptides used were as follows (in single-letter code): amino acids 29 to 42 (YIGSDKYFHARGNY), amino acids 28 to 40 (NYIGSDKYFHARG), and amino acids 83 to 104 (NEWGRSGKDPNHFRPAGLPEKY), all synthesized, purified by high-performance liquid chromatography, and characterized by amino acid composition analysis as previously described.21 Additional control peptides included scrambled RGD (DGPSGR), reverse RGD (PSDGRG), scrambled SAA peptide 29 to 42 (NYAGRKFHYSGDYI), reverse 29 to 42 (YNGRAHFYKDSGIY), and peptide-enclosing RGN (AARGNAA), all prepared and purified as described above.
Other materials
Human recombinant SAA (PeproTech, Rocky Hill, NJ), previously used in in vitro studies, was dissolved in d-H2O10,22; human plasma fibronectin was from GibcoBRL; and human fibrinogen (type 1), human thrombin, and human recombinant C5a were purchased from Sigma (St Louis, MO). The 96-well titer plates (Maxi Sorp) were from Nunc (Naperville, IL). Transfected human melanoma cells, MO-αIIb (expressing αIIbβ3 integrin receptor) and M21L4 (expressing αVβ3 integrin receptor) were a gift from Dr Mark H. Ginsberg (The Scripps Research Institute, La Jolla, CA).23 They were maintained in Dulbecco modified Eagle medium (Biological Industries, Kibbutz Beit Haemek, Israel), supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal calf serum (GibcoBRL). Other chemicals were of reagent grade from Sigma.
Preparation of platelets
Platelets were purified from fresh blood obtained from healthy human donors (men and women, aged 27 to 51 years) who had not taken any medication for at least 2 weeks. Blood was drawn in 3.8% buffered citrate solution (7:1 vol/vol). Washed platelets were prepared as described24 and resuspended in Tyrode buffer (NaCl 140 mM, KCl 2.7 mM, NaHCO3 11.9 mM, NaH2PO40.36 mM, CaCl2 2 mM, MgCl2 1 mM, glucose 5.4 mM, bovine serum albumen [BSA] 1 mg/mL, pH 7.3). Platelets were studied microscopically to ensure the absence of platelet aggregates and minimal presence of contaminating cells. They were counted (COULTER STKS, Coulter Electronics, Hialeah, FL) and adjusted to 3 to 4 × 108 platelets/mL in Tyrode buffer. The white blood cell count was 1 to 2 cells per 1000 platelets.
Adhesion of platelets
Platelet adhesion was carried out as described.25Wells of 96-well titer plates were precoated (overnight at 4°C) with 50 μL of 50 μg/mL solution of each of the adhesive proteins, BSA, SAA, fibronectin, or fibrinogen and blocked (1 hour at 37°C) with Tyrode buffer containing 1% BSA. Platelet suspension (100 μL containing 3 to 4 × 107 platelets) was added to the precoated wells, platelets were allowed to adhere (1 hour at 37°C), and wells were then washed with Tyrode buffer to remove unattached platelets. Adherent platelets were fixed and stained in 2% toluidine blue/4% paraformaldehyde (30 minutes at 37°C), and, after rinsing in tap water until the washing was clear, solubilized with 100μL 1% sodium dodecyl sulfate (SDS). Absorbance at 595 nm was read in a spectrophotometer (UVICON 930; Kontron Instruments, Zurich, Switzerland). This method of staining25 was adapted after initial experiments showed its comparability to other methods (staining platelet-derived protein26 and measuring platelet acid phosphatase activity27). The acid phosphatase assay was used in some experiments (Figure1) to quantitate the number of adherent platelets. Briefly, after the adhesion and washing procedure, as described above, the substrate solution (0.1 M citrate buffer pH 5.4, containing 5 mM p-nitrophenyl phosphate and 0.1% Triton X-100; 150 μL per well) was added and incubated for 1 hour at room temperature. The reaction was stopped, and the color was developed by the addition of 100 μL 2N NaOH, and absorbance at 405 nm was measured with a microplate reader (DYNATECH MR 5000; Dynatech Laboratories).
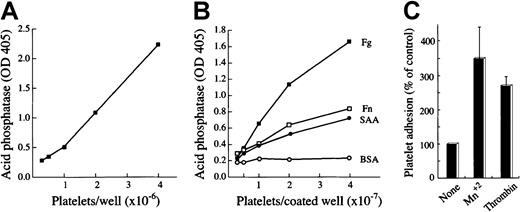
Human washed platelets adhere to SAA.
(A) Acid phosphatase activity as a function of cell number. Platelet suspensions containing the indicated platelet number were dispensed in triplicate wells of uncoated plates after which the acid phosphatase activity was measured as optical density (OD) increase at 405 nm. (B) Adhesion of human platelets to immobilized proteins. Platelet suspensions containing the indicated platelet number were dispensed in triplicate wells of plates coated with BSA, SAA, fibronectin (Fn), or fibrinogen (Fg) after which the adhesion assay was performed. Values are means of triplicates from one representative experiment of 3 performed. (C) Effect of Mn2+ and thrombin on platelet adhesion to SAA. Platelets were preincubated (15 minutes at 37°C) without (None) or with Mn2+ (1 mM) or thrombin (1 U/mL) and then added onto BSA- or SAA-coated wells. Values are means from 5 independent experiments (after subtracting adhesion to BSA).P < .05 when comparing adhesion with and without Mn2+/thrombin.
Human washed platelets adhere to SAA.
(A) Acid phosphatase activity as a function of cell number. Platelet suspensions containing the indicated platelet number were dispensed in triplicate wells of uncoated plates after which the acid phosphatase activity was measured as optical density (OD) increase at 405 nm. (B) Adhesion of human platelets to immobilized proteins. Platelet suspensions containing the indicated platelet number were dispensed in triplicate wells of plates coated with BSA, SAA, fibronectin (Fn), or fibrinogen (Fg) after which the adhesion assay was performed. Values are means of triplicates from one representative experiment of 3 performed. (C) Effect of Mn2+ and thrombin on platelet adhesion to SAA. Platelets were preincubated (15 minutes at 37°C) without (None) or with Mn2+ (1 mM) or thrombin (1 U/mL) and then added onto BSA- or SAA-coated wells. Values are means from 5 independent experiments (after subtracting adhesion to BSA).P < .05 when comparing adhesion with and without Mn2+/thrombin.
To test the effect of various agents, platelets were preincubated (15 minutes at 37°C) with the indicated agent before application onto the precoated wells. All experiments were performed in triplicates and repeated at least 3 times.
Adhesion of melanoma cells
Adhesion of the transfected human melanoma cells was carried out as described earlier with modifications. Cells were detached by minimal trypsinization (Trypsin 0.25%/EDTA 0.05%, 1 to 2 minutes), washed, and resuspended in serum-free Dulbecco modified Eagle medium supplemented with 1 mg/mL BSA. Cell suspension (100 μL containing 1 × 105 cells) was added to wells of 96-well titer plates precoated with each of the adhesive proteins, BSA, SAA, and fibronectin and was allowed to adhere for 1 hour at 37°C. Nonadherent cells were rinsed off with phosphate-buffered saline (PBS) containing CaCl2 2 mM, MgCl2 1 mM (warmed to 37°C), and adherent cells were fixed and stained in 2% toluidine blue/4% paraformaldehyde (30 minutes at 37°C). Wells were then rinsed in tap water. Stained cells were solubilized in 100μL 1% SDS, and absorbance of the solution was measured at 595 nm in microplate reader. Experiments were performed in triplicates and repeated at least 3 times. When indicated, cells were preincubated (15 minutes at 37°C) with 2 mM MnCl2 and/or anti-αIIbβ3 antibody (c7E3Fab), and then applied onto the precoated titer plate wells.
Western blot analysis
Recombinant human SAA and C5a (control) proteins were loaded onto 15% SDS-polyacrylamide gel and run at reduced conditions. After electrophoretic separation, the proteins were transferred to nitrocellulose membrane. The membrane was probed with anti-SAA monoclonal antibody (clone mc29), treated with horseradish peroxidase–conjugated goat anti–mouse IgG, and developed with Chemiluminescence Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA).
Flow cytometry analysis
Transfected human melanoma cells were stained for flow cytometry by using standard indirect procedure. Briefly, cells were detached by minimal trypsinization, washed, and adjusted to 1.5 × 106 cells/50 μL PBS containing CaCl22 mM, MgCl2 1 mM, and MnCl2 2 mM. Fifty-microliter cell suspensions were incubated (60 minutes at 4°C) with anti-αIIb/CD41 (clone 5B12), anti-αVβ3 (clone LM609), or control isotype-matched mouse IgG. Cells were then washed and stained with FITC-conjugated goat anti–mouse IgG. The samples were then washed, fixed in 1% formaldehyde, and analyzed in EPICS XL-MCL flow cytometer with System II (Beckman Coulter, Miami, FL) and EXPO32 software programs (Applied Cytometry Systems, Sheffield, United Kingdom). In experiments detecting the binding of soluble SAA, 50-μL cell suspensions were incubated (60 minutes at 37°C) with SAA or control BSA (each at 200 μg/mL). Suspensions were then washed and incubated with a mixture of anti-SAA antibodies (clones mc29 and mcl) or control mouse IgG, and the assay proceeded as above.
Statistical analysis
Unless otherwise indicated, data are presented as mean ± SEM from at least 3 independent experiments. Statistical significance was calculated by using the paired t test.
Results
Human washed platelets adhere to immobilized SAA
Human washed platelets were prepared by standard procedure24 and tested for their adhesion to immobilized proteins by using the microtiter plate adhesion assay.25,27 BSA served as a negative control for background adhesion, whereas fibronectin and fibrinogen served as positive controls. The number of platelets that adhered to the coated wells was quantified by using the acid phosphatase assay27in which 1 optical density (absorbance at 405 nm) represents approximately 2 × 106 platelets (Figure 1A). Significant adhesion of platelets to SAA was observed; the level of adhesion to SAA was comparable to fibronectin and lower than to fibrinogen (Figure 1B). The number of adherent platelets to all 3 adhesive substrates was proportional to the number of platelets added to the coated wells and was similar to the number reported by others.28 Adhesion to SAA was further enhanced by Mn2+ ions (1 mM) or the physiologic agonist thrombin (1 U/mL) (Figure 1C). Mn2+ at lower concentration was less effective in enhancing platelet adhesion to SAA (3.8-, 1.7-, and 0.8-fold enhancement for 1, 0.5, and 0.1 mM Mn2+, respectively [representative experiment]). Adhesion was not enhanced by treatment with 10 μM adenosine diphosphate (102% ± 8.2% compared with nontreated platelets [defined as 100%]). Platelets prepared by the Mustard method and resuspended in Tyrode buffer without apyrase become insensitive to adenosine diphosphate, as previously reported.24
Specificity of platelet adhesion to SAA
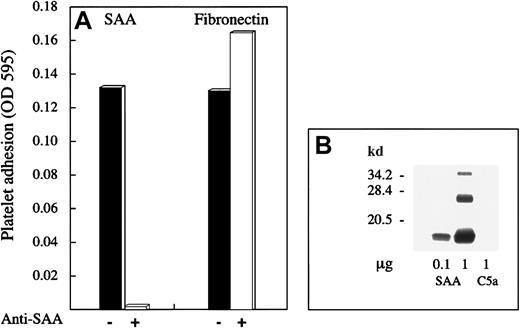
The specificity of platelet adhesion to SAA was studied using various approaches. In the first approach, SAA-coated wells preincubated with monoclonal anti-SAA antibody, mc29 (500 μg/mL for 1 hour) failed to support platelet adhesion. However, platelet adhesion to fibronectin-coated wells, similarly pretreated with mc29, was not inhibited (Figure 2A). The specificity of mc29 antibody19 20 was confirmed by Western blot analysis, demonstrating its recognition of SAA (more then 90% of the recombinant SAA consisted of a SAA monomer of expected size 12.5 kDa) but not of C5a, an unrelated protein of similar size (Figure 2B).
Effect of anti-SAA antibody on platelet adhesion to SAA.
(A) SAA- and fibronectin-coated microtiter plate wells were preincubated with monoclonal anti-SAA antibody mc29 (500 μg/mL) for 1 hour, after which washed platelets were added and adhesion assay was performed, as described. One representative experiment of 2 performed is shown. (B) SAA (0.1 μg and 1 μg) and C5a (1 μg) were subjected to Western blot analysis by using mc29 antibody. C5a is an unrelated protein that is not recognized by this antibody.
Effect of anti-SAA antibody on platelet adhesion to SAA.
(A) SAA- and fibronectin-coated microtiter plate wells were preincubated with monoclonal anti-SAA antibody mc29 (500 μg/mL) for 1 hour, after which washed platelets were added and adhesion assay was performed, as described. One representative experiment of 2 performed is shown. (B) SAA (0.1 μg and 1 μg) and C5a (1 μg) were subjected to Western blot analysis by using mc29 antibody. C5a is an unrelated protein that is not recognized by this antibody.
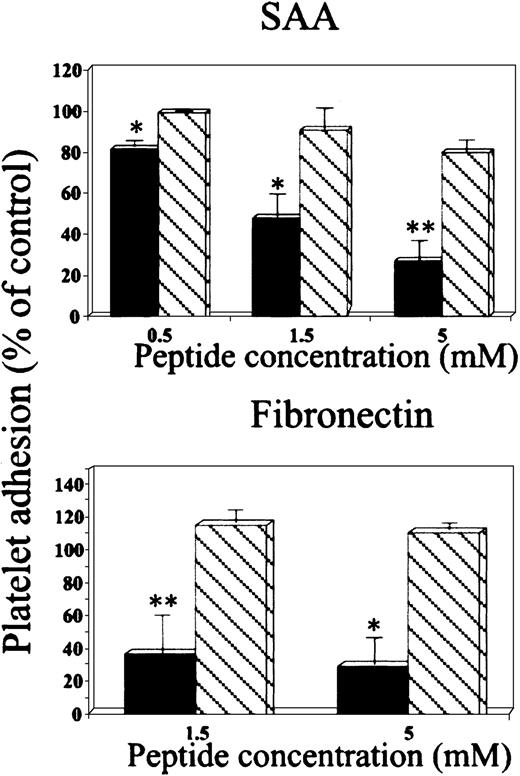
In the second approach, the peptide GRGDSP inhibited platelet adhesion to SAA in a dose-dependent manner, whereas the peptide GRGESP had no significant effect (Figure 3, upper panel). Furthermore, these peptides had similar differential effect on platelet adhesion to the control adhesion protein, fibronectin (Figure 3, lower panel). Additional control peptides, scrambled RGD (DGPSGR) and reverse RGD (PSDGRG), had no significant inhibitory effect on platelet adhesion to SAA (89% ± 7.2% and 96% ± 7.6% of control adhesion, respectively) or to fibronectin (90.6% ± 9.6% and 115% ± 7.3% of control adhesion, respectively).
Effect of RGD peptides on platelet adhesion to SAA.
Platelets were preincubated (15 minutes at 37°C) with peptides GRGDSP (▪) or GRGESP (▧) at the indicated concentrations and then added to BSA-, SAA- or fibronectin-coated wells. The results are expressed as a mean ± 1 SEM for 3 experiments, each designed with triplicates for each peptide concentration. In each experiment, 100% adhesion (control) is the adhesion achieved in the absence of the tested peptide after subtracting adhesion to BSA. *P < .05, **P < .01 when comparing adhesion with and without the tested peptide.
Effect of RGD peptides on platelet adhesion to SAA.
Platelets were preincubated (15 minutes at 37°C) with peptides GRGDSP (▪) or GRGESP (▧) at the indicated concentrations and then added to BSA-, SAA- or fibronectin-coated wells. The results are expressed as a mean ± 1 SEM for 3 experiments, each designed with triplicates for each peptide concentration. In each experiment, 100% adhesion (control) is the adhesion achieved in the absence of the tested peptide after subtracting adhesion to BSA. *P < .05, **P < .01 when comparing adhesion with and without the tested peptide.
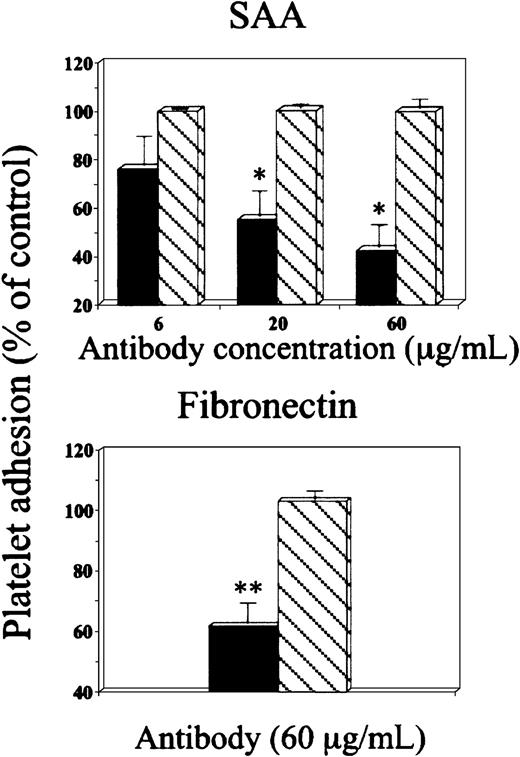
In the third approach, antibody recognizing the platelet receptor αIIbβ3 (c7E3Fab)29 inhibited platelet adhesion to SAA in a dose-dependent manner, whereas control IgG had no significant effect (Figure 4, upper panel). This antibody had similar effect on platelet adhesion to fibronectin (Figure4, lower panel). In a separate set of experiments the inhibitory effect of this antibody on adhesion of Mn2+-treated platelets to SAA was compared with its effect on adhesion of untreated platelets and was found to be significantly greater (53.1% ± 9.2% versus 33.2% ±15.1%, respectively, P < .02). Because antibody c7E3Fab interacts with both αIIbβ3 and αVβ3 on platelets,30 a monoclonal antibody specific for αVβ3 (clone LM609) was tested and had no inhibitory effect on adhesion of platelets, whether untreated or Mn2+ treated, to SAA (not shown). Furthermore, 10E5, a monoclonal antibody specific for αIIbβ3, completely inhibited platelet adhesion to fibrinogen but was found less effective than antibody c7E3Fab in inhibiting adhesion to SAA. It partially inhibited adhesion of Mn2+-treated platelets (inhibition of 35% ± 8.5%, P < .05) and had no inhibitory effect on adhesion of nontreated platelets to SAA (not shown). The antibody 10E5 was found less potent than c7E3Fab in inhibiting other platelet functions.31
Effect of anti-αIIbβ3 antibody on platelet adhesion to SAA.
Platelets were preincubated with either anti-αIIbβ3 antibody (c7E3Fab) (▪) or control IgG (▧), at the indicated concentrations and then added to BSA-, SAA- or fibronectin-coated wells. For details see legend to Figure 3.
Effect of anti-αIIbβ3 antibody on platelet adhesion to SAA.
Platelets were preincubated with either anti-αIIbβ3 antibody (c7E3Fab) (▪) or control IgG (▧), at the indicated concentrations and then added to BSA-, SAA- or fibronectin-coated wells. For details see legend to Figure 3.
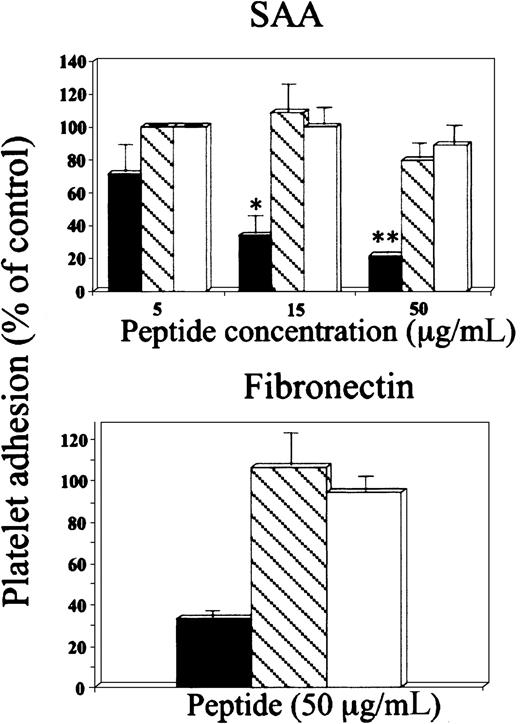
In the fourth approach, SAA-derived peptide 29 to 42 (YIGSDKYFHARGNY) inhibited platelet adhesion to SAA in a dose-dependent manner, whereas SAA peptides 28 to 40 (NYIGSDKYFHARG) and 83 to 104 (NEWGRSGKDPNHFRPAGLPEKY) had no significant effect (Figure 5, upper panel). Furthermore, SAA-derived peptide 29 to 42 also inhibited platelet adhesion to wells coated with the control adhesion protein, fibronectin (Figure 5, lower panel). Additional control peptides, scrambled 29 to 42 (NYAGRKFHYSGDYI), reverse 29 to 42 (YNGRAHFYKDSGIY), and peptide enclosing RGN (AARGNAA), had no significant inhibitory effect on platelet adhesion to SAA (88% ± 5%, 93.4% ± 6%, and 93.8% ± 7.3% of control adhesion, respectively) or to fibronectin (82.7% ± 9.2%, 96.7% ± 3.2%, and 93% ± 3.8% of control adhesion, respectively).
Effect of SAA-derived peptides on platelet adhesion to SAA.
Platelets were preincubated with SAA-derived peptides 29 to 42 (YIGSDKYFHARGNY) (▪), 28 to 40 (NYIGSDKYFHARG) (▧), or 83 to 104 (NEWGRSGKDPNHFRPAGLPEKY), (■) at the indicated concentrations and then added to BSA-, SAA-, or fibronectin-coated wells. For details see legend to Figure 3.
Effect of SAA-derived peptides on platelet adhesion to SAA.
Platelets were preincubated with SAA-derived peptides 29 to 42 (YIGSDKYFHARGNY) (▪), 28 to 40 (NYIGSDKYFHARG) (▧), or 83 to 104 (NEWGRSGKDPNHFRPAGLPEKY), (■) at the indicated concentrations and then added to BSA-, SAA-, or fibronectin-coated wells. For details see legend to Figure 3.
Adhesion and flow cytometry of human melanoma cells expressing platelet receptors
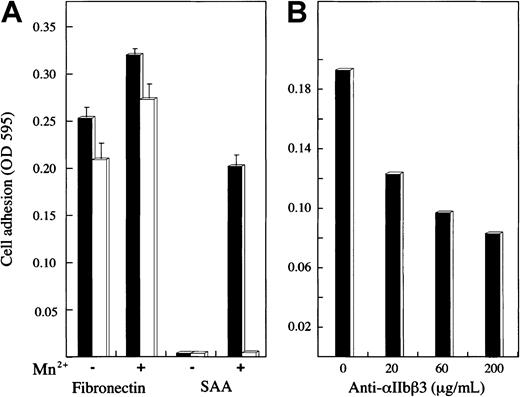
Adhesion of 2 transfected human melanoma cell lines expressing either the αIIbβ3 or αVβ3 integrin receptor on their surface23 was studied (Figure6). The control protein, fibronectin, supported the adhesion of both cell lines, and the adhesion was somewhat enhanced by Mn2+. SAA supported the adhesion of αIIbβ3-expressing cells but not αVβ3-expressing cells; the adhesion of αIIbβ3-expressing cells was dependent on Mn2+ (Figure 6A). The adhesion of αIIbβ3-expressing cells to SAA (in the presence of Mn2+) was inhibited, in a dose-dependent manner, by the anti-αIIbβ3 antibody, c7E3Fab (Figure 6B).
Adhesion of human melanoma cells expressing platelet integrin receptors.
(A) Two transfected human melanoma cell lines expressing integrin receptor αIIbβ3 (▪) or αVβ3 (■) were added to SAA-, fibronectin-, or BSA-coated wells and tested for their adhesion as described. Before addition onto the wells, cells were preincubated (15 minutes at 37°C) with or without Mn2+ (2 mM). The results are expressed, after subtracting adhesion to BSA, as a mean ± 1 SEM for 3 experiments. (B) The αIIbβ3 cells were added to SAA-coated wells after preincubation with Mn2+ and anti-αIIbβ3 antibody (c7E3Fab) at the indicated concentrations and tested for their adhesion. One representative experiment of 2 performed is shown.
Adhesion of human melanoma cells expressing platelet integrin receptors.
(A) Two transfected human melanoma cell lines expressing integrin receptor αIIbβ3 (▪) or αVβ3 (■) were added to SAA-, fibronectin-, or BSA-coated wells and tested for their adhesion as described. Before addition onto the wells, cells were preincubated (15 minutes at 37°C) with or without Mn2+ (2 mM). The results are expressed, after subtracting adhesion to BSA, as a mean ± 1 SEM for 3 experiments. (B) The αIIbβ3 cells were added to SAA-coated wells after preincubation with Mn2+ and anti-αIIbβ3 antibody (c7E3Fab) at the indicated concentrations and tested for their adhesion. One representative experiment of 2 performed is shown.
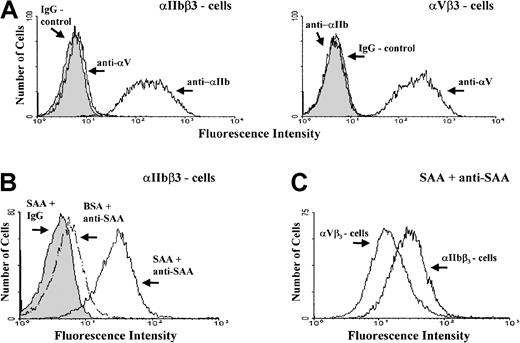
The surface expression of either αIIbβ3 or αVβ3 receptors on the studied cells was confirmed by flow cytometry (Figure7A). Furthermore, flow cytometry revealed that αIIbβ3-expressing cells bind soluble SAA (Figure 7B) at higher levels compared with the αVβ3-expressing cells (Figure 7C).
Flow cytometry of human melanoma cells expressing platelet integrin receptors.
(A) Flow cytometry of cells expressing integrin αIIbβ3 (left panel) or αVβ3 (right panel) after incubation with either one of the antibodies, anti-αIIb (clone 5B12), anti-αVβ3 (clone LM609), or control IgG. (B) Flow cytometry of αIIbβ3-expressing cells after incubation with SAA or control BSA followed by staining with a mixture of anti-SAA antibodies (clones mc29 and mc1) or control IgG. (C) Flow cytometry of αIIbβ3- and αVβ3-expressing cells, after incubation with SAA followed by staining with anti-SAA antibodies. Controls for αVβ3 cells were done as described in Figure B (not shown). One representative experiment of 3 performed is shown.
Flow cytometry of human melanoma cells expressing platelet integrin receptors.
(A) Flow cytometry of cells expressing integrin αIIbβ3 (left panel) or αVβ3 (right panel) after incubation with either one of the antibodies, anti-αIIb (clone 5B12), anti-αVβ3 (clone LM609), or control IgG. (B) Flow cytometry of αIIbβ3-expressing cells after incubation with SAA or control BSA followed by staining with a mixture of anti-SAA antibodies (clones mc29 and mc1) or control IgG. (C) Flow cytometry of αIIbβ3- and αVβ3-expressing cells, after incubation with SAA followed by staining with anti-SAA antibodies. Controls for αVβ3 cells were done as described in Figure B (not shown). One representative experiment of 3 performed is shown.
Discussion
SAA is an acute phase plasma protein, and its level in the blood is elevated to 1000-fold in response of the body to various insults, including tissue injury and inflammation. SAA is an ECM-bound protein12,13 that induces migration and adhesion of human leukocytes,10-12 and its derived peptides inhibit adhesion of leukocytes to ECM proteins.16 Because SAA expression was found in atherosclerotic lesions, including endothelial cells at the luminal sites of the vessels, it was postulated that SAA may modulate platelet aggregation or adhesion at the endothelial cell surface, thereby controlling platelet activity at vascular injury sites.3 Indeed, SAA was reported to inhibit thrombin-induced platelet aggregation9; however, its role in platelet adhesion was not studied. Here we describe that immobilized SAA supports the adhesion of human platelets and its derived peptide inhibits platelet adhesion to the subendothelial ECM protein, fibronectin. These findings identify a novel adhesive ligand for platelets and implicate its role in platelet adhesion thereby in hemostasis and thrombosis.
The specificity of platelet adhesion to SAA is supported by the following findings. First, the level of adhesion to SAA was comparable to other platelet adhesive proteins, ie, fibronectin and fibrinogen. Furthermore, the physiologic agonist thrombin, as well as the divalent ion Mn2+ (an artificial stimulus that mimics physiologic agonists), could enhance platelet adhesion to SAA. The enhancement of platelet adhesion by these 2 agents is well documented and may result from an increase in the binding affinity of the platelet receptor(s) toward SAA, as reported for other adhesive proteins.28 32-34
Second, anti-SAA antibody completely inhibited SAA-induced platelet adhesion and had no inhibitory effect on fibronectin-induced adhesion. This antibody (clone mc29) is likely to bind to a functionally relevant epitope of SAA, because it was prepared against the invariant segment of SAA containing the RGD-like motif.19 20
Third, the peptide GRGDSP but not GRGESP inhibited platelet adhesion to SAA and to fibronectin, suggesting that the adhesion to both proteins is RGD dependent. The differential inhibitory effect of these 2 peptides on platelet adhesion to fibronectin, as well as other adhesive proteins including vitronectin and fibrinogen, is well documented.35 36
Fourth, antibody recognizing the platelet receptor αIIbβ3 (c7E3Fab) inhibited platelet adhesion to SAA and to fibronectin. Thus, αIIbβ3 may function as a platelet receptor for SAA as it does for fibronectin, fibrinogen, vitronectin, and von Willebrand factor.17 High concentrations of c7E3Fab did not completely block platelet adhesion to SAA or fibronectin, suggesting the involvement of additional adhesion receptor(s). Although the adhesion of platelets to fibronectin is probably mediated by both αIIbβ3 and α5β1,17 the identity of other receptor (s) involved in platelet adhesion to SAA is not yet known. However, platelet adhesion to SAA is not mediated by the receptor αVβ3, because the anti-αVβ3 antibody LM609 had no inhibitory effect. Finally, the receptor αIIbβ3 may be more important in supporting adhesion of activated platelets to SAA, as compared with unactivated platelets, because anti-αIIbβ3 antibodies (c7E3Fab and 10E5) were more effective in inhibiting adhesion of Mn2+-treated platelets.
Fifth, the SAA-derived peptide 29 to 42, containing an RGD-like motif (aspartic acid [D] in the typical RGD motif is substituted by asparagine [N]), inhibited platelet adhesion to SAA and to fibronectin; the peptides 28 to 40, containing incomplete RGD, and 83 to 104, the C-terminus of the protein lacking adhesion motif, had no effect. Although the aspartic acid is very important for interactions with integrins, the asparagine, a noncharged amino acid, is isosteric and capable of establishing efficient interactions, ie, by hydrogen bonding. Furthermore, the presence of the RGD-like motif in SAA at the turn of an assumed β-sheet8 suggests structural similarity to the RGD ligand-recognition motif of integrins.18 Finally, the peptides 29 to 42 and YIGSD and RGNY, which are included in it, were reported to inhibit adhesion of T lymphocytes to laminin and fibronectin.16 According to these findings, it was suggested that proteolysis of SAA occurring at inflammatory sites by lysosomal enzymes of polymorphonuclear origin may yield potent peptides inhibiting lymphocyte adhesion to subendothelial ECM proteins.16 Whether this suggestion applies for the adhesion of platelets to subendothelial ECM proteins requires further investigation.
The role of αIIbβ3 integrin in the adhesion of human platelets to SAA was further supported by examining the adhesion of 2 human melanoma cell lines transfected to express either αIIbβ3 or αVβ3 integrin on their surface.23 Although fibronectin supported the adhesion of both cell lines, SAA supported the adhesion of αIIbβ3-expressing cells only. Adhesion was Mn2+dependent and could be inhibited by anti-αIIbβ3 antibody. The αIIbβ3-expressing cells interact not only with immobilized SAA but also with soluble SAA and bind SAA at higher levels compared with αVβ3-expressing cells as revealed by flow cytometry. Although soluble SAA also binds to cells lacking αIIbβ3, ie, αVβ3 cells, this binding is not sufficient to induce adhesion to immobilized SAA. These results give further support to the suggestion that αIIbβ3, but not αVβ3, is a receptor associated with adhesion of platelets and nucleated cells to SAA.
In conclusion, our data indicate that human platelets specifically adhere to SAA in an RGD- and αIIbβ3-dependent manner. Furthermore, SAA-derived peptide inhibits platelet adhesion to SAA and to the subendothelial ECM protein fibronectin. Thus, SAA may have a role in modulating platelet adhesion at vascular injury sites by sharing platelet receptors with other platelet-adhesive proteins. Further investigation is required to elucidate the physiologic relevance of our findings in hemostasis and thrombosis.
Dr Yaacov Matzner tragically passed away in a plane crash on Saturday, November 24, 2001, returning home from a scientific meeting in Germany; his many contributions to hematology will be remembered, and his presence in our community will be greatly missed.—Ed
We thank Dr Mark H. Ginsberg, The Scripps Research Institute, for the transfected human melanoma cells and Dr Barry S. Coller, Mount Sinai School of Medicine, for the 10E5 antibodies.
Yaacov Matzner died on November 24, 2001.
Supported by the Israel Cancer Research Fund, the Israel Science Foundation (grant 686/00-1), the Israel Cancer Association, the Israel Ministry of Absorption, and the Deutsche Forschungsgemeinschaft, Bonn, Germany (grant Li 247/12-3).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Simcha Urieli-Shoval, Hematology Unit, Hadassah University Hospital, Mount Scopus, Jerusalem 91240, Israel; e-mail:matzner@cc.huji.ac.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal