Enforced expression of c-mpl in embryonic stem (ES) cells inactivated for this gene results in protein expression in all the ES cell progeny, producing cells that do not belong to the megakaryocytic lineage and are responsive to PEG-rhuMGDF, a truncated form of human thrombopoietin (TPO) conjugated to polyethylene glycol. These include a primitive cell called BL-CFC, thought to represent the equivalent of the hemangioblast, and all myeloid progenitor cells. In this model, PEG-rhuMGDF was able to potentiate the stimulating effects of other growth factors, including vascular endothelial growth factor, on BL-CFC and a combination of cytokines on the growth of granulocyte macrophage–colony-forming units. The importance of the C-terminal domain of Mpl and of mitogen-activated protein kinase (MAPK) activation in TPO-dependent megakaryocytic differentiation has been well studied in vitro. Here, the role of this domain and the involvement of MAPK in upstream and nonmegakaryocytic cells are examined by using 2 truncated mutants of Mpl (Δ34, deletion of residues 71 to 121 in the C-terminal domain; and Δ3, deletion of residues 71-94) and specific inhibitors of the MAPK pathway. The 2 deleted regions support different functions, mediated by different signals. Residues 71 to 121 were required for PEG-rhuMGDF–dependent growth of BL-CFC, for megakaryocytic and other myeloid progenitors, and for megakaryocyte polyploidization. These responses were mediated by the ERK1–ERK2 MAPK pathway. In contrast, the only function of the sequence comprising residues 71 to 94 was to mediate the synergistic effects of PEG-rhuMGDF with other hematopoietic growth factors. This function is not mediated by MAPK activation.
Introduction
Thrombopoietin (TPO), also termed Mpl ligand (Mpl-L) or megakaryocyte growth and development factor (MGDF), is a specific regulator of megakaryocytopoiesis.1-3 However, TPO, in synergy with interleukin-3 (IL-3), Steel factor (KL), and Flt-3 ligand (FL), has a pleiotropic role in hematopoiesis,4-9 including regulation of the hematopoietic stem cell compartment.10
Mpl belongs to the cytokine receptor superfamily.11-14 The cytoplasmic domain of Mpl is 121 amino acids long, and there is no recognizable kinase domain or enzymatic motif in this region.13 On ligand binding, however, Mpl transmits biochemical signals such as activation of the Janus family of protein tyrosine kinases (JAKs), which in turn phosphorylate Mpl tyrosine residues. This is followed by the activation of other target molecules, including members of the latent transcription factor family named signal transducers and activators of transcription (STATs), phosphatidyl-inositol 3-kinase, Shc, mitogen-activated protein kinases (MAPK), and proto-oncogenes such as Cbl and Vav.15-26
Various cell lines have been engineered to express murine or human c-mpl on their surfaces, and they respond to TPO by proliferating, differentiating, or both. Analysis of mutations in the cytoplasmic domain of Mpl in these cell lines has shown that the conserved membrane-proximal box1 and box2 domains are required for TPO-induced phosphorylation of JAK2 and activation of STAT5 and for TPO-induced megakaryocytic proliferation and differentiation.15,16,23 In the C-terminal domain, the tyrosine residue (Y) 112 is necessary for activation of the MAPK pathway through Shc activation.27 Activation of the MAPK pathway is required for TPO-induced megakaryocytic cell line differentiation.15,16,24,25,28 In the multifactorial in vivo context, mice lacking the C-terminal 60 amino acids of Mpl have normal platelet and megakaryocyte numbers, such as the wild type, but their megakaryocytes display a defective response to TPO in vitro and in vivo.29 In addition, these megakaryocytes displayed a low ploidy on TPO treatment,29 in agreement with the decreased megakaryocyte ploidy in cultures containing MAPK inhibitors.30
MAPKs, also known as extracellular regulated kinases (ERK), are a class of serine–threonine kinases that are activated by many cytokines. They are key regulators of cell proliferation and differentiation in numerous cell types.28,31 32 A common pathway leading from cell surface receptors to ERKs involves p21ras, which activates c-Raf protein kinase. c-Raf phosphorylates 2 MAPK kinases (MEK1 and MEK2), which in turn phosphorylate ERK1 and ERK2 (p44MAPK and p42MAPK), respectively. On activation, ERKs translocate to the nucleus and phosphorylate transcription factors such as Elk-1.
Embryonic stem (ES) cells are a good model for studying the consequences of genetic manipulations on hematopoiesis in vitro. They are nontransformed cell lines in which targeted mutations can be created readily, and they can differentiate in vitro to hematopoietic cells. Several methods have been proposed for the generation of hematopoietic cells from ES cells. In this work we used a 2-step method that allows direct evaluation of the ability of ES cells to generate hematopoietic progenitors33 and a technique34 that gives access to a primitive cell called blast colony-forming cells (BL-CFCs). BL-CFCs are able to form blast cell colonies composed of hematopoietic and endothelial cells. These cells may represent the hemangioblast, the long-suspected common progenitor of the hematopoietic and endothelial lineages.35 Thus, ES cell hematopoietic differentiation allows us to study the commitment process of BL-CFCs to hematopoietic and endothelial tissues in vitro and the mechanisms of regulation of myeloid lineage differentiation and maturation.
In theory, enforced expression of genes in ES cells leads to protein expression in all ES-derived cell types. In a previous paper,36 we show that wild-type ES cells do not respond to PEG-rhuMGDF (a truncated form of human TPO conjugated to polyethylene glycol), the generation of megakaryocytic progenitors and the formation of platelets excepted. Enforced expression of full-length c-mpl in ES cells inactivated for c-mpl resulted in PEG-rhuMGDF–dependent responses of ES cell progeny, namely, the development of all myeloid progenitors and the maturation of megakaryocytic and other myeloid lineages. Interestingly, blast cell colony development from BL-CFCs, which normally depends strictly on vascular endothelial growth factor (VEGF), also occurred in the presence of PEG-rhuMGDF. Here, we showed that a synergistic effect of PEG-rhuMGDF on the VEGF-dependent blast cell colony and on the growth factor–dependent granulocyte macrophage progenitor development also exists, and we investigated the signaling pathway involved in these responses. Using Mpl mutants with truncated cytoplasmic domains and specific inhibitors of the MAPK pathway, we examined the role of MAPK activation in PEG-rhuMGDF–dependent development of ES cell–derived hematopoietic cells, extending from the hemangioblast to myeloid mature cells. We show that the MAPK pathway is required not only for megakaryocytic and all other myeloid progenitor cell development but also for BL-CFC development. In contrast, this pathway is not involved in the synergistic effects of PEG-rhuMGDF with VEGF or a combination of cytokines.
Materials and methods
Cells
The 129/SV-derived wild-type D3 ES cell line (mpl+/+) and a subclone of the D3 cell line inactivated for the c-mpl gene (mpl−/−) have been described.37
Growth factors, antibodies, and reagents
Murine recombinant leukemia inhibitory factor was purchased from Life Technologies (Cergy-Pontoise, France). Human recombinant IL-1α and murine recombinant IL-3 were obtained from Immunex (Seattle, WA). KL and murine IL-6 were obtained from Valbiotech (Paris, France) and R&D Systems (Oxon, United Kingdom), respectively. Human granulocyte–colony-stimulating factor (G-CSF) and a pegylated form of human thrombopoietin (PEG-rhuMGDF) were donated by Amgen-Roche (Neuilly sur Seine, France) and Kirin-Brewery (Tokyo, Japan). Human erythropoietin (EPO) was a gift from Cilag (Levallois-Perret, France).
Fluorescein isothiocyanate (FITC)–anti-CD41 (gpIIb) was obtained from PharMingen (San Diego, CA). Monoclonal antibody specific for the Flag epitope tag sequence (M1) was purchased from Eastman Kodak Company (New Haven, CT). The second-step reagent was FITC-conjugated goat F(ab′)2 fragment, specific for mouse immunoglobulin (Ig)G (Silenius, Hawthorn, Australia). Various purified IgG was used as irrelevant control antibodies: anti–mouse IgG1 (PharMingen) and purified mouse IgG2b (DAKO, Glostrup, Denmark). Propidium iodide was from Sigma (Saint-Quentin Fallavier, France).
Specific MEK inhibitors, PD98059 and U0126, were purchased from Promega France (Lyon, France). Stock solutions were prepared in dimethyl sulfoxide (DMSO; Sigma) at 20 mM for PD98059 and 10 mM for U0126.
Plasmid constructions
Full-length murine c-mpl (flmpl) and 2 mutant cDNAs, inserted into the retrovirus expression vector pBabepuro, have been previously described.23 Mutant Δ3 (Δ3mpl) lacks residues 576 to 599, and mutant Δ34 (Δ34mpl) lacks residues 576 to 622 (Figure 1A). All constructs were transferred to the EcoRI site of the expression vector pEF-BOS,38 containing the promoter of the human elongation factor hEF-1α.
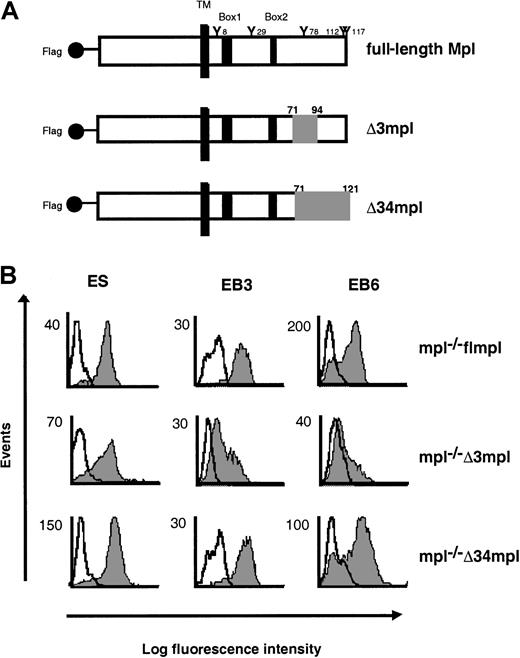
Deletions from the cytoplasmic domain of Mpl.
(A) Schematic representation of the full-length c-mpl(flmpl) and 2 deletion mutants (Δ3mpl, Δ34mpl). The intracellular domain of flmpl contains 121 amino acids, including conserved box1 and box2 motifs (black area) and 5 tyrosine residues. Deleted regions are shown in gray. TM, transmembrane domain. The flmpl and the mutants have the Flag epitope tag sequence at the N-terminus. (B) Flow cytometry analysis of the expression of Flag-Mpl or Flag-mutants in undifferentiated ES cells and during their differentiation. Cells were stained with the M1 anti-Flag antibody (gray peak) or isotype control antibody (open peak). Second-step antibody was FITC-conjugated goat F(ab′)2 antimouse immunoglobulins.
Deletions from the cytoplasmic domain of Mpl.
(A) Schematic representation of the full-length c-mpl(flmpl) and 2 deletion mutants (Δ3mpl, Δ34mpl). The intracellular domain of flmpl contains 121 amino acids, including conserved box1 and box2 motifs (black area) and 5 tyrosine residues. Deleted regions are shown in gray. TM, transmembrane domain. The flmpl and the mutants have the Flag epitope tag sequence at the N-terminus. (B) Flow cytometry analysis of the expression of Flag-Mpl or Flag-mutants in undifferentiated ES cells and during their differentiation. Cells were stained with the M1 anti-Flag antibody (gray peak) or isotype control antibody (open peak). Second-step antibody was FITC-conjugated goat F(ab′)2 antimouse immunoglobulins.
Embryonic stem cell electroporation and selection of transfected cells
Mpl−/− ES cells were maintained in an undifferentiated state and were electroporated as previously described.36 Three days after electroporation, cells expressing Flag were sorted by flow cytometry (FACS-Vantage; Becton Dickinson) to be enriched in transfected cells as previously described.36 A second round of sorting was performed 15 days later to select stable pools of transfected mpl−/−ES cells. In some cases, a third round was necessary to obtain cell populations with purity greater than 80%. Each type of transfectant (mpl−/− flmpl, mpl−/− Δ3mpl, and mpl−/− Δ34mpl) was expanded and frozen.
Embryonic stem cell differentiation
To study the responses of myeloid progenitors to various cytokines, we used the 2-step method described by Keller et al.33 Embryoid bodies (EBs) were formed from ES cells, and their progenitor content was determined in the presence of various combinations of cytokines: 6 growth factors (6GF) (5 U/mL EPO + 5 U/mL KL + 1000 U/mL IL-1α + 50 U/mL IL-3 + 10 ng/mL (nM) IL-6 + 10 ng/mL [nM] G-CSF) or 10 ng/mL (nM) PEG-rhuMGDF or 6GF+PEG-rhuMGDF.36 Myeloid colonies including megakaryocyte colony-forming units (CFU-MK), granulocyte macrophage CFUs (CFU-GM), erythroid burst-forming units (BFU-E), and mixed colonies (CFU-mix) were counted on day 7 of culture. Clones larger than 50 cells were scored as colonies except for CFU-MK, for which one unit consisted of at least a 3-megakaryocyte cluster.
We used the 3-step method described by Kennedy et al34 to study the development of BL-CFC. Three-day EBs (EBs3) were collected and plated as previously described with cytokines consisting of either 5 ng/mL (nM) VEGF or 10 ng/mL (nM) PEG-rhuMGDF or VEGF+PEG-rhuMGDF.36 Blast cell colonies were scored after 4 days of culture. Myeloid progenitors in day 4 blast cell colonies were assessed in the presence of 6GF.
To study the role of MAPK in the formation of ES cell–derived hematopoietic cells, EBs3 or EBs6 cells were incubated with various concentrations of the MEK inhibitor PD98059 or U0126 or with DMSO for 1 hour before cell stimulation. Blast cell colony and progenitor assays were subsequently assessed.
Maturation of hematopoietic cells
To study the terminal differentiation of ES cell–derived myeloid progenitors in the presence of PEG-rhuMGDF, day 4 blast cell colonies were cultured as previously described.36 After 5 days of culture, platelet numbers were quantified in triplicate, and megakaryocyte DNA content was determined by flow cytometry.
Flow cytometry analysis
To quantify platelets produced in the cultures, cells from one well were collected and incubated for 20 minutes with FITC–anti-CD41, and the samples were adjusted to a final volume of 300 μL. The acquisition rate was 1 μL/s for 60 seconds. Events were gated based on normal murine blood platelets and collected using a log-scale forward scatter and side scatter. Samples were analyzed with a FACSort flow cytometer (Becton Dickinson).
To examine the DNA content of megakaryocytes, cells were labeled with anti-FITC CD41 antibody in phosphate-buffered saline containing 5% fetal calf serum, 3 mM EDTA, 25 mM HEPES, 3.5% bovine serum albumin, and 8 μM prostaglandin 1 (Sigma), washed with the same buffer, and resuspended in 0.1% citrate sodium solution containing 50 μM propidium iodide and stored at 4°C for 18 hours. RNase (50 μg/mL [μM]) was added 10 minutes before analysis. Data were analyzed on a logarithmic scale by CellQuest software (Becton Dickinson).
To purify hematopoietic cells contained in mpl−/− flmpl, mpl−/− Δ3mpl, and mpl−/− Δ34mpl EBs6, EBs6 were collected and dissociated by trypsinization. After labeling with anti-Flag M1 antibody as previously described,36 the cells were incubated with phycoerythrin–anti-IgG2b and FITC–anti-CD41 for 20 minutes. After 2 washes, double-positive CD41+Flag+ cells were sorted with a FACS-Vantage.
Assay for MAPK activation
The total EBs6 cell population or CD41+Flag+ double-positive EBs6-sorted cells were deprived of PEG-rhuMGDF for several hours in cytokine-free medium and then were stimulated with 100 ng/mL (nM) PEG-rhuMGDF for various times at 37°C. Whole-cell lysates were prepared by resuspending cell pellets in Laemmli sample buffer (Tris 62.5 mM, pH 6.8, sodium dodecyl sulfate [SDS] 2%, glycerol 10%) and boiling them for 10 minutes. Proteins were separated on SDS–12% polyacrylamide gels and were transferred to nitrocellulose membranes. ERK activity was measured by probing the membranes with an activation-specific antibody that recognizes the dual phosphorylated forms of p42 ERK2 and p44 ERK1 (Promega, Madison, WI). Total ERK amounts were determined by reprobing the same membranes with an antibody recognizing ERK1 and ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA). Antibody binding was revealed with secondary antibodies coupled to horseradish peroxidase and the enhanced chemiluminescence system (Amersham).
Statistical methods
Data are presented as mean ± SEM. Student ttest was used for statistical analysis.
Results
Role of the C-terminal domain of Mpl in the response of embryonic stem cell–derived progenitors and BL-CFC to PEG-rhuMGDF
Response of progenitors to PEG-rhuMGDF.
The first step of ES cell hematopoietic differentiation is the formation of EBs. EBs6 contain all myeloid progenitors, identifiable by clonogenic assays. To investigate the signaling pathway involved in the response of ES cell–derived progenitors to PEG-rhuMGDF, we used ES cells inactivated for c-mpl (mpl−/−): the full-length and mutated forms of c-mpl were introduced into these cells, and we compared the response to PEG-rhuMGDF of ES cell–derived progenitors. Then mpl−/− ES cells were transfected by electroporation with constructs encoding the Flag full-length (mpl−/− flmpl) or 2 Flag mpl mutants of the intracellular domain of c-mpl (mpl−/− Δ3mpl and mpl−/− Δ34mpl) reported to affect PEG-rhuMGDF responses in cell lines.23 The Δ3 mutant (Δ3mpl) lacks amino acids 71 to 94. The Δ34 mutant (Δ34mpl) lacks the 51 C-terminal amino acids 71 to 121 (Figure 1A). Cell surface expression of flMpl and of the mutant proteins on ES cells and on EBs6 was verified by flow cytometry analysis after labeling with an antibody to the Flag epitope (Figure 1B). Eighty percent of ES cells expressed each of the various transgenes. On EBs6 cells, the Flag epitope was detected on 65% of cells transfected with flmpl and 70% of those transfected with Δ34mpl. In contrast, only 30% of the cells expressed Δ3mpl despite repeated transfections.
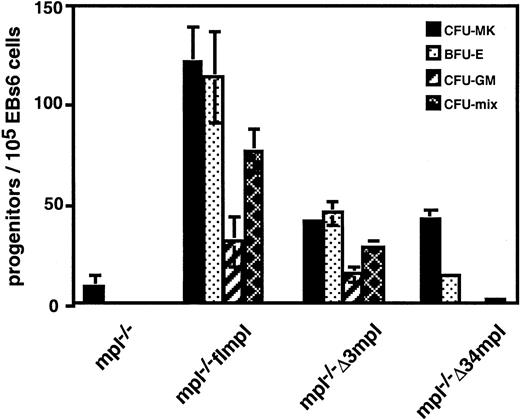
Few CFU-MK developed from mpl−/− ES cells in response to PEG-rhuMGDF (Figure 2). Reintroduction of flmpl into mpl−/− ES cells restored this response. It also allowed the differentiation of all types of myeloid progenitors (a total of 345 ± 21 per 105 EBs6 cells), whereas the wild type only gave rise to CFU-MK.36 EBs6 expressing Δ34mpl generated few progenitors (61 ± 3 per 105 EBs6 cells) (Figure 2), and these were significantly different from those generated by mpl−/−. They included CFU-MK and nonmegakaryocytic progenitors (BFU-E, CFU-mix). However, no CFU-GM developed. Hematopoietic colonies were smaller than those derived from mpl−/− flmpl (not shown). Thus, EBs6 cells expressing Δ34mpl were impaired in their capacity to generate progenitors in response to PEG-rhuMGDF. EBs6 cells expressing Δ3mpl gave rise to progenitors (134 ± 8 per 105 EBs6 cells). Although fewer than those obtained with flmpl, they included megakaryocytes and all types of nonmegakaryocyte colonies including CFU-GM (Figure 2). However, the Δ3mpl protein was expressed by a smaller proportion of EBs6 cells than flmpl, suggesting that the hematopoietic activity of the mutant Δ3 might have been underestimated. To overcome this problem, we decided to sort the Flag+ hematopoietic progenitors. Because all ES cell–derived myeloid progenitors express the CD41 antigen (Mitjavila, manuscript submitted), we sorted CD41+ Flag+ double-positive cells from mpl−/− Δ3mpl and mpl−/− flmpl EBs6 cells and then plated them for progenitor assays. Although the number of progenitors was diminished after sorting because of the use of a less efficient batch of methylcellulose in these experiments, there was no difference between the progenitor contents of these 2 cell populations (96 ± 2 and 105 ± 10 per 105 cells for Δ3mpl and flmpl, respectively). The signals transmitted by the region deleted from the mutant Δ3 (amino acids 71-94) do not appear to be required for progenitor development.
PEG-rhuMGDF–dependent ES cell–derived hematopoietic progenitors.
mpl−/−, mpl−/− flmpl, mpl−/−Δ3mpl, or mpl−/− Δ34mpl EBs6 were dissociated and replated for progenitor assay in the presence of PEG-rhuMGDF. Values represent the mean of duplicate dishes in 3 independent experiments.
PEG-rhuMGDF–dependent ES cell–derived hematopoietic progenitors.
mpl−/−, mpl−/− flmpl, mpl−/−Δ3mpl, or mpl−/− Δ34mpl EBs6 were dissociated and replated for progenitor assay in the presence of PEG-rhuMGDF. Values represent the mean of duplicate dishes in 3 independent experiments.
Response of BL-CFC to PEG-rhuMGDF.
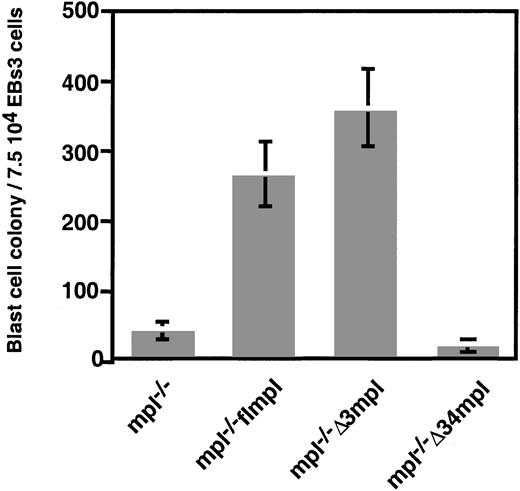
BL-CFC emerges in EBs after 3 days of differentiation. BL-CFC generates blast cell colonies in which hematopoietic progenitors develop concomitantly with endothelial cells.35 The growth of blast cell colonies is strictly dependent on the presence of VEGF.34 We demonstrated36 that the ubiquitous expression of flmpl allowed the growth of blast cell colonies in the presence of PEG-rhuMGDF. These colonies had hematopoietic and endothelial potential, similar to colonies grown with VEGF. The Δ34 deletion abolished the PEG-rhuMGDF–dependent formation of blast cell colonies (Figure 3), whereas they developed normally in the presence of VEGF (not shown). In contrast, the Δ3 mutant allowed the formation of a large number (361 ± 56) of blast cell colonies in the presence of PEG-rhuMGDF (Figure 3). Hematopoietic potential of these blast cell colonies was quantitatively and qualitatively similar to that of colonies developed in the presence of VEGF (Table 1). Endothelial potential of these colonies was also indistinguishable from that of colonies grown with VEGF (not shown). Although similar proportions of all types of EBs3-transfected cells expressed the Flag epitope (more than 60%), the fluorescent intensity of Δ3mpl cells was lower than the others (Figure 1B). To verify that the number of Δ3mpl-derived blast cell colonies was not underestimated, we sorted Flag+mpl−/− Δ3mpl and mpl−/− flmpl EBs3 cells and then plated them for blast cell colony assays in the presence of PEG-rhuMGDF. There was no difference between these 2 cell populations (91 ± 5 and 106 ± 6 colonies per 105 cells for Δ3mpl and flmpl, respectively). These findings demonstrate that the Δ34 region, but not the Δ3 region, is required for PEG-rhuMGDF signaling in BL-CFC development. Presumably, the amino acid sequence at positions 94 to 121 constitutes the C-terminal domain of Mpl required for the response of BL-CFC to PEG-rhuMGDF.
Mpl−/− flmpl ES cell–derived blast cell colonies develop in the presence of PEG-rhuMGDF.
The Δ3 region is dispensable for this effect. EBs3 derived from mpl−/−, mpl−/− flmpl, mpl−/−Δ3mpl, or mpl−/− Δ34mpl ES cells were dissociated and plated for blast cell colony assay in the absence of VEGF and in the presence of PEG-rhuMGDF. Values represent the mean of duplicate dishes in 3 independent experiments.
Mpl−/− flmpl ES cell–derived blast cell colonies develop in the presence of PEG-rhuMGDF.
The Δ3 region is dispensable for this effect. EBs3 derived from mpl−/−, mpl−/− flmpl, mpl−/−Δ3mpl, or mpl−/− Δ34mpl ES cells were dissociated and plated for blast cell colony assay in the absence of VEGF and in the presence of PEG-rhuMGDF. Values represent the mean of duplicate dishes in 3 independent experiments.
Hematopoietic potential of blast cell colonies grown in the presence of PEG-rhuMGDF alone
| Cells . | Stimuli . | CFU-MK . | BFU-E . | CFU-GM . | CFU-mixed . | Total . |
|---|---|---|---|---|---|---|
| mpl−/−flmpl | VEGF | 144 ± 25 | 32 ± 8 | 36 ± 9 | 11.3 ± 2.7 | 237 ± 20 |
| PEG-rhuMGDF | 127 ± 23 | 49 ± 20 | 37 ± 11 | 18.3 ± 7.3 | 231 ± 50 | |
| mpl−/−Δ3mpl | VEGF | 101 ± 16 | 66 ± 9 | 5.3 ± 2.0 | 20 ± 5.0 | 193 ± 30 |
| PEG-rhuMGDF | 87 ± 15 | 60 ± 14 | 14.3 ± 2.4 | 11.3 ± 2.3 | 172 ± 24 |
| Cells . | Stimuli . | CFU-MK . | BFU-E . | CFU-GM . | CFU-mixed . | Total . |
|---|---|---|---|---|---|---|
| mpl−/−flmpl | VEGF | 144 ± 25 | 32 ± 8 | 36 ± 9 | 11.3 ± 2.7 | 237 ± 20 |
| PEG-rhuMGDF | 127 ± 23 | 49 ± 20 | 37 ± 11 | 18.3 ± 7.3 | 231 ± 50 | |
| mpl−/−Δ3mpl | VEGF | 101 ± 16 | 66 ± 9 | 5.3 ± 2.0 | 20 ± 5.0 | 193 ± 30 |
| PEG-rhuMGDF | 87 ± 15 | 60 ± 14 | 14.3 ± 2.4 | 11.3 ± 2.3 | 172 ± 24 |
Mpl−/− flmpl and mpl−/−Δ3mpl-derived blast cell colonies grown in the presence of PEG-rhuMGDF were analyzed for their content in myeloid progenitors with a combination of 6GF and compared to that of blast cell colonies grown in the presence of VEGF. Results represent the mean number of progenitors per 105 cells of blast cell colonies (duplicate dishes in 3 independent experiments).
Role of the C-terminal domain of Mpl in the terminal hematopoietic differentiation of embryonic stem cells
In a previous paper,36 we show that mpl−/− flmpl ES cells can develop into mature megakaryocytic, erythroid, macrophagic, and granulocytic cell lineages in serum-free liquid cultures initiated from blast cell colonies in the presence of PEG-rhuMGDF. Here, we focused on consequences of the Δ3 and Δ34 deletions on megakaryocyte maturation. Figure4A illustrates megakaryocytes that developed from mpl−/− flmpl, mpl−/−Δ3mpl, and mpl−/− Δ34mpl ES cells in the presence of PEG-rhuMGDF.
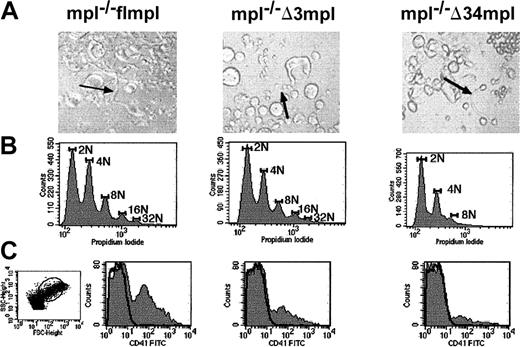
ES cell–derived megakaryocyte maturation.
Blast cell colony cells derived from mpl−/− flmpl, mpl−/− Δ3mpl, mpl−/− Δ34mpl ES cells were plated in serum-free liquid medium in the presence of PEG-rhuMGDF and were cultured for 5 days. (A) Megakaryocyte displaying proplatelets (arrows) (magnification × 200). (B) Ploidy analysis of megakaryocytes. After labeling with anti-CD41 antibody to identify megakaryocytes, cells were labeled with propidium iodide to evaluate DNA content. DNA content was scored on CD41+ cells. Images were made with Adobe Photoshop 5.0. (C) Flow cytometry quantification of platelets, defined as CD41+ elements with the same scatter properties as murine blood platelets (dot plot). SSC indicates side scatter; FSC, forward scatter.
ES cell–derived megakaryocyte maturation.
Blast cell colony cells derived from mpl−/− flmpl, mpl−/− Δ3mpl, mpl−/− Δ34mpl ES cells were plated in serum-free liquid medium in the presence of PEG-rhuMGDF and were cultured for 5 days. (A) Megakaryocyte displaying proplatelets (arrows) (magnification × 200). (B) Ploidy analysis of megakaryocytes. After labeling with anti-CD41 antibody to identify megakaryocytes, cells were labeled with propidium iodide to evaluate DNA content. DNA content was scored on CD41+ cells. Images were made with Adobe Photoshop 5.0. (C) Flow cytometry quantification of platelets, defined as CD41+ elements with the same scatter properties as murine blood platelets (dot plot). SSC indicates side scatter; FSC, forward scatter.
To examine ploidy distribution of megakaryocytes, cells were labeled with anti-CD41, which specifically labels megakaryocytes, and with propidium iodide to evaluate their DNA content. Like the wild type (not shown), mpl−/− flmpl ES cell–derived megakaryocytes displayed a maximum DNA content of 32N with 2 modal peaks at 2N and 4N (Figure 4B). Ploidy distribution was lower than that of adult mouse bone marrow–derived megakaryocytes in vitro (a maximum of 128N). This is probably because of the embryonic context of ES cell differentiation. Indeed, megakaryocytes derived from fetal liver or cord blood have lower ploidy than those derived from adult bone marrow,39 suggesting that there are differences in megakaryocyte ploidization dependent on the ontogenic stage of cells. The Δ3 deletion did not significantly change the ploidy distribution observed in mpl−/− flmpl megakaryocytes (Figure 4B). In contrast, the Δ34 deletion induced a reduction in DNA content with maximum at 8N and a modal peak at 2N (Figure 4B).
Cultures initiated from mpl−/− flmpl blast cell colonies in the presence of PEG-rhuMGDF gave rise to megakaryocytes bearing platelets (Figure 4A). CD41+ elements with the same scatter properties as blood platelets were produced (Figure 4C), suggesting that ES cell–derived megakaryocytes produce platelets. Because other cell lineages developed concomitantly with megakaryocytes in the cultures, as demonstrated in progenitor assays, we could not evaluate the number of CD41+ elements produced per megakaryocyte. The Δ3 and Δ34 deletions did not prevent the formation of megakaryocytes bearing proplatelets (Figure 4A) or the production of CD41+ elements (Figure 4C). The number of CD41+elements produced by mpl−/− Δ3mpl and mpl−/− Δ34mpl per 105 blast cell colony cells initiating the cultures was lower than that produced by mpl−/− flmpl (not shown). In conclusion, the C-terminal region of Mpl is not essential for the maturation of megakaryocytes to platelets, but it is necessary for optimal megakaryocyte polyploidization, as previously shown in other models.29The Δ3 region is not required for maturation or endomitosis of megakaryocytes, demonstrating that the amino acid region 94 to 121 is involved in polyploidization.
Role of the C-terminal domain of Mpl in the synergistic effect of PEG-rhuMGDF with VEGF or a combination of 6GF
Enforced expression of flmpl in ES cells resulted in a potentialization by PEG-rhuMGDF of the stimulating effect of a mixture of 6GF on the growth of CFU-GM (Table 2). In contrast, no potentiation was observed with mpl−/−Δ3mpl, and Δ34mpl EBs6-derived CFU-GM (Table 2). Because the Δ3mpl protein was expressed by a smaller proportion of EBs cells than fmpl and Δ34mpl, we decided to verify the absence of potentiation in Flag+-sorted Δ3mpl EBs6 cells. No synergistic effect of PEG-rhuMGDF with 6GF was observed: 52 ± 3, 61 ± 8, 133 ± 7 CFU-GM grew from 105 sorted Δ3 mpl cells cultured with 6GF, PEG-rhuMGDF, or 6GF+PEG-rhuMGDF, respectively, (NS) versus 35 ± 2, 99 ± 10, and 274 ± 16 (P < .05) for sorted flmpl cells grown in similar conditions, respectively). Therefore, the C-terminal region of Mpl, and the region deleted from the mutant Δ3 were necessary for the synergistic effect of PEG-rhuMGDF with 6GF.
Synergistic effect of PEG-rhuMGDF on the combination of 6GF-dependent growth of CFU-GM
| EBs6 cells . | 6GF . | Growth factors PEG-rhuMGDF . | 6GF + PEG-rhuMGDF . |
|---|---|---|---|
| mpl−/− flmpl | 37 ± 16 | 32 ± 12 | 204 ± 35* |
| mpl−/− Δ3mpl | 53 ± 13 | 16 ± 4 | 74 ± 7 |
| mpl−/− Δ34mpl | 24 ± 3 | 0 ± 0 | 28 ± 2 |
| EBs6 cells . | 6GF . | Growth factors PEG-rhuMGDF . | 6GF + PEG-rhuMGDF . |
|---|---|---|---|
| mpl−/− flmpl | 37 ± 16 | 32 ± 12 | 204 ± 35* |
| mpl−/− Δ3mpl | 53 ± 13 | 16 ± 4 | 74 ± 7 |
| mpl−/− Δ34mpl | 24 ± 3 | 0 ± 0 | 28 ± 2 |
Six-day EBs were dissociated and replated for progenitor assay in the presence of a combination of 6GF or PEG-rhuMGDF or 6GF + PEG-rhuMGDF. Data represent the mean of numbers of CFU-GM generated by 105 EBs6 cells (duplicate dishes in 3 independent experiments).
P < .01: comparison of the numbers of colonies developed with 6GF + PEG-rhuMGDF and the sum of the numbers of colonies developed with a combination of 6GF and PEG-rhuMGDF separately.
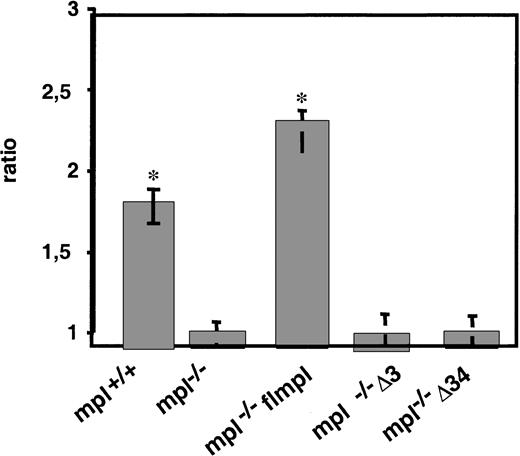
Similarly, the addition of PEG-rhuMGDF to VEGF in cultures of mpl−/− flmpl blast cell colonies resulted in a higher cloning efficiency of BL-CFCs than that in cultures with VEGF alone (ratio, 2.4 ± 0.1) (Figure5). Because the increase of blast cell colonies that developed with VEGF+PEG-rhuMGDF was higher that the sum of those that developed separately with PEG-rhuMGDF or VEGF, PEG-rhuMGDF and VEGF acted synergistically on the growth of blast cell colonies. Interestingly, a similar synergy was observed with wild-type mpl+/+ ES cells, indicating that mpl is naturally expressed in the wild-type BCFC. In contrast, no synergistic effect was observed with the mutants Δ3 or Δ34 (Figure 5). Therefore, the C-terminal region of Mpl and the region deleted from the mutant Δ3 were necessary for the synergistic effect of PEG-rhuMGDF with VEGF. Amino acid residues 71 to 94 are not required for all the other functions of PEG-rhuMGDF, suggesting that the only function of that sequence of the C-terminal domain of Mpl is to allow the synergistic effect of PEG-rhuMGDF with other growth factors on the growth of BL-CFCs and progenitor cells.
The Δ3 and Δ34 regions are necessary for the synergistic effect of PEG-rhuMGDF on the VEG-dependent growth of blast cell colonies.
EBs3 derived from mpl+/+, mpl−/−, mpl−/− flmpl, mpl−/− Δ3mpl, or mpl−/− Δ34mpl ES cells were dissociated and replated for blast cell colony assay with VEGF or VEGF+PEG-rhuMGDF. Values represent the ratio of the number of blast cell colonies generated with VEGF+PEG-rhuMGDF to the number of those with VEGF (mean of duplicate dishes in 3 independent experiments; *P < .05, comparison with mpl−/−).
The Δ3 and Δ34 regions are necessary for the synergistic effect of PEG-rhuMGDF on the VEG-dependent growth of blast cell colonies.
EBs3 derived from mpl+/+, mpl−/−, mpl−/− flmpl, mpl−/− Δ3mpl, or mpl−/− Δ34mpl ES cells were dissociated and replated for blast cell colony assay with VEGF or VEGF+PEG-rhuMGDF. Values represent the ratio of the number of blast cell colonies generated with VEGF+PEG-rhuMGDF to the number of those with VEGF (mean of duplicate dishes in 3 independent experiments; *P < .05, comparison with mpl−/−).
PEG-rhuMGDF activates MAPK in hematopoietic cells derived from mpl−/− flmpl and mpl−/− Δ3mpl, but not mpl−/− Δ34mpl ES cells
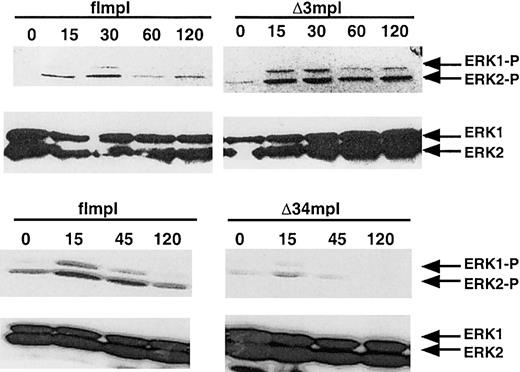
MAPK activation plays a key role in PEG-rhuMGDF–induced megakaryocytic differentiation in various cell lines and in normal progenitors.24,25,28,30,32 40-42 To determine whether the c-mpl mutations lead to impaired MAPK activation, we investigated ERK activation after PEG-rhuMGDF stimulation in hematopoietic cells derived from mpl−/− flmpl, mpl−/− Δ3mpl, and mpl−/− Δ34mpl ES cells. EBs6 are composed of hematopoietic and nonhematopoietic cells. To analyze ERK activation in the same proportion of PEG-rhuMGDF–responding hematopoietic cells, EBs6 cells were sorted into CD41+ and Flag+ cells. CD41+Flag+ EBs6 populations derived from mpl−/− flmpl, Δ3mpl, and Δ34mpl ES cells were stimulated with PEG-rhuMGDF for various times at 37°C, and ERK1–ERK2 activation was assessed in whole-cell lysates. PEG-rhuMGDF induced a transient activation of ERK1 and ERK2 in mpl−/− flmpl ES cell–derived hematopoietic cells. The Δ3 deletion did not prevent this activation, either in intensity or in duration (Figure6). Phosphorylation of ERK1–ERK2 was detected in hematopoietic cells derived from the mutant Δ34 after 15 minutes of PEG-rhuMGDF stimulation, though weak (Figure 6), but the cells contained normal amounts of ERK1 and ERK2 proteins. Thus, ERK1 and ERK2 are activated by PEG-rhuMGDF in ES cell–derived hematopoietic cells. These data suggest that most of the ERK activation can be attributed to signaling arising from the carboxyl terminus.
PEG-rhuMGDF–induced ERK activation in mpl−/− ES cells transfected with the full-length
c-mpl or the mutants Δ34 and Δ3. EBs6 cells were sorted for double Flag and CD41 expression. Whole-cell extracts were then analyzed by SDS-PAGE and Western blot analysis with phospho-specific anti-ERK1/2 or anti-ERK1/2 antibody. Images were made with Adobe Photoshop 5.0. (MGDF: PEGrhu MGDF).
PEG-rhuMGDF–induced ERK activation in mpl−/− ES cells transfected with the full-length
c-mpl or the mutants Δ34 and Δ3. EBs6 cells were sorted for double Flag and CD41 expression. Whole-cell extracts were then analyzed by SDS-PAGE and Western blot analysis with phospho-specific anti-ERK1/2 or anti-ERK1/2 antibody. Images were made with Adobe Photoshop 5.0. (MGDF: PEGrhu MGDF).
MAPK pathway is involved in PEG-rhuMGDF–dependent growth of progenitors and blast cell colonies, but not in the synergy between PEG-rhuMGDF and VEGF
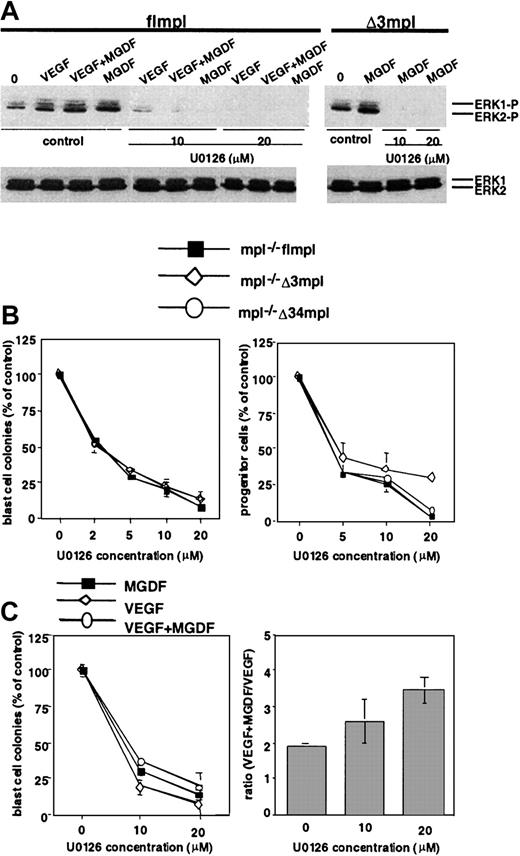
The above results show that the Δ34 deletion correlates with the loss of MAPK activation. We investigated the role of this pathway in PEG-rhuMGDF–induced development of progenitors and BL-CFCs and in the synergistic effects of PEG-rhuMGDF with VEGF or with a mixture of 6GF. Progenitors and blast cell colonies were assayed in the presence of various concentrations of the specific MEK inhibitor, U0126 (Figure7B). U0126 caused dose-dependent inhibition of the PEG-rhuMGDF–dependent growth of mpl−/−flmpl and Δ3mpl blast cell colonies. The inhibitory effect was observed at a concentration of 2 μM (50% inhibition) but reached more than 90% inhibition at 20 μM. U0126 (20 μM) completely inhibited ERK1–ERK2 phosphorylation as assessed by Western blot analysis with anti–ERK-P antibody (Figure 7A). U0126 also inhibited the PEG-rhuMGDF–dependent growth of progenitors derived from mpl−/− flmpl, Δ3mpl EBs6 cells, and the few progenitors that developed with Δ34mpl (Figure 7B). Similar inhibition was observed using another MEK inhibitor, PD98059 (not shown). Thus, the development of both progenitors and BL-CFCs in response to PEG-rhuMGDF requires the MAPK pathway.
Effect of the MEK inhibitor U0126 on the growth of BL-CFC and progenitors.
(A) EBs3 cells derived from mpl−/− flmpl or mpl−/− Δ3mpl ES cells were pretreated with 10 or 20 μM U0126 or 20 μM DMSO for 1 hour and then stimulated with VEGF, PEG-rhuMGDF, or VEGF+PEG-rhuMGDF for 15 minutes. Whole-cell extracts were then subjected to SDS-PAGE and Western blot analysis with phospho-specific anti-ERK1/2 or anti-ERK1/2 antibody. Images were made with Adobe Photoshop 5.0. (B) EBs3 or EBs6 cells were pretreated with various concentrations of U0126 or DMSO for 1 hour and then replated for blast cell colony assay (left panel) or progenitor assay (right panel) with PEG-rhuMGDF alone. (C) EBs3 derived from mpl−/− flmpl ES cells were treated as described above and replated for blast cell colony assay with PEG-rhuMGDF, VEGF, or VEGF+PEG-rhuMGDF (left panel). Ratios of the numbers of blast cell colonies stimulated with VEGF+ PEG-rhuMGDF to those stimulated with VEGF were calculated (right panel). (MGDF: PEGrhu MGDF).
Effect of the MEK inhibitor U0126 on the growth of BL-CFC and progenitors.
(A) EBs3 cells derived from mpl−/− flmpl or mpl−/− Δ3mpl ES cells were pretreated with 10 or 20 μM U0126 or 20 μM DMSO for 1 hour and then stimulated with VEGF, PEG-rhuMGDF, or VEGF+PEG-rhuMGDF for 15 minutes. Whole-cell extracts were then subjected to SDS-PAGE and Western blot analysis with phospho-specific anti-ERK1/2 or anti-ERK1/2 antibody. Images were made with Adobe Photoshop 5.0. (B) EBs3 or EBs6 cells were pretreated with various concentrations of U0126 or DMSO for 1 hour and then replated for blast cell colony assay (left panel) or progenitor assay (right panel) with PEG-rhuMGDF alone. (C) EBs3 derived from mpl−/− flmpl ES cells were treated as described above and replated for blast cell colony assay with PEG-rhuMGDF, VEGF, or VEGF+PEG-rhuMGDF (left panel). Ratios of the numbers of blast cell colonies stimulated with VEGF+ PEG-rhuMGDF to those stimulated with VEGF were calculated (right panel). (MGDF: PEGrhu MGDF).
In addition, 20 μM U0126 inhibited mpl−/− flmpl blast cell colonies cultured in the presence of VEGF or VEGF+PEG-rhuMGDF (Figure 7C), a concentration that completely abolished ERK1–ERK2 phosphorylation induced by these factors in EBs3 (Figure 7A). However, the ratio between the number of mpl−/− flmpl blast cell colonies growing with VEGF+PEG-rhuMGDF (540) and that growing with VEGF (163) remained unchanged (3.4 ± 0.4) (Figure 7C), demonstrating that the synergy between PEG-rhuMGDF and VEGF persisted. A similar result was also obtained for the synergy of PEG-rhuMGDF with 6GF on the growth of CFU-GM (not shown). Therefore, ERK1–ERK2 activation is not required for the synergy between PEG-rhuMGDF and VEGF. Indeed, ERK phosphorylation in mpl−/− flmpl EBs3 cells was not higher after VEGF+PEG-rhuMGDF than after VEGF or PEG-rhuMGDF stimulation (Figure 7A). The mechanism of the synergy between PEG-rhuMGDF and VEGF does not, therefore, require ERK1–ERK2 activation and presumably signals other than MAPK, emerging from amino acid residues 71 to 94, are involved.
Discussion
The importance of the carboxy-terminal domain of Mpl in megakaryocyte differentiation has been studied in vitro using cell lines.15,23-25 Here, we analyzed the role of this domain in response to PEG-rhuMGDF (a truncated form of human TPO conjugated to polyethylene glycol) of various ES cell–derived hematopoietic cells and the involvement of MAPK in PEG-rhuMGDF signaling. Our model was based on the re-expression of the full-length c-mpl and the expression of different mutants of c-mpl in ES cells homozygously inactivated for this gene. We evaluated the consequences of 2 deletions in the C-terminal domain of Mpl that affect the TPO response of a cell line.23 25 One deletion (Δ34) removes the 51 C-terminal amino acids of Mpl (residues 71-121), whereas the other (Δ3) only removes amino acids 71 to 94.
The deletion of the 51 C-terminal amino acids of Mpl prevented the PEG-rhuMGDF–dependent development of blast cell colonies; however, it only partially inhibited progenitor formation. All types of myeloid colonies, except CFU-GM, grew, but their total number was very much lower than that from cells expressing the full-length Mpl (18%). This deletion did not impede the final maturation, as evidenced by platelet formation in vitro, but it caused reduced megakaryocyte polyploidization (8N versus 32N) in comparison with the full-length Mpl. Finally, it inhibited the synergistic effects of PEG-rhuMGDF with VEGF or a combination of growth factors.
Therefore, in the absence of the C-terminal domain, the membrane-proximal region of Mpl allows a partial response of ES cell–derived hematopoietic cells to PEG-rhuMGDF. However, a complete biologic response requires the C-terminal domain. This suggests that the membrane-proximal domain is sufficient for CFU-MK and BFU-E differentiation, whereas the C-terminal domain is necessary for proliferation. In mice, the membrane-proximal region is sufficient to ensure megakaryocyte and platelet homeostasis.29 Signals from the distal and the proximal regions are necessary for a normal response of murine bone marrow megakaryocytes and platelets to exogenous TPO and recovery from myelosuppressive treatment.29
To discriminate between the role of the C-terminal 28 amino acids (residues 94-121) and that of the internal amino acids (sequence 71-94) in response to PEG-rhuMGDF of ES cell–derived cells, we analyzed the consequences of Δ3 deletion (71-94 residues) in these responses. The Δ3 deletion did not affect PEG-rhuMGDF–dependent colony formation from blast colony-forming cells, nonmegakaryocytic and megakaryocytic progenitors, the ploidy, and the maturation of megakaryocytes. In contrast, it prevented the synergistic effect of PEG-rhuMGDF with VEGF or a combination of growth factors. Thus, 2 regions of the C-terminal domain of Mpl have distinguishable functions—the amino acid sequence 71 to 94 appears to be required only for the synergistic action of PEG-rhuMGDF; the last 28 amino acids seem to mediate all other responses to PEG-rhuMGDF, including the development of hemangioblasts to blast cell colonies, of myeloid progenitors in numbers comparable to those formed after the binding of PEG-rhuMGDF to the full-length Mpl and megakaryocyte polyploidization. However, because the deletion of residues 94 to 121 was not tested directly, it is possible that elimination of the C-terminus by itself would have less or greater effect on the above findings.
The Δ34 deletion removes the 51 Mpl C-terminal amino acids, including the tyrosine residue 112 that drives ERK1–ERK2 MAPK pathway activation through Shc.15,24 The Δ3 deletion removes a domain located between residues 71 and 94, a sequence responsible for a prolonged activation of ERK1–ERK2 in the UT7 cell line.25MAPK activation is central to TPO-induced megakaryocytic differentiation in cell lines and, to a lesser degree, in progenitors.5,28,30,32 40-42 Our findings raise questions about the role of ERK1 and ERK2 activation in the response of ES cell–derived hematopoietic cells to PEG-rhuMGDF. ERK1–ERK2 are activated by PEG-rhuMGDF in these cells, and the amino acid sequence 94 to 121, but not 71 to 94, is required for this activation. PEG-rhuMGDF–dependent BL-CFC and progenitor development are inhibited by the specific inhibitor of MEK, U0126, showing that the growth of both cells requires the MAPK pathway. In contrast, neither the synergistic effect of PEG-rhuMGDF with VEGF nor with a combination of 6GF on the development of BL-CFC and CFU-GM was inhibited by U0126, demonstrating that the ERK1–ERK2 pathway is not involved in the synergy. Presumably, signals other than MAPK are involved in the synergy. This is further reinforced by the fact that the region deleted in the Δ3 mutant (residues 71-94) is essential for the synergistic effect of PEG-rhuMGDF.
A small number of progenitors, consisting of BFU-E and CFU-MK, can differentiate from ES cells when the C-terminal domain of Mpl is deleted. One possibility is that the weak activation of ERK1/2 in the absence of the C-terminal domain of Mpl, also detected by other groups,29,30 contributes to this differentiation. Alternatively, pathways activated by the membrane-proximal domain of Mpl, probably including STAT5, protein kinase C, or an as yet undiscovered pathway, could be involved in differentiating signals.43 44 Interestingly, no CFU-GM developed in the absence of the C-terminal domain of Mpl. This suggests that signals that allow erythroid and megakaryocytic progenitor growth are not sufficient for that of granulocyte macrophage progenitors.
Deletion of Mpl cytoplasmic residues 71 to 121, but not of 71 to 94, resulted in a reduced ploidy of ES cell–derived megakaryocytes without loss of CD41+ element production, suggesting that ERK1–ERK2 activation is involved in the ploidization of ES cell–derived megakaryocytes. This finding is consistent with previous data demonstrating that ploidy of TPO-dependent bone marrow megakaryocytes in culture is reduced by the presence of a MAPK inhibitor.30 The molecular mechanisms that regulate megakaryocyte ploidization are unknown. However, polyploidization, like cell proliferation, requires DNA replication. Thus, the effect of MAPK pathway activation on megakaryocyte polyploidization might be paralleled to its effect on cell proliferation in other cell lines. It could be the consequence of the up-regulation of cyclin D, which plays an important role in polyploidization, and the down-regulation of p27–p21.45
Deletion of residues 71 to 94 abolishes cell differentiation in the megakaryoblastic human cell line UT7.23 Loss of TPO-dependent megakaryocyte differentiation is associated with weaker and only transient ERK1–ERK2 activation compared with that of UT7 cells expressing full-length Mpl.25 Surprisingly, we found no abnormalities in ES cell–derived hematopoietic cell differentiation when this sequence was deleted and ERK1–ERK2 activation had kinetics of activation similar to that of full-length Mpl. The contribution of the amino acid sequence 71 to 94 of Mpl-to-Mpl signaling appears, therefore, to depend on the cellular context. In view of this, it may be preferable to use nontransformed cells to study the role of intracellular signals in cell proliferation and differentiation. Differences in Mpl signaling between species (murine versus human) or developmental stages (embryonic versus adult) could also explain this discrepancy.
Although not required for BL-CFC, progenitor, or megakaryocyte formation, the amino acid sequence 71 to 94 was required for the synergistic effects of PEG-rhuMGDF, either on factor-dependent progenitor or on VEGF-dependent BL-CFC development. Little is known about the mechanisms of synergy between growth factors. ERK1–ERK2 or p38MAPK activation is responsible for the synergy between EPO and KL46 or between IL-12 and IL-2,47respectively. We show here that ERK1–ERK2 activation does not contribute to the synergistic effect of PEG-rhuMGDF with other hematopoietic growth factors or VEGF. The synergy was not inhibited by the presence of specific MAPK inhibitors. At doses at which ERK activation is completely prevented, the synergy is not affected. Residues 71 to 94 may activate unknown signals necessary for the synergy. These signals could act directly or as primers of transduction pathways activated by 1 of the 2 receptors.48Alternatively, because a molecular association between EPO-R and c-Kit has been reported to be responsible for the synergy between EPO and stem cell factor on BFU-E proliferation and differentiation,49 residues 71 to 94 might allow a similar association between Mpl and other hematopoietic receptors or Flk-1, the VEGF receptor.
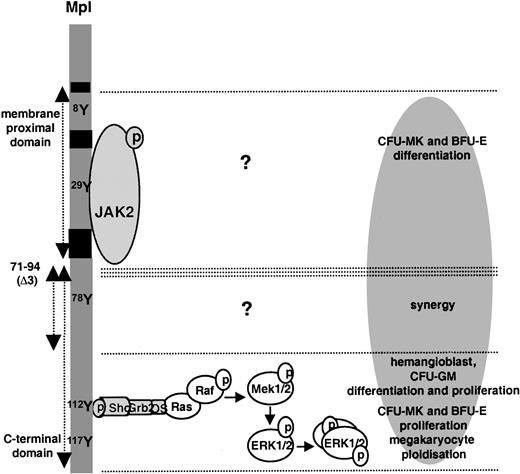
We analyzed the role of 2 regions of the Mpl C-terminal domain in a model of nontransformed hematopoietic cells derived from ES cells in vitro, in which the mpl gene is activated as early as hemangioblast emergence. As summarized in Figure8, we found that these regions mediate 2 different categories of signals, leading to different functions. MAPK ERK1–ERK2 activation, mediated by the C-terminal 28 amino acids of Mpl, supports PEG-rhuMGDF–dependent development of a cell considered the equivalent of the hemangioblast (the BL-CFC), the production of most progenitor cells, and megakaryocyte polyploidization. In contrast, the synergistic effect of PEG-rhuMGDF with other growth factors requires the amino acid sequence 71 to 94 but does not involve ERK1–ERK2 activation. Signals emerging from the latter region and responsible for growth factor synergy remain to be characterized. These give evidence that the C-terminal domain of Mpl, mostly involved in megakaryocyte differentiation,24,25,28 40-42 mediates megakaryocyte proliferation. When ectopically expressed, this domain mediates BFU-E proliferation, hemangioblast and CFU-GM differentiation and proliferation.
Scheme of function.
Various regions of the intracytoplasmic domain of Mpl in the model of ES cell–derived hematopoietic differentiation.
Scheme of function.
Various regions of the intracytoplasmic domain of Mpl in the model of ES cell–derived hematopoietic differentiation.
We thank Kirin for providing PEG-rhuMGDF, Immunex for providing IL-1α and IL-3, and Cilag for providing EPO.
Supported by INSERM and by grants from the Association pour la Recherche contre le Cancer (contract no. 5377).
F.J.d.S. is employed by Genentech.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francoise Sainteny, INSERM U362, Institut Gustave Roussy, PR1, 39 rue Camille Desmoulins, 94805 Villejuif, France; e-mail: sainte@igr.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal