Abstract
Despite the efficacy of STI571 (Glivec, Novartis, Basle, Switzerland) in treating chronic myeloid leukemia (CML), drug resistance has already been noted both in vitro and in vivo. As plasma proteins, including alpha-1-acid glycoprotein (AGP), may reduce drug efficacy through binding, AGP was investigated for its ability to interact with STI571.
At all stages of CML, AGP plasma level was significantly higher than in normal controls (P < .05). The glycoprotein was purified from normal plasma and individual chronic myeloid leukemia (CML) patients' plasma by low-pressure chromatography. The influence of α1-acid glycoprotein (AGP), in the presence of STI571, on the proliferation of Philadelphia chromosome–positive (Ph+) cells was examined. Normal AGP, even at supraphysiological concentrations, did not block the effect of STI571 on K562-cell proliferation in vitro. Moreover, CML-derived AGP failed to block the effect of STI571 on Ph+ cells in vitro. Thus, these in vitro findings suggest that AGP will not abrogate the antileukemic activity of STI571.
Introduction
Chronic myeloid leukemia (CML) has been found to be responsive to STI571.1 However, resistance to the drug, mediated through gene amplification or upregulation of P-glycoprotein, has already been noted to develop in cell lines in vitro2-4 and in vivo from preliminary reports on the clinical trial experience accruing with STI571.5 6 In vivo, drugs can be neutralized through induction of metabolic and excretory pathways. Further, plasma proteins are also known to bind drug molecules that may significantly alter the availability of pharmacologically active drug.
One such drug-binding plasma protein is α1-acid glycoprotein (AGP). The plasma concentration of AGP becomes elevated in inflammation and diseases, including cancer,7 from a population mean of 0.77 mg/mL.8 N-linked glycosylation of human AGP accounts for 42% of its molecular weight. The glycosylation pattern, although reproducible in health, is altered in disease, in which both the total AGP concentration and the relative proportions of its glycosylated variants (glycoforms) are markedly altered.9 10 AGP glycoforms differ in oligosaccharide branching, fucosylation, and sialylation.
The AGP drug-binding effect is particularly significant with lipophilic drugs such as chlorpromazine.11 AGP has high-affinity, low-capacity binding for basic drugs, an effect surpassing that of albumin.12 In light of a recent report of the ability of normal AGP to bind STI571,13 we investigated whether AGP glycoforms expressed in Phildelphia chromosome–positive (Ph+) leukemic patients would have such drug-binding capacity, which may represent a novel form of drug resistance.
Study design
AGP levels in plasma and purification
Blood was drawn, with informed consent, from 99 Ph+CML patients and 7 normal controls. AGP plasma levels were assayed by immunoturbidimetry, which was performed commercially by Clinical Pathology, Leicester Royal Infirmary (United Kingdom). AGP was isolated from plasma by our published low-pressure liquid chromatography method.10 14 The final products were assayed by standard Western blotting.
CD34+lin− enrichment
Leukapheresis samples harvested from CML patients at diagnosis were enriched for CD34+ progenitors by immunomagnetic lineage depletion.15
In vitro cell proliferation assay
Triplicate cultures of 3 × 104 K562 cells were incubated for 72 hours in serum-free media in 96-well plates with 0 to 10 μM STI571 and 0 to 2.5 mg/mL AGP. During the last 4 hours of culture, 0.25 μCi 3H-thymidine was added per well. Cells were then harvested for beta counting. Statistical analysis was performed with the Student t test.
AGP-drug binding assay
Protein drug-binding results in the quenching of the protein's intrinsic fluorescence owing to the masking of tryptophan and tyrosine residues by the drug. With excitation at 280 nm, the AGP fluorescence emission spectrum was determined over the 300 to 400 nm wavelength range in the presence of chlorpromazine or STI571.
Results and discussion
Even though the actual physiological function of AGP has remained an enigma since the 1950s, the propensity of AGP to bind basic drugs12 is a universally accepted property that merits particular attention with regard to the treatment of CML with STI571. As CML is a disseminated tumor, it may be anticipated that the efficacy of certain antineoplastic agents will be modulated by interactions with the soluble constituents of the blood. If plasma AGP were to bind significantly to STI571, or any subsequent derivatives, dose adjustment would be imperative in order to achieve the drug's minimum effective concentration.
It has been recently suggested that AGP could indeed bind out STI571, thus increasing by 90-fold the concentration of the drug that inhibits 50% in vitro.13 However, Gambacorti-Passerini et al investigated AGP commercially purified from Cohn fraction VI.16 The Cohn fractionation process risks desialylation of the protein as the labile ketosidic sialic acid linkages are acid sensitive. Moreover, the AGP was purified from normal human plasma. While the population of AGP glycoforms and its plasma concentration is found to be reproducible in health, the glycoprotein is known to change both qualitatively and quantitatively in disease. It is therefore critical to assay disease glycoforms, and any results observed with normal AGP should not be extrapolated to the disease setting. Thus, we decided to assay the plasma levels of AGP and to purify the glycoprotein from CML patients' plasma at different disease stages.
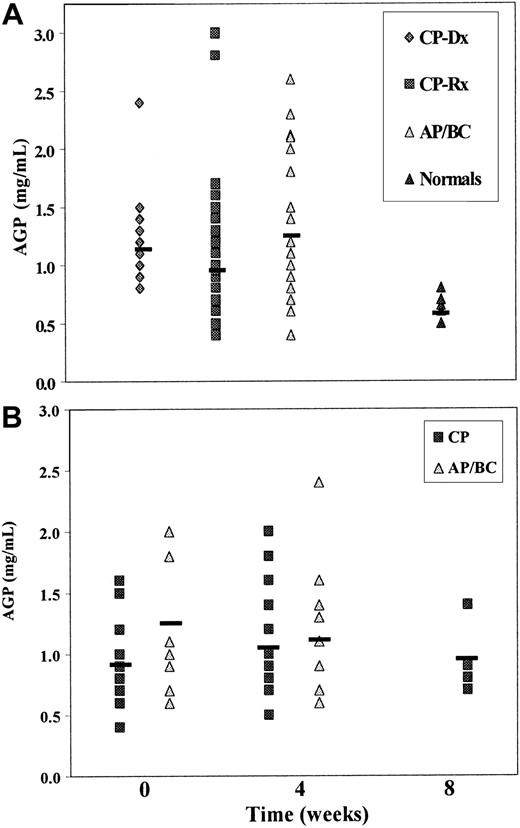
The average plasma AGP levels in our patients at each stage of CML were significantly elevated (P < .05) as compared with normal controls (Figure 1A). At diagnosis, the mean AGP level was double the level observed in healthy individuals; this is in agreement with the published literature, which describes 2- to 5-fold increases in AGP in disease.7 Despite conventional chemotherapy, eg, hydroxyurea, and control of white blood cell count, the average AGP level in the treated group did not fall significantly from diagnosis. Of note, however, was the statistically significant increase in plasma AGP on disease progression from CP to AP/BC (P = .018). Even in the face of abundant AGP throughout the course of the disease, it seems that this need not be of concern with respect to negating STI571 efficacy. Moreover, STI571 as monotherapy did not itself induce further AGP production (Figure 1B).
Plasma AGP levels in CML.
(A) AGP plasma levels in CML patients at different disease stages. At diagnosis, mean AGP level was 1.22 ± 0.12 mg/mL (mean ± SEM; n = 13); in conventionally treated CML patients, 1.02 ± 0.07 mg/mL (n = 49); and in accelerated phase/blast crisis, 1.32 ± 0.14 mg/mL (n = 21). The mean plasma AGP level in normal controls was 0.62 ± 0.04 mg/mL (n = 7). (B) The effect of STI571 treatment on plasma AGP levels in chronic phase (CP) and accelerated phase/blast crisis (AP/BC) CML patients. The mean plasma AGP levels in 16 CML patients in CP measured prior to STI571 monotherapy was 0.91 ± 0.08 mg/mL. At 4 weeks into treatment, the mean AGP plasma in these same patients was found to be 1.06 ± 0.11 mg/mL; 5 of the 16 were followed to week 8 when mean AGP plasma level was 0.94 ± 0.12 mg/mL. In 8 AP/BC patients, the baseline AGP plasma level prior to STI571 therapy was 1.25 ± 0.19 mg/mL, and 4 weeks later it was 1.10 ± 0.19 mg/mL.
Plasma AGP levels in CML.
(A) AGP plasma levels in CML patients at different disease stages. At diagnosis, mean AGP level was 1.22 ± 0.12 mg/mL (mean ± SEM; n = 13); in conventionally treated CML patients, 1.02 ± 0.07 mg/mL (n = 49); and in accelerated phase/blast crisis, 1.32 ± 0.14 mg/mL (n = 21). The mean plasma AGP level in normal controls was 0.62 ± 0.04 mg/mL (n = 7). (B) The effect of STI571 treatment on plasma AGP levels in chronic phase (CP) and accelerated phase/blast crisis (AP/BC) CML patients. The mean plasma AGP levels in 16 CML patients in CP measured prior to STI571 monotherapy was 0.91 ± 0.08 mg/mL. At 4 weeks into treatment, the mean AGP plasma in these same patients was found to be 1.06 ± 0.11 mg/mL; 5 of the 16 were followed to week 8 when mean AGP plasma level was 0.94 ± 0.12 mg/mL. In 8 AP/BC patients, the baseline AGP plasma level prior to STI571 therapy was 1.25 ± 0.19 mg/mL, and 4 weeks later it was 1.10 ± 0.19 mg/mL.
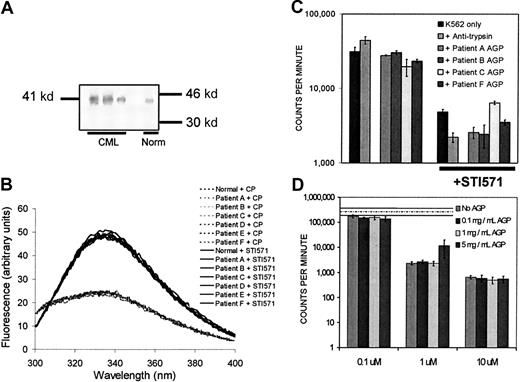
We have previously published a low-pressure liquid chromatography method for plasma AGP isolation that avoids the oligosaccharide damage resulting from standard acid-precipitation methods.10,14The identity of the CML-derived protein isolated by our method was assayed by Western blotting (Figure 2A). In a direct binding assay, the AGP isolated by our method failed to bind STI571 while still being capable of binding chlorpromazine (Figure2B). The CML-derived isolated AGP was tested for an ability to abrogate K562-cell killing induced by STI571 in an in vitro cell proliferation assay. In such assays, the cohort of CML-derived AGP tested was unable to block the effects of 1 μM STI571 on Ph+ cells (Figure 2C). AGP isolated from a pool of healthy donors did not “rescue” K562 cells from the effects of 1 or 10 μM STI571 (Figure 2D). The discordance between our observations and those of Gambacorti-Passerini et al13 may be due to the isolation methods employed, with our method assured not to damage the oligosaccharide structure resulting from harsher acid precipitation methods. Moreover, however, Gorre et al17recently reported that there is a 10-fold difference in sensitivity to STI571 between pretreatment and relapse leukemia cells, suggesting that resistance is cell intrinsic and not host mediated.
Experiments investigating AGP identity, direct drug-binding capacity, and influence on STI571 activity in vitro.
(A) Representative Western blot of isolated CML-derived AGP. Briefly, following probing with a mouse antihuman α1-acid glycoprotein monoclonal antibody (clone AGP42 Sigma, Poole, United Kingdom) and a secondary antimouse alkaline phosphatase–conjugated antibody (Sigma), the blot was developed with NitroBlue tetrazolium bromochloroindolyl phosphate (NBT-BCIP). (B) Measurement of AGP drug-binding capacity by fluorescence quenching. Neither normal-derived nor CML-derived AGP (1 mg/mL) bound 1 μM STI571, as indicated by lack of quenching of the glycoprotein's fluorescence (solid lines). Peak fluorescence of the CML-derived AGP alone at λmax (335 nm) was 49.02 ± 0.35 arbitrary units (mean ± SD; n = 5). Both normal-derived and CML-derived AGP at 1 mg/mL could bind 2.5μM chlorpromazine (dotted lines; + CP) as shown by fluorescence quenching. (C) Effect of CML-derived AGP on K562 proliferation ± STI571. CML-derived AGP was tested for its ability to block the effect of 1 μM STI571 on K562 cell proliferation in vitro by a standard3H-thymidine–uptake assay. AGP isolated from the plasma of patients A and B was tested at 1 mg/mL, and from patients C and F at 2 mg/mL final concentration. Errors are displayed as 1 SD about the mean of triplicate determinations. (D) Effect of normal AGP on K562 proliferation ± STI571. Normal AGP (0.1 to 5 mg/mL) was tested for an ability to block the effect of STI571 (0.1 to 10 μM) on K562 cell proliferation in vitro. K562 cell proliferation in the absence of STI571 or AGP is indicated by the hashed line (— · —); SEM around this mean is indicated by the dotted line (-----).
Experiments investigating AGP identity, direct drug-binding capacity, and influence on STI571 activity in vitro.
(A) Representative Western blot of isolated CML-derived AGP. Briefly, following probing with a mouse antihuman α1-acid glycoprotein monoclonal antibody (clone AGP42 Sigma, Poole, United Kingdom) and a secondary antimouse alkaline phosphatase–conjugated antibody (Sigma), the blot was developed with NitroBlue tetrazolium bromochloroindolyl phosphate (NBT-BCIP). (B) Measurement of AGP drug-binding capacity by fluorescence quenching. Neither normal-derived nor CML-derived AGP (1 mg/mL) bound 1 μM STI571, as indicated by lack of quenching of the glycoprotein's fluorescence (solid lines). Peak fluorescence of the CML-derived AGP alone at λmax (335 nm) was 49.02 ± 0.35 arbitrary units (mean ± SD; n = 5). Both normal-derived and CML-derived AGP at 1 mg/mL could bind 2.5μM chlorpromazine (dotted lines; + CP) as shown by fluorescence quenching. (C) Effect of CML-derived AGP on K562 proliferation ± STI571. CML-derived AGP was tested for its ability to block the effect of 1 μM STI571 on K562 cell proliferation in vitro by a standard3H-thymidine–uptake assay. AGP isolated from the plasma of patients A and B was tested at 1 mg/mL, and from patients C and F at 2 mg/mL final concentration. Errors are displayed as 1 SD about the mean of triplicate determinations. (D) Effect of normal AGP on K562 proliferation ± STI571. Normal AGP (0.1 to 5 mg/mL) was tested for an ability to block the effect of STI571 (0.1 to 10 μM) on K562 cell proliferation in vitro. K562 cell proliferation in the absence of STI571 or AGP is indicated by the hashed line (— · —); SEM around this mean is indicated by the dotted line (-----).
In conclusion, as the glycoprotein changes in disease, results with AGP isolated from healthy individuals, while of interest, are not of direct relevance to CML. In this study, CML-derived AGP did not appear to rescue Ph+ cells from STI571 activity. Together with the evidence from the clinic in which the majority (98%) of patients, even in late chronic phase, achieve a complete hematological remission with 400 mg STI571 daily, it would appear that AGP drug binding does not contribute to any loss of efficacy of STI571 therapy in CML.
We thank Novartis, Basle, Switzerland for the gift of STI571, and we gratefully acknowledge Stem Cell Technologies, Vancouver, Canada; L. Richmond, C. Pearson, and M. Alcorn of Glasgow Royal Infirmary; S. Graham of Glasgow University; and A. Polacchi of Strathclyde University.
Supported by the United Kingdom Leukaemia Research Fund (H.G.J. and T.L.H.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Heather G. Jørgensen, ATMU, Dept of Medicine, University of Glasgow, Glasgow Royal Infirmary, 10 Alexandra Parade, Glasgow G31 2ER, United Kingdom.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal