Abstract
A male infant with severe bleeding tendency had undetectable factor V activity. Sequence analysis of the proband's DNA revealed one base deletion in exon 13 (2952delT) and one base insertion in exon 16 (5493insG) in heterozygous form. Both mutations introduced a frameshift and a premature stop at codons 930 and 1776, respectively. The proband's father and mother were heterozygous for 2952delT and for 5493insG, respectively. Both mutations would result in the synthesis of truncated proteins lacking complete light chain or its C-terminal part. In the patient's plasma, no factor V light chain was detected by enzyme-linked immunosorbent assay. The N-terminal portion of factor V containing the heavy chain, and the connecting B domain was severely reduced but detectable (1.7%). A small amount of truncated factor V–specific protein with a molecular weight ratio of 236 kd could be immunoprecipitated from the plasma and detected by Western blotting. This protein, factor VDebrecen, corresponds to the translated product of exon 16 mutant allele.
Introduction
Blood coagulation factor V, a large (molecular weight ratio [Mr] = 330 kd) single-chain glycoprotein, is composed of 3 homologous A-type domains, 2 smaller homologous C-type domains, and a heavily glycosylated B domain that connects the N-terminal A1-A2 region with the C-terminal A3-C1-C2 region.1,2 Its plasma concentration is approximately 10 μg/mL, and approximately 20% of factor V in the blood is compartmentalized in platelet α-granules. Thrombin, the physiological activator of factor V, removes the internal B domain by limited proteolysis and the remaining heavy chain (HC) (Mr = 105 kd) and light chain (LC) (Mr = 73 kd) are associated via a calcium ion.3 The active form of factor V enhances the activation of prothrombin by several thousand-fold.4,5 The gene for human factor V has been localized to chromosome 1q21-25; it spans approximately 80 kilobases of DNA and consists of 25 exons and 24 introns. Complete complementary DNA (cDNA) and derived amino acid sequence of human factor V have been determined.6
Congenital factor V deficiency (parahemophilia)7 is a rare disorder with an incidence of about 1 in 106. It is inherited in an autosomal recessive manner, and the patients suffer a moderate to severe bleeding disorder. Most factor V–deficient patients have low factor V activity and antigen level; however, discrepancy between functional and antigenic levels has also been described.8,9 More than 200 factor V–deficient cases have been reported in the literature, but the molecular basis for factor V deficiency has been established in only a few cases.10-13Additional molecular defects in the factor V gene have been identified in patients with “pseudohomozygosity” for factor V Leiden (the Leiden allele plus the null allele). These patients may be identified on the basis of thrombotic problems (reviewed in Kane1). Here, we describe 2 novel frameshift mutations in the factor V gene that lead to severe factor V deficiency.
Study design
Case history
A male infant presented at birth with subdural hematoma when his factor V deficiency was diagnosed. He is now 2 years old and has been suffering from easy bruising and severe hematoma following intramuscular injection. Consanguinity in the family was excluded. No bleeding disorder was detected in other members of the family.
Preparation of plasma and platelet specimens
Ethical approval was obtained from the Ethics Committee of the Medical and Health Science Center, University of Debrecen (Hungary). Following informed consent, blood samples from the patient and family members were collected in a 1:10 volume of 0.105 M sodium citrate. The patient did not receive substitution therapy for 2 months prior to blood sampling for factor V antigen and activity measurement. Blood samples used for Western blotting or immunoprecipitation were immediately treated with a cocktail of protease inhibitors including 10 μg/mL benzamidine; 0.1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride; 10 μg/mL leupeptin; and 2 μg/mL aprotinin (Sigma, St Louis, MO). Plasma was separated by centrifugation at 2000g for 20 minutes, and aliquots were stored at −70°C. The washed platelet suspension was prepared as described14with a final platelet count of 1000 G/L (109platelets per liter), and lysed with 1% Triton X-100.
Factor V activity and antigen assays
Factor V coagulant activity in plasma was measured by a one-stage assay based on prothrombin time. Standard human plasma (Dade Behring, Marburg, Germany) was used for calibration. Factor V activity in platelet lysate samples prepared as described above was determined by the same assay system, but in this case the assay was calibrated against pooled normal platelet lysate. Factor V, factor V HC, and factor V LC antigen levels were determined by sandwich enzyme–linked immunosorbent assay (ELISA). Sheep antihuman factor V polyclonal antibody (The Binding Site, Birmingham, United Kingdom), monoclonal antibody directed to epitope on the 150-kd activation peptide in the connecting B domain (clone B10) (Chemicon, Temecula, CA), and monoclonal antibody directed to epitope on C2 domain of factor V LC (clone HV1) (Sigma)15 were used as capture antibodies. Rabbit antihuman factor V antiserum (Diagnostica Stago, Asnières, France) was used as second antibody and was followed by peroxidase-labeled goat antirabbit immunoglobulin (Ig)–G (Dako, Glostrup, Denmark). The assays were calibrated against standard human plasma (Dade Behring), and factor V antigen levels were expressed as a percentage of the normal average.
Immunoprecipitation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotting
Factor V was isolated from the plasma by immunoprecipitation with the use of sheep polyclonal anti–factor V antibody (The Binding Site) biotinylated at carbohydrate residues16 and streptavidin agarose (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Immunoprecipitates and whole plasma samples were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)17 and by Western blotting. The following antibodies were used as primary antibody: biotinylated sheep polyclonal antibody against factor V (see above); rabbit anti–factor V antiserum (Assera V, Diagnostica Stago); and mouse monoclonal antibody against the B domain of factor V (Chemicon). The immunoreaction with the biotinylated antibody was visualized by avidin H and biotinylated peroxidase complex (Vectastain ABC kit) (Vector, Burlingame, CA). Nonbiotinylated anti–factor V rabbit antibody and anti–factor V B-domain mouse antibody were followed by peroxidase-labeled goat antirabbit IgG and rabbit antimouse IgG (Dako), respectively. Bound peroxidase was detected by ECL-Plus (Amersham) chemiluminescent reagent.
Polymerase chain reaction amplification and sequencing
Genomic DNA was isolated from buffy coats by QIAamp Blood Mini Kit (QIAGEN, Hilden, Germany). The following oligonucleotides used in polymerase chain reaction (PCR) amplification and sequencing were designed from factor V gene sequence18: e1F, 5′-cccacagcctctagagctc-3′; e1R, 5′-cccggactccacacct-3′; e2F, 5′-ttagtttttgtattttatttccag-3′; e2R, 5′-gtttctataaattttcagtaaatgg-3′; e5F, 5′-ctgcagtgctactgaaaacat-3′; e5R, 5′-tttccttcttgatagggagttg-3′; e7F, 5′-ttcttctcttgagttatttcattg-3′; e7R, 5′-tttgcccagtggtatgaa-3′; e8F, 5′-atttgagaaagtggtttaatttt-3′; e8R, 5′-catttgaatttaaaattatatgagc-3′; e17F 5′-ctgtgtcaacagattttaattgattt-3′; e17R, 5′-aagaaatgagaaggagttacagatt-3′; e21F, 5′-gaatttaggcagtgtgtgacttgtt-3′; e21R, 5′-tctagagattcagatagaaatatgcacaca-3′; e22F 5′-taaactttcctcttttcttctag-3′; e22R, 5′-tcccaaatcttgattcttt-3′; e24F, 5′-caaaggttttaacatcttccttatct-3′; e24R, 5′-gcacagtcttcagattgctttc-3′; e25F, 5′-tttctcttatttggctttcag-3′; e25R 5′-attctaaatggtttgaggtctt-3′. Exon 13 was amplified in 4 overlapping fragments by means of the following primers: e13aF, 5′-gattattgtgttttcatgtct-3′; e13aR, 5′-cttgggtcccttatgctta-3′; e13bF, 5′-atacgtctactttcacttg-3′; e13bR, 5′-tgggaagagatgtttcatt-3′; e13cdF, 5′-caacacattttcagaaagaag-3′, e13cdR, 5′-cattgagagtaggagatg-3′; e13eF, 5′-ttgatcagatattctacc-3′; e13eR, 5′-tcagcagtaatggaaaaatg-3′. The remaining exons were amplified by the use of published primer sequences.11 PCR products purified by ultrafiltration were sequenced by ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Results and discussion
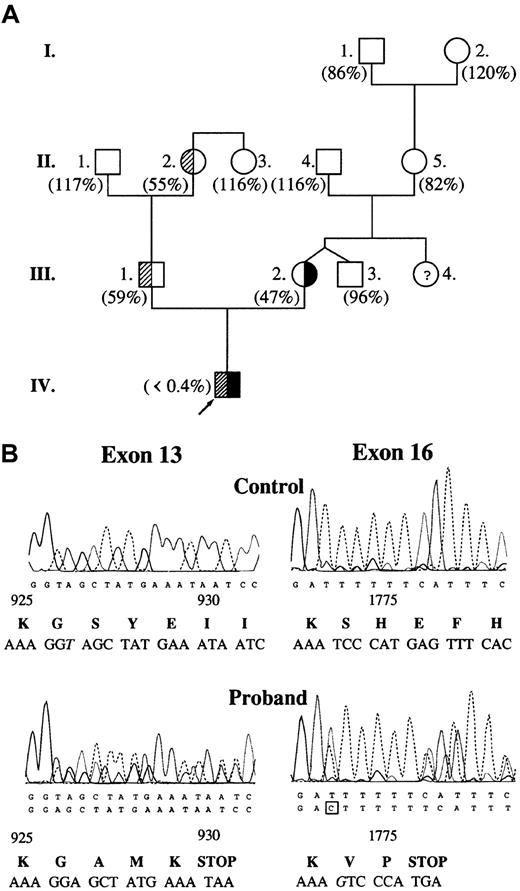
The proband had highly prolonged prothrombin time (58.1 seconds; control, 8.7-11.5 seconds) and activated partial thromboplastin time (198.8 seconds; control, 29.5-42.7 seconds). Plasma clotting factor activities were within the reference (control) range with the exception of factor V. Factor V activity was undetectable in the proband's plasma (Figure 1A) and platelet lysate. The mother, the father, and one grandmother had moderately decreased factor V activity corresponding to a heterozygous state. DNA sequence analysis revealed 2 causative mutations in heterozygous form: one base deletion (thymine) in exon 13 at nucleotide position 2952, and one base insertion (guanine) in exon 16 at nucleotide position 5493 (Figure 1B). The proband's mother was heterozygous for 5493insG while his father was heterozygous for 2952delT. In the proband's DNA, there were 2 further noncausative mutations: a homozygous silent A327→G substitution in exon 2 and a heterozygous A6250→T substitution in exon 22.10
Molecular genetic analysis of factor V deficiency.
(A) Pedigree of factor V–deficient family. Values in parentheses represent plasma factor V activities. Reference interval for factor V activity is 70% to 120%. Subjects with 2952delT and 5493insG mutations are demonstrated with shaded and solid areas, respectively. There was no indication of the presence of heterozygous factor V deficiency in the mother's family. (B) Identification of causative mutations in the DNA sequence of the proband. Exon 16 was sequenced on the reverse strand. Altered nucleotides are shown in italics. Predicted amino acid sequences around mutations are shown below the electropherograms. Amino acid residue numbering is based on the cDNA sequence published by Jenny et al.6
Molecular genetic analysis of factor V deficiency.
(A) Pedigree of factor V–deficient family. Values in parentheses represent plasma factor V activities. Reference interval for factor V activity is 70% to 120%. Subjects with 2952delT and 5493insG mutations are demonstrated with shaded and solid areas, respectively. There was no indication of the presence of heterozygous factor V deficiency in the mother's family. (B) Identification of causative mutations in the DNA sequence of the proband. Exon 16 was sequenced on the reverse strand. Altered nucleotides are shown in italics. Predicted amino acid sequences around mutations are shown below the electropherograms. Amino acid residue numbering is based on the cDNA sequence published by Jenny et al.6
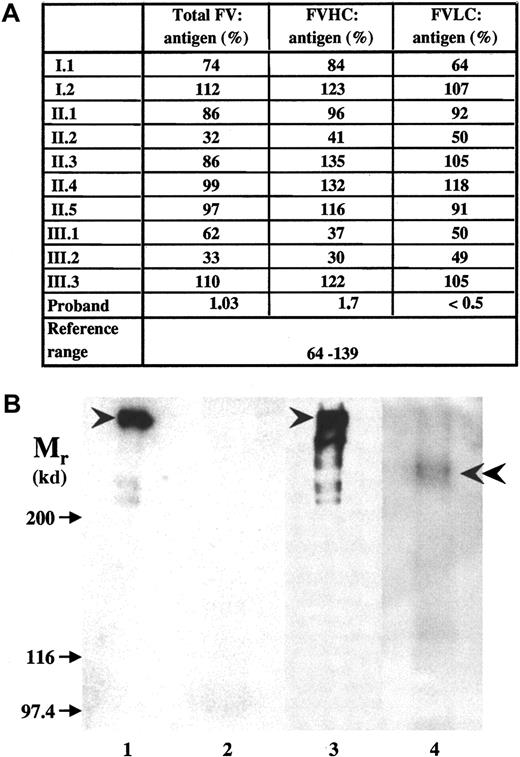
Both causative mutations introduced a frameshift and predicted novel stop codons at positions 930 and 1776, respectively, that would lead to the synthesis of truncated factor V molecules. Factor V antigen level in the patient's plasma was severely reduced, but detectable (Figure 2A). In ELISA systems using B-domain or LC-specific monoclonal antibodies, only the N-terminal portion of factor V containing HC plus B domain could be detected. The parents had antigen values around 50%. No factor V was detected in the patient's plasma by Western blotting (Figure 2B, lane 2); however, when factor V antigen was concentrated 100-fold by immunoprecipitation, a faint band with an Mr of 236 kd reacted with monoclonal anti–factor V B-domain antibody (Figure 2B, lane 4) or polyclonal anti–factor V antibodies (results with the latter antibodies are not shown). No intact factor V could be seen, and a further 4-fold increase in the amount of immunoprecipitate obtained from the patient's plasma did not change the situation (not shown). The predicted protein resulting from the exon 13 mutation would lack part of the B domain and the complete LC. The exon 16 mutant protein would lack a significant part of the LC, and this 1775 amino acid–long polypeptide would have an Mr of 200 kd. Considering that it contains the heavily glycosylated B domain, it is very likely that the 236-kd protein that we now designate factor VDebrecen represents this larger truncated protein. The absence of smaller truncated protein and the highly reduced amount of factor VDebrecen could be due to reduced synthesis of mutant messenger RNAs,12 to the instability and intracellular degradation of mutant proteins, and to the accelerated plasma clearance of truncated factor V.19
Factor V antigen in the plasma of the proband and family members.
(A) Measurement of factor V antigen by 3 different sandwich ELISAs. The following capture antibodies were used: a polyclonal anti–factor V antibody (total factor V antigen); a monoclonal antibody against an epitope on factor V B-domain that detects the N-terminal portion of the molecule containing factor V HC plus the connecting region (FVHC antigen); and a monoclonal antibody against an epitope on factor V light chain (FVLC antigen). The reference interval obtained by Montefusco et al12 was used for comparison. (B) The detection of factor V by Western blotting. SDS-PAGE was carried out in 5% gel. The sample loaded onto the gel contained 0.625 μL normal (lane 1) and 12.5 μL patient (lane 2) plasma or factor V immunoprecipated from 2.5 μL normal plasma (lane 3) and 250 μL patient's (lane 4) plasma. Biotinylated polyclonal antibody against factor V and Vectastain ABC kit were used for the detection of factor V in plasma samples (lanes 1,2), while the blots of immunoprecipitates (lanes 3,4) were developed by monoclonal anti–factor V B-domain antibody and peroxidase-labeled antimouse IgG. Arrows indicate the position of Mr marker proteins; arrowheads point to intact factor V molecule; and double arrowhead points to the faint band representing truncated factor V present in the patient's sample. In the immunoprecipitate from normal control plasma, some breakdown product of factor V could also be seen.
Factor V antigen in the plasma of the proband and family members.
(A) Measurement of factor V antigen by 3 different sandwich ELISAs. The following capture antibodies were used: a polyclonal anti–factor V antibody (total factor V antigen); a monoclonal antibody against an epitope on factor V B-domain that detects the N-terminal portion of the molecule containing factor V HC plus the connecting region (FVHC antigen); and a monoclonal antibody against an epitope on factor V light chain (FVLC antigen). The reference interval obtained by Montefusco et al12 was used for comparison. (B) The detection of factor V by Western blotting. SDS-PAGE was carried out in 5% gel. The sample loaded onto the gel contained 0.625 μL normal (lane 1) and 12.5 μL patient (lane 2) plasma or factor V immunoprecipated from 2.5 μL normal plasma (lane 3) and 250 μL patient's (lane 4) plasma. Biotinylated polyclonal antibody against factor V and Vectastain ABC kit were used for the detection of factor V in plasma samples (lanes 1,2), while the blots of immunoprecipitates (lanes 3,4) were developed by monoclonal anti–factor V B-domain antibody and peroxidase-labeled antimouse IgG. Arrows indicate the position of Mr marker proteins; arrowheads point to intact factor V molecule; and double arrowhead points to the faint band representing truncated factor V present in the patient's sample. In the immunoprecipitate from normal control plasma, some breakdown product of factor V could also be seen.
Complete factor V deficiency is lethal in knockout mice; however, they can be rescued by a very low level (less than 0.1%) of transgene factor V expression.20,21 Although we were unable to detect intact factor V in the patient's plasma, it cannot be excluded that the patient expresses a very low level of factor V as a result of ribosomal slippage or somatic reversion. Such a low level of factor V might be undetectable on the Western blot, even by the highly sensitive chemiluminescent technique. Alternatively, intact factor V, owing to its extreme protease sensitivity, could have been degraded during the immunoprecipitation procedure. A further possibility is that the truncated protein possesses some residual procoagulant activity22 that is sufficient to rescue the patient from fatal consequences.
Supported by grants from the Higher Education Development Programs of the Ministry of Education, Hungary (FKFP 0214/2001); from the Hungarian National Research Fund (OTKA T030406); and from the Hungarian Academy of Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
László Muszbek, Dept of Clinical Biochemistry and Molecular Pathology, University of Debrecen Medical and Health Science Center, PO Box 40, Debrecen 4012, Hungary; e-mail:muszbek@jaguar.dote.hu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal