Abstract
Pax5-deficient pre-B I–cell clones, transplanted into natural killer (NK)–cell–deficient RAG2−/−IL-2Rγ−/−hosts, populate the NK-cell compartment with functional NK cells. NK-cell generation fromPax5−/−pre-B I cells is also observed in NK-cell–proficient Balb/c RAG2−/− hosts. In the same Balb/c RAG2−/− hosts,Pax5−/− pre-B I–cell clones not only populate the pre-B I–cell compartment and fill the deficient T-cell–lineage compartment in the thymus and the periphery of all hosts, as shown before, they also generate CD8α− and CD8α+ dendritic cells (DCs), macrophages, and granulocytes in vivo in approximately half the hosts. In some recipients, practically all the mature myeloid cells are ofPax5−/− origin, indicating the effectiveness by which Pax5−/−pre-B I cells can compete with endogenous myeloid precursors. In a smaller percentage of hosts, the generation of Pax5−/−pre-B I–cell–derived erythrocytes is observed 4 to 6 months after transplantation. The results indicate that Pax5−/−pre-B I cells can develop in vivo in hosts that have undergone transplantation to erythroid, myeloid, and lymphoid cell lineages. Hence, the Pax5−/−mutation introduces an unusual instability of differentiation in pre-B I cells so that they appear to dedifferentiate as far back as the pluripotent hematopoietic stem cell.
Introduction
A rare cell in bone marrow is responsible for the sustained production of the different erythroid, myeloid, and lymphoid cells of blood throughout life.1 This hematopoietic stem cell (HSC) is characterized by 2 capacities, the potential to give rise to all cells of the blood and the capacity to self-renew. Recently, it was reported that HSCs also contribute to the production of cells of brain, liver, and skeletal muscle.2-9 Moreover, muscle- and central nervous system–derived stem cells have been reported to generate blood cells.9-11 Thus, HSCs and other stem cells appear to have even greater plasticity than previously thought.
The differentiation of HSCs into the various hematopoietic lineages is usually pictured in a hierarchical fashion in which these cells develop first into progenitors and then into precursors, with decreasing pluripotency and increasing commitment to single differentiation pathways. This idea is supported by the recent identification of a common lymphoid progenitor (CLP)12 and a common myeloid progenitor (CMP).13 CLP can differentiate into T, B, and natural killer (NK) cells but not into myeloid cells. CMP, on the other hand, can give rise to various cells of the myeloid lineage but not of the lymphoid lineage. In other studies with a series of transcription factor–deficient mouse strains, a slightly different hierarchy of blood cell development has been proposed.14
Progenitors and intermediate stages of hematopoietic development have been shown to exhibit some plasticity in their developmental program when they are genetically modified. Thus, Kondo et al15have recently shown that CLPs isolated from wild-type mice transfected with the interleukin-2 receptor β (IL-2Rβ) gene or fromIL-2Rβ transgenic mice can, under the appropriate in vitro conditions, differentiate into cells of the myeloid lineage. The bipotential B lymphocyte–macrophage progenitor isolated from adult bone marrow is another example of plasticity of a more differentiated hematopoietic precursor.16 Moreover, it has been reported that DCs can be generated from CD19+ B-lymphoid precursors.17 Perhaps the greatest plasticity of this kind yet demonstrated is the in vitro and in vivo differentiation ofPax5-deficient pre-B I cells into various hematopoietic lineages.18 19
Transcription factor Pax5-deficient mouse is blocked in B-cell development at the transition from DHJH-rearranged pre-B I to VHDHJH-rearranged pre-B II cells.20,21Pax5−/− pre-B I cells express various lymphoid and B-cell–specific genes, includingRAG1, RAG2, TdT, λ5,VpreB, immunoglobulin (Ig)α, Igβ,E2A, and EBF, similar to wild-type pre-B I cells.21,22 Moreover, like wild-type cells,Pax5−/− pre-B I cells have the long-term capacity to grow in vitro on stromal cells in the presence of IL-7.21 However, and in marked contrast to wild-type pre-B I cells, Pax5-deficient B-cell precursors can develop into myeloid cells under appropriate in vitro conditions—that is, in the presence of different cytokines known to induce development of the various myeloid cell lineages.18 Transfer ofPax5−/− pre-B I cells into osteoclast-deficient c-fos−/− mice results in a partial but rapid restoration of the osteoclast compartment.18 Moreover, again unlike wild-type pre-B I cells, the transfer of in vitro–grown Pax5−/−pre-B I–cell clones into lymphoid-deficientRAG2−/− mice results in full and long-term reconstitution of T-cell development and in repopulation of the bone marrow with Pax5−/− pre-B I cells.19
Here we analyzed the in vivo reconstitution capacity of in vitro–grownPax5-deficient pre-B I–cell clones in more detail. We extended our analysis by using one other deficient mouse strain, that lacking the common γ-chain of the IL-2/IL-7/IL-9/IL-15 receptor (cγ−/−) in combination with a RAG-2 deficiency, to show that NK cells can develop in these hosts that received transplants ofPax5−/− pre-B I–cell clones. Furthermore, we investigate the development of various hematopoietic lineages inRAG2−/− recipients that received transplants of Pax5−/− pre-B I–cell clones for extended periods of time, such as in hosts with normal differentiation capacities for myeloid and erythroid cell development. After the rapid reconstitution of bone marrow with Pax5−/−pre-B I cells and T-cell compartments with thymocytes, we found reconstitution of NK cells, DCs, myeloid cells, and even erythrocytes. Thus, the in vivo plasticity in hematopoietic cell differentiation of in vitro–grown Pax5−/− pre-B I cells is much greater than previously anticipated. Our findings suggest thatPax5−/− pre-B I cells have the capacity to dedifferentiate into HSCs.
Materials and methods
Mice
RAG2−/− mice were originally provided by Dr F. Alt (Boston, MA). Balb/c RAG2−/− mice were generated at the Basel Institute for Immunology by backcrossing the RAG2 mutation into Balb/c mice for 15 generations.RAG2/IL-2Rγ-chain double-deficient (RAG2cγ−/−) mice were purchased from Taconic Farms (Germantown, NY).
Cells
Transfer of cells
Balb/c RAG2−/− orRAG2cγ−/− mice at 8 to 12 weeks of age were γ-irradiated with 4 Gy, and 107 in vitro–grown Pax5−/− pre-B I cells were injected intravenously 6 to 8 hours after irradiation. Cell suspensions of various lymphoid organs were prepared by collagenase-DNase digestion at different time points after cell transfer, as described.24
Flow cytometry
Flow cytometric analysis was performed using a FACScalibur (Becton Dickinson, Silicon Valley, CA). Biotinylated CD11c (HL3), Ly49A (A1), TER-119, Gr-1, phycoerythrin (PE)–labeled Ly49A+D (12A8) PE- or biotin-labeled NK1.1, allophycocyanin (APC)– or fluorescein isothiocyanate (FITC)–labeled CD8α (53-6.7) and CD3ε (2C11), and APC-labeled Ly49G2 (LGL-1) were purchased from PharMingen (San Diego, CA). Biotinylated antibodies were visualized with streptavidin-PE (PharMingen) or streptavidin-APC (Molecular Probes, Leiden, The Netherlands). Anti–H-2Kb monoclonal antibody (mAb) Y-3 (HB-176) and F4/80 hybridoma (HB-198) were obtained from American Type Culture Collection. Y-3 mAb was labeled with Cy5 as recommended by the provider (Amersham, Little Chalfont, United Kingdom). Stained cells were sorted with a MoFlo (Cytomation, Fort Collins, CO) or a FACStar Plus (Becton Dickinson).
Cytotoxicity assay and mixed-lymphocyte reaction
Sorted NK1.1+CD3− cells were stimulated with IL-2 for 6 days. Thereafter, their cytotoxic activity was tested on YAC-1 cells and lipopolysaccharide-stimulated spleen cells of β2m−/− mice as previously described.25
For the mixed lymphocyte reaction, 3 × 103 sorted and γ-irradiated CD11c+CD8α+ and CD11c+CD8α− cells and 2 × 106γ-irradiated C57Bl/6 or Balb/c splenocytes were cocultured with 2 × 105 C57Bl/6 or Balb/c lymph node cells for 5 days. [3H]-thymidine uptake was measured during the last 14 hours of culture.
Results
In vivo NK-cell development from Pax5−/−pre-B I–cell clones in NK-cell–deficient mice
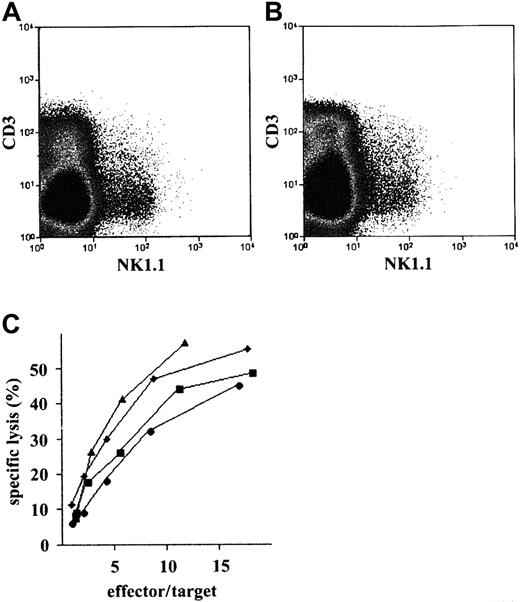
Pax5−/− pre-B I–cell clones can be induced to develop to osteoclasts in vitro by the removal of IL-7 and the presence of tumor necrosis factor–related activation-induced cytokine (TRANCE)–expressing stromal cells.18Furthermore, Pax5−/− pre-B I cells have been found to give rise to osteoclasts in osteoclast-deficient c-fos−/− mice.18 These experiments showed that the transplantation ofPax5−/− pre-B I cells can, at least in part, reconstitute a deficient hematopoietic lineage compartment. Therefore, we attempted to repair other hematopoietic deficiencies.Pax5−/− pre-B I–cell clones have been shown to develop into NK cells in vitro with the removal of IL-7 and the presence of IL-2.18 Because NK-cell development is deficient in cγ−/− mice,26 we transplanted in vitro–grown Pax5−/− pre-B I–cell clones into RAG2cγ−/−recipients. Within weeks, a small population of NK1.1+CD3− cells appeared in thymus, spleen, and bone marrow of the NK-cell–deficient recipients (Figure1A). However, appreciable numbers of NK cells were only detectable 6 weeks after the transplantation ofPax5−/− pre-B I cells. We conclude thatPax5−/− pre-B I–cell clones can, to a degree, reconstitute NK-cell development in NK-cell–deficient hosts.
Generation of NK cells.
Flow cytometric analysis of (A)RAG2cγ−/−and (B) Balb/cRAG2−/−thymi 2 months after reconstitution with a Pax5−/−pre-B I–cell clone. (C) Cytotoxic activity of sorted NK1.1+CD3− cells was sorted from RAG2cγ−/−(▴ and ▪) and Balb/c RAG2−/−(♦ and ●) thymi transplanted 2 months earlier with Pax5−/−pre-B I–cell clones 4 (▴ and ♦) and 5 (▪ and ●). Sorted cells were activated for 7 days with IL-2 and then were assayed for their cytotoxicity against YAC-1 targets.
Generation of NK cells.
Flow cytometric analysis of (A)RAG2cγ−/−and (B) Balb/cRAG2−/−thymi 2 months after reconstitution with a Pax5−/−pre-B I–cell clone. (C) Cytotoxic activity of sorted NK1.1+CD3− cells was sorted from RAG2cγ−/−(▴ and ▪) and Balb/c RAG2−/−(♦ and ●) thymi transplanted 2 months earlier with Pax5−/−pre-B I–cell clones 4 (▴ and ♦) and 5 (▪ and ●). Sorted cells were activated for 7 days with IL-2 and then were assayed for their cytotoxicity against YAC-1 targets.
In vivo NK-cell development from Pax5−/−pre-B I–cell clones in NK-cell–competitive mice
Most of our previous in vivo analyses of T-cell development fromPax5−/− pre-B I cells inRAG2−/− recipients were performed 3 to 6 weeks after transplantation. The slow kinetics of reconstitution of NK-cell development in RAG2cγ−/−recipients prompted us to test the potential ofPax5−/− pre-B I cells to give rise to NK cells in vivo in a competitive situation. Therefore,Pax5-deficient pre-B I– cell clones were transferred into Balb/c RAG2−/− mice, which have normal NK-cell development. Donor-derived NK-cell development can be distinguished from that derived from endogenous progenitors because only transplanted but not endogenous NK cells express the NK1.1 marker. In vitro–grownPax5−/− pre-B I–cell clones can also give rise to NK cells when transplanted into Balb/cRAG2−/− recipients (Figure 1B).
To test the functional capacity of the Pax5−/−pre-B I–cell–derived NK cells, NK1.1+CD3− cells were FACS-purified fromRAG2cγ−/− and from Balb/cRAG2−/− recipients and then stimulated in vitro with IL-2 for 6 days. NK cells developed in vivo from 2 transplanted Pax5−/− pre-B I–cell clones, PC-4 and PC-5 and, when stimulated with IL-2 in vitro, were found to lyse the classical NK target YAC-1 efficiently (Figure 1C). This was the case for both types of NK cells, isolated either fromRAG2cγ−/− or Balb/cRAG2−/− recipients.
In the mouse, NK cells express various receptors for major histocompatibility complex class I molecules. These receptors prevent NK cells from killing major histocompatibility complex class I–expressing target cells. The Ly49 gene family encodes at least 9 closely related receptors of this kind. Recently, it has been shown that NK cells acquire these receptors with development.27 We compared Ly49A, G2, and A+D receptor expression on C57Bl/6 and Pax5−/−pre-B I–cell–derived NK cells. A small fraction of each NK-cell population expresses Ly49A, and approximately half express Ly49G2, Ly49A+D, or both (Figure 2A). Thus, with respect to Ly49 receptor expression, NK cells derived from transplantedPax5−/− pre-B I cells do not seem to differ from endogenous wild-type NK cells. Moreover, FACS-purified, IL-2–stimulated Pax5−/−-derived NK cells lyse YAC-1 and β2m−/− targets as efficiently as endogenous wild-type C57Bl/6 NK cells do (Figure 2B). We conclude that functional NK cells can develop in vivo from transplantedPax5−/− pre-B I cells in NK-cell–deficient or NK-cell–competitive hosts.
Generation of NK cells.
(A) Flow cytometric analysis of wild-type C57Bl/6 spleen cells andRAG2cγ−/−spleen cells 3 months after reconstitution with a Pax5−/−pre-B I–cell clone. (B) Cytotoxicity assay. NK1.1+CD3− cells were sorted from C57Bl/6 spleen (●), and reconstitutedRAG2cγ−/− (○) were activated for 7 days in vitro with IL-2 and then assayed for their cytotoxicity against YAC-1 and lipopolysaccharide-stimulated spleen cells of β2m−/− mice.
Generation of NK cells.
(A) Flow cytometric analysis of wild-type C57Bl/6 spleen cells andRAG2cγ−/−spleen cells 3 months after reconstitution with a Pax5−/−pre-B I–cell clone. (B) Cytotoxicity assay. NK1.1+CD3− cells were sorted from C57Bl/6 spleen (●), and reconstitutedRAG2cγ−/− (○) were activated for 7 days in vitro with IL-2 and then assayed for their cytotoxicity against YAC-1 and lipopolysaccharide-stimulated spleen cells of β2m−/− mice.
In vivo development of dendritic cells, macrophages, and granulocytes from Pax5−/−pre-B I–cell clones
Our findings that NK cells develop fromPax5−/− pre-B I–cell clones in vivo in a mouse with NK-proficient progenitors prompted us to test thePax5−/− pre-B-cell–transplantation hosts for the development of donor-derived DCs, macrophages, and granulocytes.Pax5−/− pre-B I cells have previously been found capable, under appropriate stimulatory conditions of cytokines and cell contacts, to develop in vitro into CD8α− DCs, phagocytic macrophages, and granulocytes.18
Pax5−/− pre-B I–cell clones of H-2b/d haplotype were transplanted into Balb/c (H-2d) RAG2−/− mice. To test for the development of DCs, 3-color FACS analyses were performed 6 to 12 weeks after transplantation, and cell suspensions were prepared by enzymatic digestion of thymus (data not shown) and spleen (Figure3A). Results of these analyzes show that 30% to 40% of the CD11c+CD8α− and the CD11c+CD8α+ cells are H-2b-positive (ie, of Pax5−/−origin). They also carry the clone-characteristic DHJH rearrangements (data not shown). Similar findings were made with the transfer of GFP-markedPax5−/− pre-B I–cell clones (data not shown).
Generation of DCs.
(A) Flow cytometric analysis on splenic cells of Balb/cRAG2−/−(H-2d) mice that received transplantations 3 months earlier of Pax5−/−H-2b/d pre-B I cells. (B) Mixed lymphocyte reaction. CD11c+CD8α+ and CD8α− cells ofPax5−/−origin (H-2b) and of host Balb/c RAG2−/−(H-2d) were sorted and γ-irradiated, and 3 × 103 of these were incubated for 5 days with 2 × 105 Balb/c or C57Bl/6 lymph node cells. γ-Irradiated spleen cells (1 × 106) of Balb/c or C57Bl/6 mice were used as controls. [3H]-thymidine incorporation was measured during the last 14 hours of incubation. Stimulators were CD11+CD8α+H-2b/d, CD11+CD8α+H-2d, CD11+CD8α−H-2b/d, CD11+CD8α−H-2d, and Balb/c H-2d splenocytes, and C57Bl/6 H-2b splenocytes.
Generation of DCs.
(A) Flow cytometric analysis on splenic cells of Balb/cRAG2−/−(H-2d) mice that received transplantations 3 months earlier of Pax5−/−H-2b/d pre-B I cells. (B) Mixed lymphocyte reaction. CD11c+CD8α+ and CD8α− cells ofPax5−/−origin (H-2b) and of host Balb/c RAG2−/−(H-2d) were sorted and γ-irradiated, and 3 × 103 of these were incubated for 5 days with 2 × 105 Balb/c or C57Bl/6 lymph node cells. γ-Irradiated spleen cells (1 × 106) of Balb/c or C57Bl/6 mice were used as controls. [3H]-thymidine incorporation was measured during the last 14 hours of incubation. Stimulators were CD11+CD8α+H-2b/d, CD11+CD8α+H-2d, CD11+CD8α−H-2b/d, CD11+CD8α−H-2d, and Balb/c H-2d splenocytes, and C57Bl/6 H-2b splenocytes.
To test the functionality of these CD11c+ cells, endogenous Balb/c (H-2d–positive) andPax5−/−-derived (H-2b/d–positive) CD11c+CD8α− and CD8α+ DCs were sorted and used in a mixed lymphocyte reaction. As few as 3000 H-2b–positive (Pax5−/−-derived H-2b/d–positive) CD11c+CD8α−and CD8α+ cells elicited a strong proliferative T-cell response from Balb/c and C57Bl/6 (H-2b–positive) mice (Figure 3B). On the other hand, endogenous H-2d–positive, CD11c+CD8α+ and CD8α− cells induced a strong response of allogeneic C57Bl/6 T cells but not of syngeneic Balb/c T cells (Figure 3B). Hence,Pax5−/− pre-B I–cell clones can develop in vivo into functional CD8α− DCs. In contrast to development in vitro, Pax5−/− pre-B I–cell clones can also differentiate in vivo to functional CD8α+DCs.
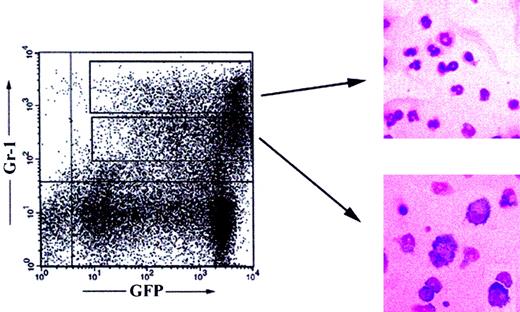
Next, we analyzed Balb/c RAG2−/− hosts that received transplants of GFP-marked Pax5−/−pre-B I–cell clones from 6 weeks to 6 months after transplantation for GFP+CD11b+ (Mac-1), GFP+Gr-1+, and GFP+Ter119+ cells. Because granulocytes express high levels of Gr-1 and CD11b, whereas macrophages are Gr-1int and CD11bhigh, we FACS-sorted GFP+Gr-1high and Gr-1int cells. After a 30-hour culture period in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), all cells still expressed high levels of GFP. May-Grünwald-Giemsa staining of cytospin preparations of these cells then revealed large numbers of cells with segmented nuclear morphology, typical for granulocytes, in the GFP+Gr-1high population (Figure4). On the other hand, GFP+Gr-1int cells showed typical macrophage morphology (Figure 4). We conclude that Pax5−/−pre-B I–cell clones can give rise to granulocytes and macrophages not only in vitro but also in vivo.
Generation of granulocytes and macrophages.
Flow cytometric analysis of splenocytes of Balb/cRAG2−/−mice reconstituted 5 months earlier with a GFP+Pax5−/−pre-B I–cell clone. May-Grünwald-Giemsa staining of cytospin preparations of sorted GFP+Gr-1high (right, upper panel) and Gr-1int (right, lower panel) spleen cells. Magnification × 400.
Generation of granulocytes and macrophages.
Flow cytometric analysis of splenocytes of Balb/cRAG2−/−mice reconstituted 5 months earlier with a GFP+Pax5−/−pre-B I–cell clone. May-Grünwald-Giemsa staining of cytospin preparations of sorted GFP+Gr-1high (right, upper panel) and Gr-1int (right, lower panel) spleen cells. Magnification × 400.
In vivo development of erythrocytes fromPax5−/−pre-B I–cell clones
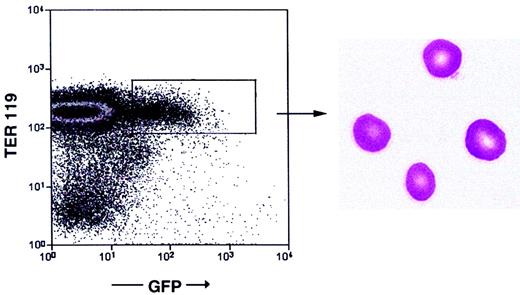
Finally, we analyzed by flow cytometry Balb/cRAG2−/− mice that received transplants of GFP-expressing Pax5−/− pre-B I–cell clones for the presence of GFP+Ter119+ erythrocytes.Pax5−/− pre-B I cells have so far not been seen to develop into erythrocytes in vitro. Up to 4 months after transplantation, none of the recipients had detectable numbers of GFP+Ter119+ erythrocytes in their circulatory systems. However, in 4 of 25 recipients analyzed as late as 4 to 6 months after transplantation, between 0.15% and 3% of all erythroid cells were GFP+Ter119+ erythrocytes (Figure5). May-Grünwald-Giemsa staining of cytospins of GFP+Ter119+ cells confirmed their erythrocyte nature (Figure 5). Our results thus show thatPax5−/− pre-B I–cell clones can give rise even to cells of the erythroid lineage.
Generation of erythrocytes.
Flow cytometric analysis of peripheral blood of Balb/cRAG2−/−mice reconstituted 6 months earlier with a GFP+Pax5−/−pre-B I–cell clone. May-Grünwald-Giemsa staining of sorted GFP+Ter119+ cells (right panel). Magnification × 630.
Generation of erythrocytes.
Flow cytometric analysis of peripheral blood of Balb/cRAG2−/−mice reconstituted 6 months earlier with a GFP+Pax5−/−pre-B I–cell clone. May-Grünwald-Giemsa staining of sorted GFP+Ter119+ cells (right panel). Magnification × 630.
Variations in Pax5−/−pre-B I–cell–derived hematopoietic engraftment
We have observed previously that all RAG2−/−recipients become populated by donor-derived thymocytes and mature CD4+ and CD8+ T cells in normal numbers19 and with normal antigen-recognizing repertoires of T-cell receptors (data not shown) within 3 to 5 weeks of transplantation. In the blood, donorPax5−/−-derived T cells constitute 5% to 30% of all leukocytes at 3 months after transplantation (Table1). Furthermore, all recipients are engrafted with approximately 5% to 10% of their total nucleated bone marrow cells by donor-derived cells, which, in most, are of original B220+c-Kit+CD19−Pax5−/−pre-B I–cell phenotype. The engraftments in the thymus, the peripheral T-cell compartments, and the pre-B I–cell compartments of the bone marrow appear to be stable in phenotype and cell number for several months after transplantation.
Percentage of total, granulocyte/macrophage, and T cells ofPax5−/− origin
| Months . | % of total cells* . | granulocytes/macrophages . | % T cells of total cells1-153 . | ||||
|---|---|---|---|---|---|---|---|
| % of total cells† . | % of total Gr/Mac‡ . | ||||||
| 3 . | 4 . | 3 . | 4 . | 4 . | 3 . | 4 . | |
| Mouse | |||||||
| 1 | 42.5 | 73.6 | 15.5 | 65.7 | 76.0 | 28.5 | 7.1 |
| 2 | 59.0 | 84.7 | 47.3 | 64.2 | 87.6 | 9.7 | 18.6 |
| 3 | 13.6 | 9.2 | 1.5 | 3 | 2.1 | 11.5 | 7.2 |
| 4 | 8.2 | 2.5 | ND | ND | ND | 6.9 | 2.5 |
| 5 | 25.1 | 16.4 | 10.9 | 10.2 | 15.2 | 15.1 | 6.2 |
| 6 | 8.4 | 10.3 | 1.0 | 2.0 | 2.0 | 8.0 | 8.0 |
| 7 | 3.3 | 4.4 | ND | ND | ND | 3.1 | 3.3 |
| 8 | 8.5 | 8.1 | ND | ND | ND | 6.7 | 5.9 |
| 9 | 40.4 | 75.2 | 42.2 | 64.2 | 84.3 | 18.2 | 16.2 |
| 10 | 75.0 | 87.0 | 69.1 | 74.5 | 92.9 | 8.1 | 12.7 |
| Months . | % of total cells* . | granulocytes/macrophages . | % T cells of total cells1-153 . | ||||
|---|---|---|---|---|---|---|---|
| % of total cells† . | % of total Gr/Mac‡ . | ||||||
| 3 . | 4 . | 3 . | 4 . | 4 . | 3 . | 4 . | |
| Mouse | |||||||
| 1 | 42.5 | 73.6 | 15.5 | 65.7 | 76.0 | 28.5 | 7.1 |
| 2 | 59.0 | 84.7 | 47.3 | 64.2 | 87.6 | 9.7 | 18.6 |
| 3 | 13.6 | 9.2 | 1.5 | 3 | 2.1 | 11.5 | 7.2 |
| 4 | 8.2 | 2.5 | ND | ND | ND | 6.9 | 2.5 |
| 5 | 25.1 | 16.4 | 10.9 | 10.2 | 15.2 | 15.1 | 6.2 |
| 6 | 8.4 | 10.3 | 1.0 | 2.0 | 2.0 | 8.0 | 8.0 |
| 7 | 3.3 | 4.4 | ND | ND | ND | 3.1 | 3.3 |
| 8 | 8.5 | 8.1 | ND | ND | ND | 6.7 | 5.9 |
| 9 | 40.4 | 75.2 | 42.2 | 64.2 | 84.3 | 18.2 | 16.2 |
| 10 | 75.0 | 87.0 | 69.1 | 74.5 | 92.9 | 8.1 | 12.7 |
ND indicates not detectable.
Percentage of blood nucleated cells ofPax5−/− origin as determined by H-2b- and GFP-expression.
Percentage of blood nucleated cells ofPax5−/− origin expressing Gr-1 and Mac-1.
Percentage of Gr-1 and Mac-1 expressing cells ofPax5−/− origin.
Percentage of blood nucleated cells ofPax5−/− origin expressing CD3.
By contrast, only half the recipients that received transplants become populated in the myeloid (macrophage and granulocyte) compartments. The contribution of donor Pax5−/−-derived cells in the blood increases with time to 75% to 95% of total myeloid cells within 4 months of transplantation (Table 1). The slow, but continued, increase of donor-derived cells suggests that the Pax5−/−-progenitor compartment has a stronger repopulation capacity than does the endogenous host. Because no myeloid tumors have been observed in hosts that received transplants within 6 months of transplantation, this stronger repopulation capacity does not appear to be the result of a neoplastic transformation of thePax5−/− progenitors.
Discussion
It has been demonstrated thatPax5−/−-derived pre-B I–cell clones can differentiate in vitro, in the proper environments of cytokines and cell contacts, into various myeloid cell lineages—macrophages and granulocytes, CD8α− DCs and osteoclasts, and NK cells.18 Moreover, in vivo transplantation ofPax5−/− pre-B I–cell clones into lymphoid-deficient RAG2−/− mice results in rapid and full reconstitution of T-cell lineage compartments and the precursor B-cell compartment in bone marrow,19 whereas transplantation into osteoclast-deficient c-fos−/−mice gives rise to at least partial reconstitution withPax5−/−-derived osteoclasts.18 In these earlier experiments, the other hematopoietic cell lineages into which Pax5−/− pre-B I cells can develop in vitro were not detected in vivo. Hence, it was argued thatPax5−/− pre-B I cells could only populate those hematopoietic compartments for which the host carried a genetic deficiency, resulting in the absence or in reduced numbers of that hematopoietic cell lineage.18
However, in this study we demonstrated that Pax5−/−pre-B I– cell clones can also repopulate hematopoietic cell lineages for which host endogenous progenitors and mature cell compartments are not deficient. Thus, we show here that the transplantation of Pax5−/− pre-B I–cell clones into RAG2−/− mice can result in the development of NK cells, DCs, macrophages, granulocytes, and, in some cases, even erythrocytes—that is, into lineages in which theRAG2−/− host has no obvious defects. It should be noted, however, that the kinetics of development of these cell lineages is slower than that of the development of T cells, which is why they were missed in previous studies.18 19
We also conclude from the results presented here that the in vivo developmental potential of Pax5−/− pre-B I cells exceeds even the previously observed in vitro differentiation capacity. It has been observed that Pax5−/−pre-B I cells can differentiate in vitro in the presence of M-CSF and GM-CSF into CD8α− but not into CD8α+DCs (into myeloid but not lymphoid DCs).18 Here we show that Pax5−/− pre-B I cells can give rise to CD8α+ DCs in vivo. This now allows study of the genetic program of development of these 2 DC lineages and of their functions and their relations to each other from a clone ofPax5−/− pre-B I cells.
Moreover, and again for the first time, we show thatPax5−/− pre-B I–cell clones can give rise to erythrocytes. We detected cells of the erythroid lineage as Ter119+ cells and those generated fromPax5−/− pre-B I cells as GFP+Ter119+. If GFP had a short half-life in erythrocytes and if it was shorter than that of Ter119, we might not have scored all Pax5−/− pre-B I–cell–derived erythroid cells but might have considered them to be host derived. This could partially have explained the apparently slow kinetics and low frequency of erythroid development. We plan to use a different form of the enzyme glucose-phosphate-isomerase for the detection of erythroid cells in transplantation experiments with Pax5−/−pre-B I cell–clones. Alternatively, it is also possible that the slow and inefficient development of erythrocytes is a result of an inefficient competition between Pax5−/− pre-B I–cell–derived erythroid progenitors with those of the host. The low efficiency of Pax5−/− pre-B I cells to generate erythroid cells might be another reason these cells cannot be used to rescue lethally irradiated recipients from death in a bone marrow transplantation–like situation.
One obvious question arising from previous studies and the current study is why Pax5−/−, but not wild-type pre-B I, cells can differentiate into various hematopoietic lineages.Pax5−/− pre-B I cells, unlike their wild-type counterparts, express multiple genes known to play key roles in the development of hematopoietic lineages other than B cells, such asM-CSF-receptor and MPO for myeloid andGATA-1 for erythroid development. Retroviral introduction ofPax5 into Pax5−/− pre-B I cells results in the complete down-regulation of expression of those genes.18 It has been shown that the transgenic expression of the M-CSF-receptor in early pre-B-cell lines enables these cells to differentiate into macrophages in the presence of M-CSF,28 whereas the introduction of GATA-1reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts.29 Thus, the expression of genes such as M-CSF-receptor, MPO, orGATA-1 in Pax5−/− pre-B I cells might be expected to allow these cells, under appropriate in vitro and, as shown here, in vivo conditions, to dedifferentiate and develop into various hematopoietic lineages other than B cells.
Why does the differentiation of Pax5−/− pre-B I cells into the various hematopoietic lineages occur with different kinetics and with different efficiencies? Three possible reasons can be considered. The first is that it might be easier to reconstitute an empty thymus and peripheral mature T-cell compartments, even though the CD4−CD8− thymocyte progenitors are present in the RAG2−/− hosts in normal numbers. Moreover, and in contrast to myeloid and erythroid cells, during their development in the thymus, T-lineage cells go through a phase of proliferative expansion. This cellular expansion, mediated by the pre-T-cell receptor,30 31 would allow the detection of T-lineage cells in appropriate numbers much earlier (ie, shortly after transplantation), whereas it would take longer for nonproliferating myeloid precursors to accumulate detectable numbers of mature myeloid cells.
The second reason for the differential efficiencies of hematopoietic lineage developments from Pax5−/− pre-B I cells could be a result of the number of dedifferentiation and redifferentiation steps this pre-B I cell must take to develop into the various lineages of hematopoietic differentiation. Two models of differentiation of HSCs into various hematopoietic lineages have been proposed. In one, HSCs differentiate through intermediate HSC stages, with decreasing self-renewal capacity, into CLPs, which give rise to T, B, and NK cells, and into CMPs, which develop into myeloid and erythroid lineages.1 Neither CLP nor CMP has self-renewal potential; therefore, they have to be produced throughout life to keep the hematopoietic system intact. In the other model, hematopoietic differentiation is based on a series of experimentally induced mutations in genes encoding transcription factors.14 This model proposes a common progenitor with myeloid and lymphoid capacity that has lost the ability to generate erythroid and megakaryocytic cells. This progenitor then can give rise to all cell types, similar to the CLP of the first model. In each model, the choice of differentiation to T, B, or NK cells is a late event. Therefore, the dedifferentiation of Pax5−/− pre-B I cells into the T- and NK-cell lineage is the closest step and might be the easiest and most efficient. On the other hand, in the Weissman model1 of differentiation, Pax5−/−pre-B I cells would have to dedifferentiate to HSCs to become erythroid cells, whereas in the Singh model,14they would only have to dedifferentiate to an erythroid-myeloid-lymphoid progenitor. In mice in which erythrocytes ofPax5−/− origin are detectable, the myeloid compartment is to a large extent Pax5−/− pre-B I–cell derived.
The third reason for a differential hematopoietic reconstitution capacity could be the consequence of varying strengths of the various progenitors and precursors of the different hematopoietic lineages to compete with endogenous counterparts. That Pax5−/−pre-B I cells repopulate the full precursor B-cell compartments for long periods of time at stable, normal numbers argues the possibility that Pax5−/− pre-B I cells and their dedifferentiated T-lymphoid progenitors are stronger in reconstituting the host than endogenous progenitors. NK-cell and DC development appear equally effective, though it is difficult to judge whether these lineages actually outcompete the endogenously derived ones. We should be able to test this possibility by transplantingPax5−/− pre-B I cells into normal wild-type hosts rather than into severe combined immunodeficient hosts.
A similarly stronger and stable repopulation capacity of thePax5−/− pre-B I cells can be observed in the myeloid lineages. In half of all hosts that underwent transplantations that develop these myeloid cells,Pax5−/−-derived macrophages and granulocytes outgrow those of the host (Table 1). However, because only 50% of all hosts develop myeloid cells yet all of them develop T-lineage cells, the competition of Pax5 progenitors for reconstitution to the myeloid lineage compartments appears to be less efficient. Given that only 15% of all hosts that received transplants developPax5−/− pre-B-cell–derived erythrocytes, this competition for lineage development with the endogenous cells appears even less efficient. The lack of evidence that T-lymphoid or -myeloid neoplasms develop in hosts that received transplants is taken as an indication that the stronger repopulation capacity of thePax5−/− pre-B I–cell–derived cells is not the result of a malignant, oncogenic transformation as a result of thePax5 mutation.
The 3 possible reasons for differential capacities ofPax5−/− pre-B I–cell–derived progenitors to compete with endogenous counterparts for the cellular chimerisms in a given compartment and for the strength to generate mature cells are, obviously, not mutually exclusive. Pax5−/−pre-B I–cell clones and their dedifferentiated progenitors of the various hematopoietic lineages offer fascinating possibilities to quantitate hematopoietic capacities of different progenitor compartments, maybe even of pluripotent HSCs, in competition with wild-type cells of the hosts that underwent transplantations. Although the pluripotency of hematopoietic differentiation ofPax5−/− pre-B I cells is of the right quality but not yet of the right quantity, further mutations and alterations of the control of gene expression programs active in early hematopoiesis might eventually lead to a better understanding of how to manipulate hematopoietic progenitor development for bone marrow transplantation.
We thank Drs K. Karjalainen and A. Potocnik for critical reading of the manuscript.
The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche Ltd, Switzerland.
C.S. and L.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Antonius G. Rolink, Basel Institute for Immunology, Grenzacherstrasse 487, CH-4005 Basel, Switzerland; e-mail:rolink@bii.ch.


![Fig. 3. Generation of DCs. / (A) Flow cytometric analysis on splenic cells of Balb/cRAG2−/− (H-2d) mice that received transplantations 3 months earlier of Pax5−/−H-2b/d pre-B I cells. (B) Mixed lymphocyte reaction. CD11c+CD8α+ and CD8α− cells ofPax5−/− origin (H-2b) and of host Balb/c RAG2−/− (H-2d) were sorted and γ-irradiated, and 3 × 103 of these were incubated for 5 days with 2 × 105 Balb/c or C57Bl/6 lymph node cells. γ-Irradiated spleen cells (1 × 106) of Balb/c or C57Bl/6 mice were used as controls. [3H]-thymidine incorporation was measured during the last 14 hours of incubation. Stimulators were CD11+CD8α+H-2b/d, CD11+CD8α+H-2d, CD11+CD8α−H-2b/d, CD11+CD8α−H-2d, and Balb/c H-2d splenocytes, and C57Bl/6 H-2b splenocytes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.472/6/m_h80222030003.jpeg?Expires=1769178562&Signature=A4c1yA6CKYaLgl7XS6GkBebQ1RM7LgXcv1Wj-0WfPe2TyfrXzUHElWpDvgs1jwxaTZSoWgKmoucSy8kbyE~iUCRgePbMU~9Qa~dGdAJliSrsrxb396Zyppt5Kx6nGfP6tEZP66Bh~ipJXSOfpGrjmiY7TfdvG82EIHyvVwthSZI-XjN5di1KGzi5BlhqFzmouqBgH76Vt7Ob5Wb8q6nm3zyWx9FYqujY5nt6c5Zkv5o8UTLq9ywIWCh-LbKTj6R4YTgtsT18eu81zaOyZ9BMeq93fIMOu~OC0QEGach70e8SbnWohsRZ4IG3UYmQQRbtJYd2JCxGMcTxnFF6ecfa7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal