The interaction between platelet glycoprotein (GP) Ibα and von Willebrand factor (VWF) is essential for initiation of hemostasis. The sulfation of the 3 tyrosine residues 276, 278, and 279 in GPIbα is an important posttranslational modification that seems to promote the interaction with VWF. The environment where sulfation of tyrosines occurs has been proposed to contain highly acidic residues. This investigation has examined the highly acidic region from Asp249 to Asp287 in the mature GPIbα protein. Changes to most of the carboxylic acids in this region resulted in decreased reactivity to VWF. Only 3 mutants (Glu270Gln, Asp283Asn, Asp283Asn/Glu285Gln/Asp287Asn) resulted in the abolition of sulfation. Two novel mutations were also created. First, a deletion of the 7 amino acids from Tyr276 to Glu282 led to a loss of sulfation and totally abolished VWF binding in the presence of botrocetin. This confirms that it is these 3 tyrosines that undergo sulfation and that this region is crucial for botrocetin-mediated VWF binding. The second mutation involves changing the lysine residues at 253, 258, and 262 to alanine. This also led to distinct changes in VWF binding and abolition of sulfation.
Introduction
Posttranslational modifications are important for the expression and function of proteins. Sulfation of tyrosine residues is one such modification and is catalyzed by tyrosylprotein sulfotransferase in the trans-Golgi network.1,2 This modification is crucial in the interaction between many plasma proteins such as fibronectin and fibrin,3 hirudin and thrombin,4 coagulation factor VIII, and von Willebrand factor (VWF)5 and glycoprotein (GP) Ibα with both VWF and thrombin.6-10
The interaction between the platelet membrane protein GPIbα and VWF is essential for initiation of hemostasis. GPIbα is the ligand-binding subunit in the GPIb-V-IX receptor complex.11-15 Each of the 4 polypeptides of this complex spans the platelet membrane once and is a member of the leucine-rich motif family.16-19 The binding site for VWF is located in the amino-terminal domain of GPIbα20-22and the proposed sulfated tyrosine residues are located within this region at positions 276, 278, and 279.6-8
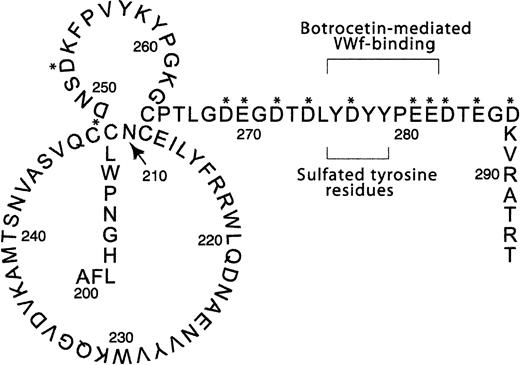
The consensus sequence for tyrosine sulfation has not been fully defined, but the presence of acidic amino acids adjacent to the tyrosine residues seems to be important.23-25 Three of the 9 tyrosine residues within GPIbα are located in a region with 43.5% acidic residues encompassed by Asp249 to Asp287 (Figure1). A previous examination of the carboxylic acids in this region has demonstrated how these amino acids affect both the conformation of the amino-terminal region of GPIbα and the electrostatic interaction with VWF in the presence of both ristocetin and botrocetin.26
GPIbα from amino acid 200-294.
This is a representation of part of the amino-terminal region of GPIbα containing the high concentration of acidic amino acids (indicated by the asterisk) surrounding the 3 sulfated tyrosine residues at positions 276, 278, and 279. Also shown is the 7 amino acid region from Tyr276 to Glu282 possibly involved in botrocetin-mediated VWF binding as demonstrated by Ward et al.8 This figure is an adaptation from Hess et al.34
GPIbα from amino acid 200-294.
This is a representation of part of the amino-terminal region of GPIbα containing the high concentration of acidic amino acids (indicated by the asterisk) surrounding the 3 sulfated tyrosine residues at positions 276, 278, and 279. Also shown is the 7 amino acid region from Tyr276 to Glu282 possibly involved in botrocetin-mediated VWF binding as demonstrated by Ward et al.8 This figure is an adaptation from Hess et al.34
In this investigation, specific mutations were introduced into GPIbα complementary DNA (cDNA) as outlined in Table1 and the mutant DNA was expressed in Chinese hamster ovary (CHO) cells already expressing GPIbβ and GPIX. Previous studies examining recombinant proteins in CHO cells have demonstrated that tyrosine sulfation occurs in this cell system.6 To determine the effect of changing the aspartic and glutamic acids to asparagine and glutamine, respectively, VWF binding to the mutant proteins was examined, in a membrane-bound and complexed form of GPIbα. Examination of VWF binding to mutated GPIbα indicated that specific changes led to decreased ligand binding. Further examination of those mutations that led to a decrease in VWF binding revealed 2 carboxylic acids that, after mutation, prevented sulfation of the mutant GPIbα receptor.
Summary of the mutations of GPIbα
| Mutant cell line . | Codon changes . |
|---|---|
| D249N/D252N (Comb-3) | GAC to AAC |
| GAC to AAC | |
| K253A/K258A/K262A (Comb-2) | AAG to GCG |
| AAA to GCA | |
| AAG to GCG | |
| DEL* | Deletion of nc 874-894† |
| D269N | GAT to AAC |
| E270Q | GAA to CAA |
| D272N | GAC to AAC |
| D274N | GAC to AAC |
| D277N | GAT to AAT |
| D277A | GAT to GCT |
| E281Q | GAA to CAA |
| E282Q | GAG to CAG |
| D283N | GAC to AAC |
| E285Q | GAG to CAA |
| D287N | GAT to AAT |
| D283N/E285Q/D287N (Comb-1) | GAC to AAC |
| GAG to CAA | |
| GAT to AAT |
| Mutant cell line . | Codon changes . |
|---|---|
| D249N/D252N (Comb-3) | GAC to AAC |
| GAC to AAC | |
| K253A/K258A/K262A (Comb-2) | AAG to GCG |
| AAA to GCA | |
| AAG to GCG | |
| DEL* | Deletion of nc 874-894† |
| D269N | GAT to AAC |
| E270Q | GAA to CAA |
| D272N | GAC to AAC |
| D274N | GAC to AAC |
| D277N | GAT to AAT |
| D277A | GAT to GCT |
| E281Q | GAA to CAA |
| E282Q | GAG to CAG |
| D283N | GAC to AAC |
| E285Q | GAG to CAA |
| D287N | GAT to AAT |
| D283N/E285Q/D287N (Comb-1) | GAC to AAC |
| GAG to CAA | |
| GAT to AAT |
Single-letter amino acid codes used in the table.
This mutant contains a deletion of the 7 amino acid region from Tyr276 to Glu282.
The nucleotide (nc) count starts from the ATG site.
Materials and methods
Reagents
The monoclonal antibodies (mAbs) AK2 (anti-GPIbα), AK3 (anti-GPIbα), and WM23 (anti-GPIbα) and purified human VWF and botrocetin were a kind gift from Professor Michael Berndt (Baker Medical Research Institute, Melbourne, Victoria, Australia).
The plasmid pZeoSV and the antibiotic zeocin were from Invitrogen (Carlsbad, CA). The pAlter-1 vector was from Promega (Madison, WI). The QIAfilter plasmid Maxi kit was from Qiagen (Hilden, Germany). The fluorescein isothiocyanate (FITC)–conjugated rabbit antimouse IgG antibody was from Silenus Laboratories (Melbourne, Victoria, Australia) and the FITC-conjugated antihuman VWF was from Serotec (Oxford, United Kingdom). The cell culture reagents fetal bovine serum (FBS) and Dulbecco modified Eagle medium (DMEM) were from Trace Bioscientific (Melbourne, Victoria, Australia). Ristocetin was from Sigma Chemical (St Louis, MO). Pansorbin beads were from Calbiochem (La Jolla, CA).
Site-directed mutagenesis
The full-length cDNA for GPIbα was subcloned into the mutagenesis vector pAlter-1. Site-directed mutagenesis was performed on single-stranded DNA as described by the manufacturer. The amino acid changes are outlined in Table 1. The oligonucleotides used to change the specified amino acids were custom made by Life Technologies (Gaithersburg, MD). The DNA was sequenced to confirm the presence of the directed mutations and to identify any nonspecific mutations.
Transfection of CHO cells
The CHO-βIX cells were grown in DMEM containingl-glutamine and 4.5 g/L glucose, supplemented with 3.7 g/L sodium bicarbonate and 10% FBS. Cells were incubated at 37°C in an atmosphere of 5% CO2 and 90% humidity.
Mediated VWF-binding assay
Cells were incubated with increasing concentrations of VWF (0-8 μg/mL) as described previously.27 Binding was initiated with the addition of either ristocetin sulfate (0.75 mg/mL) or botrocetin (2 μg/mL). The data are expressed as a ratio of the mean fluorescence for VWF binding over the mean fluorescence of AK3 binding, to normalize for the level of GPIbα expressed by the various recombinant cells.
Metabolic labeling of cells
Cells were grown to 90% confluency, then washed with serum-free DMEM, and incubated with [35S]-sulfur (100 μCi/dish; 3.7 MBq) in sulfate-free DMEM containing 5% dialyzed FBS and 2% of normal concentration of methionine for 4 hours at 37°C. The metabolically labeled cells were washed with ice-cold phosphate-buffered saline and lysed in a buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, 1 μg/mL leupeptin, 1.6 μg/mL benzamidine, 0.1 mg/mL soybean trypsin inhibitor, 1 mM phenylmethylsulfonyl fluoride, and 1% digitonin for 20 minutes at 4°C. The cell lysates were centrifuged at 10 000g for 5 minutes to remove cellular debris and then incubated for 2 hours at 4°C with Pansorbin beads (protein A) to remove proteins that bind the beads nonspecifically. After centrifugation to remove the beads, cell lysates were first incubated with the GPIbα mAb WM23 (2 μg/mL) overnight and then with a rabbit antimouse IgG (Zymed, South San Francisco, CA) for 2 hours at 4°C. The antigen-antibody complex was then precipitated by incubating the cell lysates with 50 μL Pansorbin beads for 2 hours at 4°C followed by 5 minutes of centrifugation at 10 000g. The bead pellets were washed 3 times in lysis buffer by resuspension and centrifugation. Immunoprecipitated proteins were released from the beads by boiling for 5 minutes in sodium dodecyl sulfate (SDS)–sample buffer containing 2% β-mercaptoethanol (final concentration). Equal amounts of protein were loaded and separated by electrophoresis on 7.5% SDS-polyacrylamide gels. Dried gels were exposed to a phosphorimager plate for 72 hours at room temperature. The absence or presence of the incorporated [35S]-sulfur was detected by scanning the plate on a Fuji BAS-1000 Bio-Imaging Analyzer (Fuji, Tokyo, Japan) and the data were analyzed with MacBAS software.
Statistical analysis
Student t test was used to test for differences between the cell lines. A P < .05 was considered to be statistically significant.
Results
Generation of recombinant GPIbα-expressing cell lines
To examine the effects of the mutations on both VWF binding and the sulfation of the tyrosine residues, plasmids subcloned with either wild-type or mutant cDNA for GPIbα were constructed. Cell lines were generated expressing wild-type GPIbα associated with GPIbβ and GPIX (wild-type), a vector-only control cell line (βIX-zeo) and the 14 cell lines described in Table 1.
Effect of mutation on expression of GPIbα
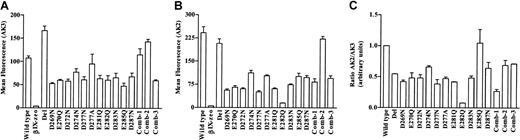
The surface expression of GPIbα was determined by incubating cells with mAb AK3, followed by flow cytometric analysis. The epitope for AK3 is within the macroglycopeptide region and therefore binding should not be affected by the mutations.27 The data for 3 clones of each mutant cell line are presented in Figure2A. Wild-type and all the mutant cells bound AK3 indicating that these cells expressed the GPIbα receptor on their surface. The binding to the mutants ranged from 50% to 150% compared to wild-type. The binding was specific to GPIbα because βIX-zeo cells failed to bind AK3.
Expression of GPIbα on recombinant cell lines.
Transfected cell lines were incubated with the mAbs to GPIbα (A) AK3 and (B) AK2 and cell autofluorescence was detected using a mouse isotype control antibody. Bound mAbs were detected using a FITC-labeled antimouse IgG antibody, followed by flow cytometric analysis (n = 2-3). (C) The data for GPIbα expression are also presented as a ratio of bound AK2 to AK3 binding for the mutants normalized to wild-type expression.
Expression of GPIbα on recombinant cell lines.
Transfected cell lines were incubated with the mAbs to GPIbα (A) AK3 and (B) AK2 and cell autofluorescence was detected using a mouse isotype control antibody. Bound mAbs were detected using a FITC-labeled antimouse IgG antibody, followed by flow cytometric analysis (n = 2-3). (C) The data for GPIbα expression are also presented as a ratio of bound AK2 to AK3 binding for the mutants normalized to wild-type expression.
To determine if the mutations had caused conformational changes to the amino-terminal region of GPIbα, cells were also incubated with mAb AK2. The epitope for AK2 has been localized to residues within the first leucine-rich repeat of GPIbα28 and is known to block VWF binding to both platelets8 and recombinant GPIbα.28 The data presented in Figure 2B demonstrate that only Glu285Gln behaved similarly to wild-type, whereas all the other mutant clones bound significantly less AK2 than wild-type, indicating that these mutations induced a conformational change to the amino-terminal region of GPIbα. This conformational change is also demonstrated by a reduction in the AK2/AK3 ratio compared to wild-type cells (Figure 2C).
Ristocetin-mediated VWF binding
To examine the effect of the mutations on VWF binding, cell lines were treated with human VWF in the presence or absence of ristocetin. We have previously demonstrated that wild-type cells bind VWF in a dose-dependent manner, whereas VWF does not bind to βIX-zeo cells.27 Specificity was confirmed by blocking the binding reaction using the mAb AK2 known to inhibit VWF binding.8 28
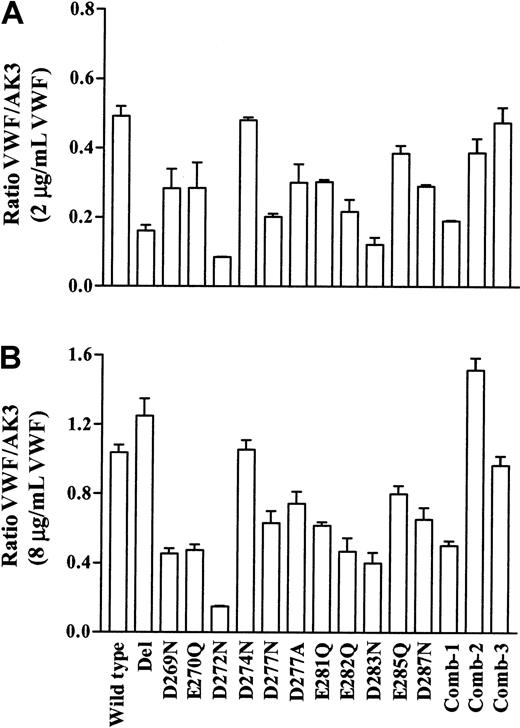
The mutant cells also bound VWF in the presence of ristocetin. The data presented in Figure 3 show binding of VWF normalized for GPIbα expression. At all concentrations of VWF examined, the Asp274Asn and Comb-3 (Asp249Asn/Asp252Asn) mutants bound similar levels of ligand as wild-type cells. At low concentrations of ligand (Figure 3A), statistical analysis showed no significant decrease in VWF binding with the Glu270Gln and Comb-2 (Lys253Ala/Lys258Ala/Lys262Ala) mutants. Cells that bound less than 50% of VWF compared to wild-type included the Del (33%), Asp272Asn (17%), Asp277Asn (41%), Glu282Gln (44%), Asp283Asn (25%), and Comb-1 (Asp283Asn/Glu285Gln/Asp287Asn, 39%) mutants (P < .005, n = 3). The remaining mutants bound 60% to 80% (P < .05, n = 3) of the ligand compared to wild-type at 2 μg/mL VWF.
Ristocetin-induced VWF binding to GPIbα mutant cell lines.
Transfected cell lines were incubated with increasing concentrations of VWF (0-8 μg/mL) in the presence of ristocetin. Bound VWF was detected using an FITC-conjugated antihuman VWF antibody, followed by flow cytometric analysis. The data are expressed as a ratio of the mean fluorescence of VWF binding to that of AK3 binding and 2 points are presented: (A) 2 μg/mL VWF representing results obtained for the range of 0.5 to 4 μg/mL VWF and (B) 8 μg/mL VWF. The mean ratio ± SEM from 3 experiments is shown.
Ristocetin-induced VWF binding to GPIbα mutant cell lines.
Transfected cell lines were incubated with increasing concentrations of VWF (0-8 μg/mL) in the presence of ristocetin. Bound VWF was detected using an FITC-conjugated antihuman VWF antibody, followed by flow cytometric analysis. The data are expressed as a ratio of the mean fluorescence of VWF binding to that of AK3 binding and 2 points are presented: (A) 2 μg/mL VWF representing results obtained for the range of 0.5 to 4 μg/mL VWF and (B) 8 μg/mL VWF. The mean ratio ± SEM from 3 experiments is shown.
At the highest ligand concentration of 8 μg/mL VWF (Figure 3B), statistical analysis showed that the Del mutant bound similar levels of VWF as wild-type. The Comb-2 mutant (Lys253Ala/Lys258Ala/Lys262Ala) bound 146% of VWF compared to wild-type (P < .005, n = 3). This mutation was the only one in this series that resulted in increased binding to VWF at high concentrations of ligand. Cells that bound less than 50% of VWF compared to wild-type included the Asp269Asn (44%), Glu270Gln (46%), Asp272Asn (15%), Glu282Gln (45%), Asp283Asn (39%), and Comb-1 (Asp283Asn/Glu285Gln/Asp287Asn, 49%) mutants (P < .005, n = 3). The remaining mutants bound 60% to 80% (P < .05, n = 3) VWF compared to wild-type.
Botrocetin-mediated VWF binding
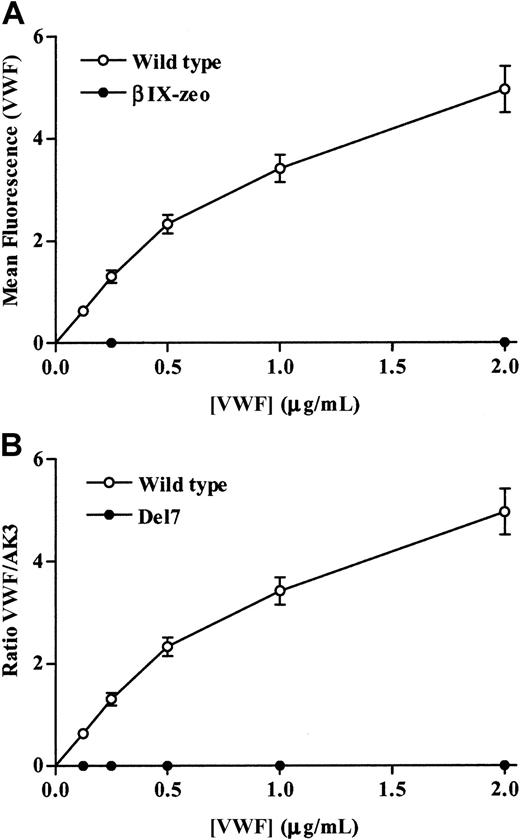
Transfected cells were also incubated with human VWF in the presence of 2 μg/mL botrocetin. Wild-type cells bound VWF (0-2 μg/mL) in a dose-dependent manner (Figure4A), whereas no VWF was detected on the surface of the βIX-zeo cells. The Del mutant also did not bind VWF in the presence of botrocetin (Figure 4B).
Botrocetin-induced VWF binding to control and the Del mutant cells.
(A) Control cells and (B) wild-type and Del cells were incubated with increasing concentrations of VWF (0-2 μg/mL) in the presence of 2 μg/mL botrocetin. Bound VWF was detected using an FITC-conjugated antihuman VWF antibody, followed by flow cytometric analysis. (A) The data are presented as mean fluorescence plotted against increasing VWF concentration. (B) The data are expressed as a ratio of the mean fluorescence of VWF binding to that of AK3 binding. The mean ratio ± SEM from 3 experiments is shown.
Botrocetin-induced VWF binding to control and the Del mutant cells.
(A) Control cells and (B) wild-type and Del cells were incubated with increasing concentrations of VWF (0-2 μg/mL) in the presence of 2 μg/mL botrocetin. Bound VWF was detected using an FITC-conjugated antihuman VWF antibody, followed by flow cytometric analysis. (A) The data are presented as mean fluorescence plotted against increasing VWF concentration. (B) The data are expressed as a ratio of the mean fluorescence of VWF binding to that of AK3 binding. The mean ratio ± SEM from 3 experiments is shown.
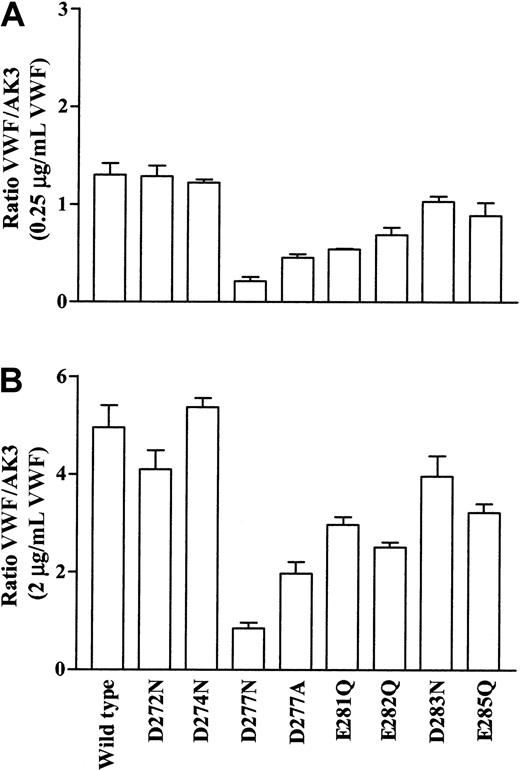
Other mutant cells, however, did bind VWF in the presence of botrocetin. The data presented in Figure5 show binding of VWF normalized for GPIbα expression. At all concentrations of VWF examined, the Asp272Asn, Asp274Asn, and Asp283Asn mutants bound similar levels of ligand as wild-type cells. At low concentrations of ligand (Figure 5A), statistical analysis showed no significant decrease in VWF binding with the Glu285Gln mutant. Cells that bound less VWF compared to wild-type included the Asp277Asn, Asp277Ala, Glu281Gln, and Glu282Gln mutants (P < .01, n = 3).
Botrocetin-induced VWF binding to GPIbα mutant cell lines.
Transfected cell lines were incubated with increasing concentrations of VWF (0-2 μg/mL) in the presence of botrocetin. Bound VWF was detected using an FITC-conjugated antihuman VWF antibody, followed by flow cytometric analysis. The data are expressed as a ratio of the mean fluorescence of VWF binding to that of AK3 binding. Two points are presented: (A) 0.25 μg/mL VWF representing results obtained for the range of 0.125 to 0.5 μg/mL VWF and (B) 2 μg/mL VWF representing results obtained for the range of 1 to 2 μg/mL VWF. The mean ratio ± SEM from 3 experiments is shown.
Botrocetin-induced VWF binding to GPIbα mutant cell lines.
Transfected cell lines were incubated with increasing concentrations of VWF (0-2 μg/mL) in the presence of botrocetin. Bound VWF was detected using an FITC-conjugated antihuman VWF antibody, followed by flow cytometric analysis. The data are expressed as a ratio of the mean fluorescence of VWF binding to that of AK3 binding. Two points are presented: (A) 0.25 μg/mL VWF representing results obtained for the range of 0.125 to 0.5 μg/mL VWF and (B) 2 μg/mL VWF representing results obtained for the range of 1 to 2 μg/mL VWF. The mean ratio ± SEM from 3 experiments is shown.
At the highest ligand concentration of 2 μg/mL VWF (Figure 5B), statistical analysis showed that the Glu285Gln mutant bound slightly less VWF than wild-type (P < .03, n = 3). Cells that also bound significantly less VWF compared to wild-type included the Asp277Asn, Asp277Ala, Glu281Gln, and Glu282Gln mutants (P < .01, n = 3).
Examination for tyrosine sulfation
The mutant GPIbα proteins were further examined for the presence or absence of sulfation by metabolically labeling cells with [35S]-sulfur in sulfate-free media. Analysis of the polyacrylamide gel electrophoresis (PAGE; Figure6) shows that wild-type cells did incorporate [35S]-sulfur and the βIX-zeo cells did not incorporate [35S]-sulfur. The Del mutant, which contains a deletion of the region containing the 3 tyrosine residues, also did not incorporate [35S]-sulfur. The mutants that also did not incorporate [35S]-sulfur and therefore must have an affect on sulfation were Glu270Gln, Asp283Asn, Comb-1 (Asp283Asn/Glu285Gln/Asp287Asn), and Comb-2 (Lys253Ala/Lys258Ala/Lys262Ala).
The effect of the GPIbα mutations on the sulfation of the tyrosine residues.
Transfected cell lines were metabolically labeled with [35S]-sulfur in sulfate-free media, immunoprecipitated, and then equal amounts were loaded onto 7.5% SDS-PAGE as described in “Materials and methods.” (+) indicates incorporation and (−) indicates no incorporation of [35S]. The image shown is a representation of 4 experiments.
The effect of the GPIbα mutations on the sulfation of the tyrosine residues.
Transfected cell lines were metabolically labeled with [35S]-sulfur in sulfate-free media, immunoprecipitated, and then equal amounts were loaded onto 7.5% SDS-PAGE as described in “Materials and methods.” (+) indicates incorporation and (−) indicates no incorporation of [35S]. The image shown is a representation of 4 experiments.
Discussion
This investigation has examined the charged residues from Asp249 to Asp287 (Figure 1) and their effect on the sulfation of tyrosines 276, 278, and 279. The role in regulating the VWF-binding function of the GPIb-V-IX complex was also examined and these data are summarized in Table 2.
Summary of binding data and tyrosine sulfation status for the GPIbα mutants
| Mutant cell line . | AK2 . | Ristocetin . | Botrocetin . | Sulfation* . |
|---|---|---|---|---|
| D249N/D252N (Comb-3) | ↓↓ | NC | NA | NA |
| K253A/K258A/K262A (Comb-2) | ↓↓ | ↑ | NA | No |
| DEL | ↓↓ | ↓↓↓† | – | No |
| D269N | ↓↓↓ | ↓↓ | NA | Yes |
| E270Q | ↓↓↓ | ↓↓↓ | NA | No |
| D272N | ↓↓↓ | ↓↓↓↓ | NC | Yes |
| D274N | ↓↓ | NC | NC | NA |
| D277N | ↓↓↓ | ↓↓ | ↓↓↓↓ | Yes |
| D277A | ↓↓↓ | ↓↓ | ↓↓↓ | Yes |
| E281Q | ↓↓↓ | ↓↓ | ↓↓↓ | Yes |
| E282Q | ↓↓↓ | ↓↓↓ | ↓↓ | Yes |
| D283N | ↓↓↓ | ↓↓↓ | NC | No |
| E285Q | NC | ↓ | NC | Yes |
| D287N | ↓↓ | ↓↓ | NA | Yes |
| D283N/E285Q/D287N (Comb-1) | ↓↓↓ | ↓↓↓ | NA | No |
| Mutant cell line . | AK2 . | Ristocetin . | Botrocetin . | Sulfation* . |
|---|---|---|---|---|
| D249N/D252N (Comb-3) | ↓↓ | NC | NA | NA |
| K253A/K258A/K262A (Comb-2) | ↓↓ | ↑ | NA | No |
| DEL | ↓↓ | ↓↓↓† | – | No |
| D269N | ↓↓↓ | ↓↓ | NA | Yes |
| E270Q | ↓↓↓ | ↓↓↓ | NA | No |
| D272N | ↓↓↓ | ↓↓↓↓ | NC | Yes |
| D274N | ↓↓ | NC | NC | NA |
| D277N | ↓↓↓ | ↓↓ | ↓↓↓↓ | Yes |
| D277A | ↓↓↓ | ↓↓ | ↓↓↓ | Yes |
| E281Q | ↓↓↓ | ↓↓ | ↓↓↓ | Yes |
| E282Q | ↓↓↓ | ↓↓↓ | ↓↓ | Yes |
| D283N | ↓↓↓ | ↓↓↓ | NC | No |
| E285Q | NC | ↓ | NC | Yes |
| D287N | ↓↓ | ↓↓ | NA | Yes |
| D283N/E285Q/D287N (Comb-1) | ↓↓↓ | ↓↓↓ | NA | No |
Single-letter amino acid codes used in the table.
Arrows indicate increases or decreases in binding; NC, no change in binding compared to wild-type; −, no binding detected; and NA, not analyzed.
Yes indicates the recombinant cells have incorporated [35S], and no indicates no incorporation of [35S].
In the presence of ristocetin, the Del mutant bound less VWF at low ligand concentrations yet bound similar levels of VWF at high concentrations, compared to wild-type cells.
Two mutations to individual carboxylic acids resulted in the abolition of sulfation. These were glutamic acid 270 to glutamine (Glu270Gln) and aspartic acid 283 to asparagine (Asp283Asn). There was also loss of sulfation with the Comb-1 mutation (Asp283Asn/Glu285Gln/Asp287Asn), which confirmed that Asp283 is an important residue for this posttranslational effect. These mutations also resulted in significant changes in ristocetin-mediated VWF binding and changes to the AK2 binding epitope. The decreased binding to VWF could therefore be accounted for by both the loss of sulfation and also to the conformational change in the amino-terminus of GPIbα. This reduced binding to VWF associated with the loss of sulfation is in agreement with other more general studies examining tyrosine sulfation and VWF binding6-10; however, this is the first report identifying specific amino acids that affect tyrosine sulfation.
The Glu270Gln and Asp283Asn mutations are positioned −6 and +7 centered on the first tyrosine residue (Tyr276). These positions exist in other tyrosine-sulfated proteins described by Rosenquist and Nicholas25 and Hortin et al.23 There were 3 of 69 proteins containing a glutamic acid residue at −6 to a sulfated tyrosine and 4 of 54 proteins contained an aspartic acid residue +7 of a sulfated tyrosine. In relation to Tyr278, Glu270Gln is −8 and Asp283Asn is +5. These positions also agree with those published, where 1 of 54 tyrosine-sulfated proteins contain a glutamic acid at −8 and 5 of 54 proteins contain an aspartic acid at +5. In relation to the third tyrosine Tyr279, Glu270Gln is −9 and Asp283Asn is +4. There are 2 of 54 proteins with the glutamic acid at −9 and 2 of 54 proteins with an aspartic acid at +4. Because there are only a small number of proteins with sulfated tyrosines that actually contain a similar sequence to our protein, this further supports the fact that there is no set consensus sequence for tyrosine sulfation as proposed by Niehrs et al.29 However, there does seem to be a predominance of glutamic acids (48 of 69 proteins) at the COOH side of the sulfated tyrosine residue,25 which further corroborates that the result seen with the mutation to Glu270 is real.
Mutations to other carboxylic acids did not result in the abolition of sulfation; however, there was a significant decrease in ristocetin-induced VWF binding associated with decreased binding to AK2. Therefore the change in the interaction with VWF could partly be accounted for by the probable conformational change in the amino-terminus of GPIbα indicating that some of these residues may be involved in regulating VWF binding. These data do not distinguish those amino acids that may directly interact with VWF.
The current study has also highlighted differences in the adhesive properties of the different recombinant forms of GPIbα. The results obtained from this study and from both Murata et al26 and Marchese et al7 demonstrate that the carboxylic acids located before Pro280 play a role in ristocetin-mediated VWF binding. However, our results have identified specific carboxylic acids located after Pro280 that also have an effect on ristocetin-mediated VWF binding. These findings contrast those of Murata et al,26who reported minimal change in ristocetin-mediated VWF binding with their mutant 2. The 2 systems used to examine binding are distinct. Murata et al26 studied 2 mutants containing block changes to all the carboxylic acids between Ser251 and Tyr279 (mutant 1) and Pro280 and Ala302 (mutant 2). Our study has examined the individual carboxylic acids. Murata et al26 examined soluble GPIbα immobilized onto nitrocellulose. We have examined the mature GPIbα protein associated with GPIbβ and GPIX and containing the cytoplasmic domain, including those regions involved in cell signaling.30 In our model system there may be effects on the receptor complex or on cell signaling that are not evident with the block mutants or with the purified extracellular fragment of GPIbα examined by Murata et al.26
Another mutation that resulted in the loss of sulfation was Comb-2 (Lys253Ala/Lys258Ala/Lys262Ala). This mutation also resulted in increased binding of VWF in the presence of ristocetin and reduced reactivity to AK2. Interestingly, other groups who have also changed lysine residues in both GPIbα and its ligand VWF have observed this gain-of-function binding.31 32 These data indicate that the loss of the positively charged side chains from the lysines may allow the 2 proteins to come together more easily and this has had such a profound effect that it has led to an increased interaction between GPIb and VWF despite the loss of the sulfate groups.
The effect of the Comb-2 mutation on tyrosine sulfation was unexpected because this mutation is not located in the high negatively charged environment normally associated with tyrosine sulfation. This result could indicate that the change in sulfation associated with this mutation may be due to increased acidity caused by the removal of the positively charged side chains of the lysine residues. This could further imply that the electrostatic nature of the protein might be important for the posttranslational process. Alternatively, the reduction in positive charge may eliminate the requirement for tyrosine sulfation altogether, because there was no change to the net charge of the protein. Overall, these observations indicate the lysine residues seem important in regulating the affinity of GPIb to VWF. We also propose that lysine residues may regulate charge distribution in the ligand-binding domain of GPIbα, as well as the sulfation of the tyrosine residues. Further studies defining this role of the lysine residues are beyond the scope of this study.
One mutation that provided a unique insight to tyrosine sulfation and to the GPIb-VWF interaction was the Del mutant. This mutation deleted the 7 amino acids from Tyr276 to Glu282 and resulted in the loss of sulfation. This has confirmed previous studies6-8demonstrating that it is the 3 tyrosines at positions 276, 278, and 279 in GPIbα that are sulfated. Removal of these sulfated tyrosine residues has led to a slight decrease in the interaction with VWF in the presence of ristocetin, indicating that these 7 amino acids may not play as major a role in ristocetin-mediated VWF binding to GPIbα.
In contrast, these 7 amino acid residues are crucial for botrocetin-mediated VWF binding as proposed by Ward et al.8 Deletion of these residues resulted in complete abrogation of botrocetin-mediated VWF binding. Mutations to the flanking carboxylic acids did not affect botrocetin-mediated VWF binding as significantly as mutations to the carboxylic acids within this region. This included the Asp283Asn mutant, which led to the loss of sulfation but did not have an effect on botrocetin-mediated VWF binding. Only Glu285Gln led to a small decrease in reactivity to VWF, whereas Asp277Ala, Glu281Gln, and Glu282Gln led to 40% to 60% decreases in reactivity compared to wild-type. Asp277Asn bound the least VWF in the presence of botrocetin indicating that this residue plays an important role in this interaction. Similar observations were made by Murata et al26 and Marchese et al,7where the negatively charged residues between Ser251 and Glu285 were shown to be important in botrocetin-mediated VWF binding. However, this is the first report identifying specific carboxylic acids in GPIbα involved in botrocetin-mediated VWF binding. These data are consistent with a recent study examining the crystal structure of botrocetin with the A1 domain of VWF, which has implicated Lys599 as being important in the VWF-GPIb interaction.33 If positive charge residues in VWF are exposed in the proposed GPIb-binding site on VWF, then the negatively charged residues in GPIbα could likewise be important.
In conclusion, this investigation has clearly demonstrated that both Glu270 and Asp283 in the platelet GPIbα protein can directly affect sulfation of the tyrosine residues in this protein. The absence of sulfation caused by these mutations is associated with decreased interaction between the plasma ligand VWF with GPIbα, in the presence of ristocetin, which further supports the idea that sulfation promotes specific protein-protein interactions. This investigation has also confirmed that the region from Tyr276 to Glu282 is the botrocetin-mediated VWF-binding domain in GPIbα. Based on the observations from our botrocetin-binding studies and from the crystallography work of Huizinga et al,33 negatively charged residues in this region may play a role in strengthening or stabilizing the VWF-GPIb interaction.
We thank Professor Michael Berndt for supplying the various monoclonal antibodies and the purified human VWF and botrocetin used in the flow cytometry binding assay, Dr Rob Andrews for his technical advice, and Ms Leonie Gaudry for operation of the flow cytometer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sasha Tait, 2201/148 Elizabeth Street, Sydney, New South Wales 2000, Australia; e-mail: s.tait@unsw.edu.au.

![Fig. 6. The effect of the GPIbα mutations on the sulfation of the tyrosine residues. / Transfected cell lines were metabolically labeled with [35S]-sulfur in sulfate-free media, immunoprecipitated, and then equal amounts were loaded onto 7.5% SDS-PAGE as described in “Materials and methods.” (+) indicates incorporation and (−) indicates no incorporation of [35S]. The image shown is a representation of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/12/10.1182_blood.v99.12.4422/4/m_h81222685006.jpeg?Expires=1767941307&Signature=cjyyZ9HovFm~AdZX7vxuZa7v~X3XYQXQ0St-sBAegZIFNT0WyiFNRypjWn4tBIy4e7YD9ezVJBGYRaJsqsNqbPuwUnSS0yHH8xRQSUWG0aRldR26R0j0c32yy7nJdx0JXCYGpA9drFIFLnQ7pa4hybOPJ4DPXuH~hQ9~sljiYjz9w3EQl5fm30ubtQxTWN2hTOpeOOA-pDcrDKB0VpBBd5eEMvSodJxICehvfYxiJ~ejcceQnKrFLd69Cd2tc6q9KuoBbsBq8NWJoui2rK7IIOJ3uQBYWKxAQ68CK-v64fIRp08cj0S8VmYq8PKVS65jRRwRca8Syk74Oko8hcMpbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal